| R.T. Kono, corresponding author. e-mail: rtkono@kahaku.go.jp phone: +81-3-3364-7132; fax: +81-3-3364-7104 Published online 13 April 2004 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.03106 |
Dental elements in general have a greater chance to be preserved and fossilized than other body parts. Therefore, they often constitute a major part of fossil collections. This is true in the case of primate fossils, including fossil hominids.
The dentition is functionally adapted to diet and feeding behavior. Morphogenesis of the tooth crown is controlled mostly by genetic factors, rather than by environmental factors. While bones continue to remodel throughout an individual’s life, tooth enamel does not regenerate once its formation is completed. Therefore tooth morphology reflects both phylogenetic heritage and the results of selection for functional adaptation. Teeth are also suitable to the study of morphology in relation to its formational processes, since their developmental history is in part recorded as histological markings. For these reasons, dental morphology has been intensively studied, as an important source of information from both functional and phylogenetic perspectives.
Molars show great morphological diversity among taxa. This is thought to be closely related to functional requirements imposed by the physical properties of the masticated food (e.g., Janis and Fortelius, 1988). For molars of extant great apes and modern humans, morphological studies have been conducted from various perspectives. Hominoid molars share a generalized morphological pattern compared to those of other mammals, but also exhibit taxonomically informative differences in details (e.g., Hartman, 1988, 1989; Kobayashi, 2001). Molar enamel thickness is known to vary among the extant hominoids, apparently exhibiting substantial functional and/or phylogenetic information (e.g., Martin, 1983; Shellis et al., 1998).
Lawrence Martin and his colleagues discussed the evolution of hominoid enamel thickness by comparing extant great apes, modern humans, and fossil hominoids and hominids (Martin, 1983, 1985; Grine and Martin, 1988; Andrews and Martin, 1991). Martin physically sectioned molars at a plane that passes approximately through the tips of the two mesial cusps (mesial cusp section, MCS), and made various thickness measurements on those sections. He concluded that the “average enamel thickness (AET)”, calculated as enamel area divided by length of the enamel-dentine junction (EDJ), was the most reliable measure of the amount of enamel, since it was supposedly less variable than comparable measures taken at other section positions (Martin, 1983). Moreover, he devised a measure called “relative enamel thickness” as a means to compare thicknesses between taxa with different tooth sizes. Relative enamel thickness is calculated as the AET standardized by the square root of dentine area of the crown at that section. Using this relative thickness value, he proposed four categories of thickness, “thick”, “intermediate~thick”, “intermediate~thin”, and “thin”. He assigned gorilla and chimpanzee molars to “thin”, orangutan molars to “intermediate~thick”, and human molars to “thick” categories (Martin, 1983, 1985). These variables, average and relative enamel thicknesses, have since been used frequently in species comparisons (Grine and Martin, 1988; Andrews and Martin, 1991; Dumont, 1995; Beynon et al., 1998; Shellis et al., 1998).
Martin furthermore related enamel thickness with prism packing patterns, and concluded that enamel was thick and formed fast in the last common ancestor of extant large-bodied hominoids (including humans), while enamel had become thin in the gorilla and chimpanzee because of a secondary decrease in enamel formation speed (Martin, 1983, 1985; Andrews and Martin, 1991). Later, however, Beynon et al. (1991) reexamined hominoid molar histology, and showed that no such decrease in enamel formation speed is in fact seen in gorilla and chimpanzee molars. In all four species that the latter authors examined, enamel formation speed was found to gradually increase from EDJ to the outer enamel surface (OES). They therefore rejected Martin’s interpretations of enamel thickness and speed of formation. They further proposed that thin and fast-forming enamel was likely to be the ancestral condition of the last common ancestor of humans and extant great apes (Beynon et al., 1991). Recently, Shellis et al. (1998) examined, in various primate taxa, the relationship between Martin’s average enamel thickness and size measurements made on the mesial cusp section. They presented a somewhat different view of great ape enamel thickness, showing that, when empirically scaled relative to tooth size using a taxonomically broader primate data set, AET is actually not so different between chimpanzee and orangutan molars (Shellis et al., 1998). They then presented the alternative opinion that the last common ancestor possibly had “average” enamel thickness (Shellis et al., 1998).
Molnar and Gantt (1977) were the first to discuss the functional significance of enamel, by using a numerical evaluation of enamel thickness of extant great ape and human molars based on linear measurements. They reported that enamel was thicker in humans than in apes at multiple positions of the molar tooth crown. They proposed that the thick human enamel, together with the low cusps, was adapted to a crushing-grinding function, although no functional rationale was presented (Molnar and Gantt, 1977). Kay (1981) measured enamel thickness on the worn occlusal surface of molars in a wide range of primate taxa, and plotted thickness against the mesiodistal length of the tooth. He noticed that enamel tends to be thicker in frugivorous species, whereas the more folivorous taxa tend to have thinner enamel (Kay, 1981). Among great apes, according to his interpretation, Pongo has relatively thick enamel, which might be an adaptation to eating very hard fruits, while Gorilla has relatively thin enamel and well-developed shearing crests, which are likely to be adaptations to leaf-eating (Kay, 1981).
More recently, Macho, Schwartz and colleagues studied enamel thickness patterns within the molar crown, and discussed their functional significance (Macho and Berner, 1993, 1994; Spears and Macho, 1995, 1998; Schwartz, 1997, 2000a, b; Macho and Spears, 1999). Macho and Berner (1993) showed that the values of “average” and “relative enamel thickness” introduced by Martin (1983) differed between tooth types within the human molar row. They thus proposed the necessity to investigate enamel thickness patterns seen among tooth type, or within a tooth, rather than simply obtaining a representative thickness value for the entire tooth and/or species (Macho and Berner, 1993). They measured enamel thickness at multiple positions on the MCS, and suggested that the first upper molars have a pattern of enamel distribution different from that of the second and third upper molars. Human upper first molars were found to be characterized by distinctly thicker lingual and thinner buccal enamel. The posterior two molars exhibited overall thicker enamel than the first molar, and with less discrepancy between lingual and buccal enamel thicknesses, so that the “non-functional” buccal cusps have somewhat thicker enamel. This was interpreted as reflecting different functional roles of the first and posterior two molars (Macho and Berner, 1993, 1994).
Using finite element analysis, they further investigated stress distribution patterns in the MCS, under load from different directions, and compared the molars of Homo, Pan, and Pongo in their capacity to resist such mechanical loads (Spears and Macho, 1995, 1998; Macho and Spears, 1999). According to them, in human lower molars, the buccal cusps the so-called “functional cusps” better dissipate occlusal loads compared to their lingual counterparts, whereas in upper molars, the buccal (“non-functional”) cusps generally resist loads as well as the lingual cusps. Moreover, it was suggested that in the second and third upper molars, the buccal cusps even better dissipate loads than the lingual cusps. These results were interpreted by the authors that all lower molars act as “pestles”, while the upper molars especially the posterior ones are better suited to act as “mortars” (Spears and Macho, 1995, 1998). While they considered enamel thickness to be part of this structural resistance to mechanical load, such that thick enamel was considered to be an adaptation to larger magnitudes of occlusal load, they later showed that occlusal topography is more responsible for efficient load dissipation and resistance rather than enamel thickness itself (Macho and Spears, 1999).
Schwartz (1997) used computed-tomography (CT) as a non-destructive method of measuring enamel thickness in fossil hominids. Combining this with previously published data of modern great ape and human molars taken on physical sections at the MCS (Schwartz, 1997, 2000a), he interpreted the results on the assumption that regions with relatively greater functional loads would have thicker molar enamel (Schwartz, 1997, 2000a). However, by functional load, whether he meant more wear or greater mechanical load, was not made clear nor kept consistent throughout his discussions. He further suggested that it is possible to discriminate among hominoid species using enamel distribution patterns of the maxillary molars at the MCS (Schwartz, 1997, 2000a). He also analyzed enamel distribution patterns of human lower molars, and concluded that a pattern similar to the one shown by Macho and Berner (1993, 1994) for the upper molar row was not seen (Schwartz, 2000b). In human lower molars, he found that buccal enamel thickness tended to be consistently thicker in the posterior molars, but with no relative increase of thickness in the “non-functional” lingual cusp. He interpreted this in a framework of functional adaptation in relation to the helicoidal occlusal plane, as proposed by Macho and Berner, that all lower molars function as “pestles” regardless of their position along the tooth row, supporting the view that thick enamel is an adaptation to greater loads (Spears and Macho, 1995, 1998; Macho and Spears, 1999; Schwartz, 2000b).
Although several attempts have been made, as summarized above, to investigate phylogenetic and/or functional significance of molar enamel thickness in great apes and humans, the information so far gained is limited in that most previous studies measured thickness only on the specific section which runs through the two mesial cusp tips, as initially introduced by Molnar and Gantt (1977) and Martin (1983). Phylogenetically, enamel thickness of each species has often been evaluated with reference to simple categories such as “thick” or “thin”, by use of a single representative value of thickness, such as the “average enamel thickness” measured at the MCS (Martin, 1983, 1985; Shellis et al., 1998). No effort has been made to test the validity of the use of this specific section as representative of enamel thickness of the entire molar crown. It remains to be seen whether the “average enamel thickness” measured at the MCS is really a good estimator of the average thickness of the entire molar crown, as Martin (1983) had intended. Recent studies that aimed to investigate enamel distribution patterns have indicated that not only a single representative value but also within tooth distribution patterns may also be informative for phylogenetic inferences (Macho and Berner, 1993, 1994; Schwartz, 1997, 2000a, b). However, these studies were also limited to analyses of the MCS (Macho and Berner, 1993, 1994; Schwartz, 1997, 2000a, b; Spears and Macho, 1998; Macho and Spears, 1999). Such results are obviously insufficient to establish the significance of enamel distribution of the entire crown.
As for the functional significance of enamel thickness, most interpretations seem to have been based on the assumption that the thicker the enamel the greater the load it could resist (Macho and Berner, 1993, 1994; Spears and Macho, 1995, 1998; Macho and Spears, 1999; Schwartz, 2000b). However, as already mentioned, Macho and Spears (1999) themselves implied that thickness itself may not be a significant factor determining the molar’s capacity to resist mechanical load. Other factors such as resistance to wear should also be taken into account when examining the functional meaning of enamel distribution patterns.
In order to understand the significance of molar enamel thickness and its patterning, it is necessary to first examine and establish enamel distribution patterns of the entire molar crown of each tooth type of each species. Only through this approach, would it become possible to discuss the relationship between the average thickness of enamel and the characteristics of its distribution pattern, and then to offer more informed hypotheses about the evolution and adaptation of enamel thickness. The aim of this study is thus: 1) to determine a variety of “representative” values of enamel thickness of the whole molar tooth crown, using 3D volumetric measures, 2) to compare 3D-based average enamel thickness (enamel volume divided by EDJ surface area) with the widely used 2-dimensional (2D) measure (enamel cross-section area divided by EDJ length), 3) to investigate and compare the whole-crown enamel distribution patterns of four extant hominoid species (modern humans, gorillas, chimpanzees, and orangutans), and 4) to discuss the evolutionary history and functional significance of molar enamel thickness in hominoids.
Forty-one permanent molars of Homo sapiens of Jomon period Japanese (n=10), Edo period Japanese (n=12), and modern Asian (n=19), and a hominoid sample consisting of permanent molars of Pan troglodytes (n=22), Gorilla gorilla (n=4), and Pongo pygmaeus (n=7) were analyzed in this study. Molars are either first or second, and upper or lower. The details of sample compositions are given in Table 1. Sex is unknown for most of the specimens, since they were taken from juvenile individuals.
The Jomon and modern Asian human molars were taken from the skeletal collection housed in The University Museum, The University of Tokyo. The modern Asian molars were taken from educational skeletal specimens of unknown origin. The Edo period specimens derive from the skeletal collections housed in the National Science Museum, Tokyo. Although these three populations are from different ages and/or regions, they are all pooled in the present study since apparent population differences were not seen in the preliminary stages of our investigation. Besides, the aim of this study is to investigate differences and patterns seen at the species level. Therefore, it is reasonable to combine populations representing intraspecific variation.
Most of the hominoid teeth are from the collection of the Cleveland Museum of Natural History, collected as wild-shot specimens. The rest of the specimens of chimpanzee and orangutan are housed either at the University of California, Berkeley, or The University Museum, The University of Tokyo.
Molars were chosen to have no wear, or slightest wear. Although it was possible to obtain a substantial number of completely unworn human molars, this was quite difficult in the case of non-human hominoid species. Therefore molars of these taxa with slight occlusal wear and/or small chipping of enamel around the cervical region were included.
For the purpose of inter-specific comparisons, all tooth types were pooled by taxon, because of the limited number of gorilla and orangutan molars. For the human and chimpanzee samples, comparisons between tooth types were also possible.
Three-dimensional shape of the enamel cap is delineated by the topographies of the EDJ and OES. In this study, enamel cap shape of the molar crown was reconstructed by obtaining digital representations of these two surfaces. The resulting data set of the molar crown was then subjected to various measurements of enamel thickness and other parameters. In the primary acquisition of the digital morphological data, either of two sets of devices was used. These were the 3D surface digitizing system using a laser beam (Voxelan small field model, Hamano-engineering, Kanagawa), and the micro-focal X-ray CT scanning system (TX225-ACTIS, TESCO Corporation, Tokyo). Only the modern Asian specimens (n=19) were digitized by the former system (Kono et al., 2002) and a detailed explanation of this method is given elsewhere (Kono-Takeuchi et al., 1997; Kono et al., 1998, 2002; Kono, 2002).
The human molars of the Jomon and Edo period Japanese populations and all molars of the great apes were digitized by means of serial CT scanning. Each molar was scanned with a tube voltage of 90 kV (for Gorilla molars, 100 kV), filtered with a 3 mm thick aluminum plate (0.5 mm copper plate for Gorilla), with a current of 0.13 to 0.16 mA. Each molar was scanned perpendicular to its long axis, serially with intervals of either 0.056, 0.062, 0.068, 0.074, or 0.084 mm, according to its crown size. The number of scanned sections was up to 200 for each molar crown. Each section was reconstructed in a matrix size of 512×512 pixels. The size of the pixels was set to half of the slice interval. Slice thickness was kept constant at 0.05 mm regardless of tooth size and slice interval. Pixel size, slice interval and slice thickness were all calibrated to an accuracy of circa 0.1% by measuring an aluminum rod of known diameter. The boundary between enamel and dentine was determined by thresholding the CT images using the half maximum height method (Spoor et al., 1993). In scanning the molars, they were immersed in a sodium polytungstate (SPT) solution (Danhara et al., 1992), the concentration of which was empirically adjusted so that X-ray absorption was equivalent to that of dentine. This enabled both OES and EDJ to be optimally defined by the same threshold.
The digitized data of each molar was organized into a set of data formats described below, by use of 3D image analysis software (3D- and CT-Rugle, Medic Engineering Inc., Kyoto). These applications were in part developed through our research project (Kono-Takeuchi et al., 1997; Kono et al., 1998, 2000; Suwa and Kono-Takeuchi, 1998; Suwa et al., 2000). This data formatting process enables us to handle the two (laser and micro-CT based) data types equivalently in all ensuing measurement procedures. The overall accuracy of both data sets was confirmed to be sufficient and comparable (Kono et al., 2000; Suwa and Kono, 2000; Suwa et al., 2000).
The orientation of the tooth was systematically adjusted during the data formatting process, so that regions of interest could later be defined in relation to the standardized orientation. First, each molar was oriented to maximize the projected occlusal surface area (Suwa and Kono-Takeuchi, 1998). This was done with the EDJ rather than with the OES, since the occlusal fovea of the EDJ is bounded by a sharp rim of dentine that can be delineated regardless of slight differences in initial orientation (Kono et al., 2002). In orienting the maxillary molars, only the trigon basin was used. Once the occlusal view orientation of the molar was adjusted, the entire data set of the tooth was rotated around the axis of projection, the z-axis, so that the dentine tips of the two mesial cusps were aligned on the same x-coordinate.
In order to derive the different kinds of variables that were analyzed in this study, the original digitized data set of each molar was transformed into three formats: the xz- and yz-line data, the T-map data, and the VOL data. The line data were used in the calculation of surface area and EDJ length (Kono et al., 1998). Both xz- and yz-line data were prepared for each molar. Each xz- (or yz-) line data is composed of lined data points of EDJ or OES cross-section contours, represented by 640 points within each xz- (or yz-) plane, of evenly spaced serial xz- (or yz-) planes. The interval between planes was set to correspond to unit pixel size, and the number of planes would be around 150 for each xz- (or yz-) line data. The length of the EDJ contour on a particular xz- (or yz-) plane was calculated as the sum of the distances between adjacent points. Surface areas were calculated by forming triangular patches between adjacent lines, and summing their areas. The number of triangular patches used in surface area calculation was typically about 250,000 for each molar.
The T-map data format takes the form of a thickness map (Kono et al., 2002). For each data point of the OES, the minimum distance from that point to the EDJ surface was calculated and allocated to that point. A T-map data format of a molar crown in occlusal view is typically represented by about 30,000 points. This data format enables calculation of representative thickness values of certain crown regions of interest (such as the average thickness of the occlusal surface of the protoconid), by averaging or ranking thickness values of all points included in that region.
The VOL data format was used in the calculation of the volume of a certain part of the crown or enamel cap. This format is in 256×256×256 matrix, and each voxel is assigned to be either enamel, dentine (see below), or blank (outside of the tooth crown). The algorithm for the volume measurement is a simple count of the number of voxels within the region of interest, multiplied by the calibrated size of the voxel.
The accuracy of volumes, surface areas, and curvilinear distances derived from the above data formats were modeled by measuring virtual hemi-spheres. Digital data processing error was estimated to be better than 0.1% for volume, 1.5% for surface areas, and 0.5% for curvilinear distances (Suwa and Kono, unpublished study).
Variables studied in this paper are categorized into five groups according to type and objective (Table 2). Brief descriptions of each of these five groups are given below.
Group 1: Measures of molar crown size
This first group consists of several basic size measures of the tooth crown, including enamel and dentine volumes, and EDJ surface area. In this study, “tooth crown” is defined as the enamel cap and the dentine and pulp cavity portions enclosed within it, but here the latter two portions were treated not separately but together as “dentine”. This is partly because the molars used in this study were at various stages of root formation (since they were mostly unworn) and had not reached the final proportions of dentine and pulp cavity within the above defined tooth crown.
Two of the measurements, the basal and maximum horizontal areas, were taken on horizontal sections of the crown. Basal area is dentine cross section area measured at the lowest height where enamel is continuous around the entire circumference of the section. Maximum horizontal area is the maximum cross section area in standardized orientation (as defined above). Maximum horizontal area includes both enamel and dentine tissue.
Group 2: Representative enamel thickness values of the entire crown
Average enamel thickness in the strict 3D sense was derived, that is enamel volume divided by EDJ surface area. Enamel volume relative to the horizontal cross section area was calculated as a rough indicator of the durability of tooth against abrasion. The average of the minimum enamel thickness values was calculated, as an alternative way of representing mean enamel thickness.
Group 3: Variables measured on the MCS
In order to compare the 3D variables with their 2D counterparts of previous studies, “average-” and “relative enamel thickness” were calculated from enamel area, EDJ length, and dentine area of the MCS, as defined by Martin (1983).
Group 4: Variables representing enamel distribution patterns within the crown
In order to compare overall patterns of enamel distribution, molar crown enamel was divided into two portions in two different ways; cuspal and basal, or occlusal and lateral. Average thickness values were calculated for each of these respective subdivisions of the crown. For the cuspal and basal regions, average thickness was calculated as enamel volume divided by EDJ surface area. The boundary between cuspal and basal portions was set at the horizontal plane passing through the lowest point on the 3D occlusal EDJ surface. Occlusal and lateral average thicknesses were obtained by averaging the minimum distances from OES to EDJ. The occlusal region was defined on the OES as the area enclosed by the cusp tips and marginal ridges.
Group 5: Representative values of cusp-by-cusp enamel distribution
Enamel distribution patterns were investigated in a cusp-by-cusp manner, using the T-map data of occlusal and lateral surfaces of each cusp (Kono et al., 2002). For the lateral surfaces, the maximum, median, and the average of minimum thickness values were calculated for each cusp. The 95th percentile value was designated here as the “maximum” thickness for the purpose of eliminating possible effects of noise. For the occlusal surface, the average minimum thickness was calculated for each cusp.
Variables expressing molar crown size are summarized in Appendix 1. In all of these variables, Gorilla molars were significantly larger than those of the other three species. Pongo molars were significantly larger than those of Pan and Homo in all variables, except with enamel volume in Homo. Comparing Pan and Homo, enamel volume was significantly greater in Homo, but dentine volume was comparable. Human molars therefore can be described as having a dentine crown the size of Pan, covered with enamel the volume of which equals that of Pongo. EDJ surface area, basal area and maximum horizontal area were all greater in Pan than in Homo. This reflects differences in molar crown shape between these two taxa.
Figure 1 shows the relationship between these variables and dentine volume. There was a strong correlation between dentine volume and EDJ surface area (r=0.991, with both values log-transformed) (Figure 1a). Coefficient of the logarithmic regression line was 0.681±0.022, indicating that the relationship did not deviate significantly from isometry. When viewed in relation to the line of isometry passing through the average of Homo, however, a tendency characteristic of each taxon could be seen to exist. Gorilla and Pan individuals were placed a little above this line, while Pongo individuals tended to be on or below this line. Differences between taxa were more clearly shown when enamel volume was plotted against dentine volume (Figure 1b). In relation to the line of isometry passing through the average of Pan, enamel volume was relatively greater in Homo and smaller in Gorilla. Pongo was comparable, or isometric, to Pan in this regard. The basal and maximum horizontal areas were also correlated with dentine volume (data not shown).
 View Details | Figure 1. (a) Log-plot of EDJ surface area against dentine volume. The dotted line is the least squares regression line, and the solid line is the line of isometry running through the average of Homo. (b) Log-plot of enamel volume against dentine volume. The two lines are the lines of isometry running through the average of Homo and Pan, respectively. Circles, Homo; filled triangles, Pan; open triangles, Gorilla; crosses, Pongo. |
The above variables did not differ significantly between tooth type in both Homo and Pan (Appendix 2). The only exception is that, in the upper molars of Homo, enamel volume and EDJ surface area were significantly greater in the first than in the second molar. Dentine volume and maximum horizontal area were also greater in the first molar, although the differences were not significant. These results imply that the second molars are smaller overall than the first molars in Homo.
The conventional 2-dimensional average enamel thickness (2D AET) and relative enamel thickness (2D AETSTD) were compared between species (Table 3). The results were mostly the same as those of Martin (1983). The mean values of 2D AET in Pan and Gorilla, however, were greater in the present study. While 2D AETSTD of Pan and Gorilla did not differ significantly in Martin’s data, a significant difference was seen between the two in the present study (Table 3) with Pan exhibiting thicker enamel than Gorilla. To further investigate the effect of tooth size, 2D AET was log-plotted against dentine area, and examined with regard to the line of isometry running through the average of Pan (Figure 2a). The Gorilla molars were positioned below the line, and those of Homo and Pongo were distributed above it.
 View Details | Figure 2. Log-plot of 2D AET against dentine area on the MCS (a), and 3D AET against dentine volume (b). The line of isometry runs through the average point of Pan. Circles, Homo; filled triangles, Pan; open triangles, Gorilla; crosses, Pongo. |
2D AET was also compared between tooth type within Homo and Pan (Table 4). In Pan, there was no significant difference between tooth type. In Homo, the upper molars exhibited greater 2D thicknesses than the lower molars.
Among the four species included in this study, 3D AET was significantly greater in Homo, and smaller in Pan (Table 3). The difference between Gorilla and Pongo was not significant. 3D AET was log-plotted against dentine volume and examined with regard to the line of isometry passing through the average of Pan (Figure 2b). Like the pattern seen in the case of 2D AET, Homo and Gorilla molars tended to deviate above and below this line, respectively. On the other hand, Pongo molars were distributed near the line. When the 3D version of relative enamel thickness was statistically compared among taxa (Table 3), Pongo and Pan did not differ significantly. This means that, relative to tooth size, the 3D determined average thickness is comparable in Pan and Pongo, in contradistinction to the 2D results.
In order to further investigate the relationship between enamel thickness and tooth size, correlation coefficients between 3D AET and the variables representing tooth crown size were calculated (Table 5). No significant correlation was found with any of the size variables when all species were pooled, or within Homo or Pan. Several of the size variables were shown to correlate significantly with 3D AET in Gorilla and Pongo, but these results should be regarded as tentative, since the sample sizes were small.
In Homo and Pan, 3D AET was compared between tooth type (Table 4). There was no significant difference seen between jaws or tooth positions, in contradistinction to the suggested serial difference of enamel thickness on the MCS reported for human upper molars (Macho and Berner, 1993, 1994).
In order to understand the basis of the discrepancy seen between 2D and 3D results, the variables measured on the MCS were compared between taxa (Appendix 1). The patterns seen in the 2D variables were different from those of the corresponding 3D variables. For example, while EDJ surface area differed among the four taxa, EDJ contour line length was not significantly different between Homo, Pan and Pongo. Figure 3 shows that these variables are not uniformly related among the four species. In Pongo, 2D dentine area and EDJ length were smaller relative to their corresponding 3D values.
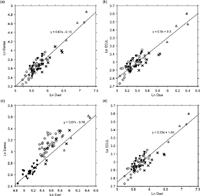 View Details | Figure 3. Log-plots of 2D variables measured on the MCS against the respective 3D counterparts: (a) dentine area against dentine volume; (b) EDJ contour length against EDJ surface area; (c) enamel area against enamel volume. To compare with Figure 1(a), EDJ contour length is log-plotted against dentine volume (d). The lines are the isometry lines running through the average of Pan. Circles, Homo; filled triangles, Pan; open triangles, Gorilla; crosses, Pongo. |
In 3D evaluation, EDJ surface area was shown to change almost isometrically with dentine volume throughout all four taxa (Figure 1a). EDJ contour line length, however, did not exhibit such constancy in relation to dentine volume (Figure 3d). EDJ length measured on the MCS was relatively smaller in Pongo than in the other species.
The 2D variables were compared between tooth type in Homo and Pan (Appendix 2). In both species, enamel area differed significantly between upper and lower first molars. The other two variables also tended to be larger in the upper molars. In Homo, EDJ length was longer in the first molars than in the second in both upper and lower jaws. Since there was no such difference seen between tooth type with the 3D variables, these differences likely reflect shape differences that are emphasized specifically on the MCS.
As an alternative to the AET, the average of the minimum thickness values taken from all OES points to EDJ was calculated for the entire tooth crown (AMT, Table 6). The results were similar to those of the 3D AET, in that Homo and Pan were shown to have the thickest and the thinnest enamel, respectively. The log plot against dentine volume was also shown to be similar to that of 3D AET, although Pongo deviated a little above the line of isometry running through the Pan average (Figure 4).
 View Details | Figure 4. Log-plot of average minimum thickness against dentine volume. The line of isometry runs through the average of Pan. Circles, Homo; filled triangles, Pan; open triangles, Gorilla; crosses, Pongo. |
Enamel volume was divided by basal or maximum horizontal areas of the tooth crown (AETBA and AETMA, Table 6). These measures represent the total amount of enamel available for wear, relative to approximate areas of occlusion available through time. When divided by the basal area, significant differences were seen between all pairs of the four species, except between Pan and Pongo. Similar results were obtained for maximum horizontal area of the tooth crown, but the exception was between Gorilla and Homo. Both values were greatest in Homo, and decreased in order of Gorilla, Pongo, and Pan. When these measures were log-plotted against dentine volume, Gorilla and Pongo molars were distributed near the line of isometry running through the Pan average, but Homo molars alone deviated from that line (Figure 5).
 View Details | Figure 5. Average enamel thickness values in relation to horizontal areas, log-plotted against dentine volume. Thickness is standardized by basal area (a) and maximum horizontal area (b). The lines are isometry lines running through the average of Pan. Circles, Homo; filled triangles, Pan; open triangles, Gorilla; crosses, Pongo. |
In Homo, enamel volume relative to the horizontal crown areas was larger in the first than in the second molars (Appendix 2), although no difference was seen between tooth type in either the 3D AET or the average minimum thickness of the entire crown. No significant difference was seen between tooth type in Pan (Appendix 2).
The molar crown was divided into two halves, in two different ways, and the average enamel thicknesses of each molar region were compared (Table 7 and Figure 6). Cuspal, occlusal, and lateral average thicknesses differed significantly between each pair of the four species, except between Gorilla and Pongo. Basal average thickness, on the other hand, did not differ significantly among taxa. When log-plotted against dentine volume, each of these four variables showed a distinctive pattern (Figure 7). In Pongo molars, cuspal and occlusal enamel were thicker, lateral enamel was comparable, and basal enamel was thinner, in relation to the line of isometry passing through the Pan mean. Gorilla molars tended to have thinner enamel than those of Pan for all molar subregions except the occlusal basin. The enamel of Homo molars was thicker than that of Pan in the cuspal, occlusal and lateral regions, but basal enamel did not differ significantly. In Homo, a weak correlation was seen between basal enamel thickness and dentine volume.
 View Details | Figure 6. Schematic depiction of the crown subdivisions. See the text for further explanations. |
 View Details | Figure 7. Log plots of regional average enamel thicknesses against dentine volume. The lines are isometry lines running through the average of Pan. Circles, Homo; filled triangles, Pan; open triangles, Gorilla; crosses, Pongo. |
These variables were then compared with the average thicknesses of the entire crown (Figure 8). When cuspal and basal thicknesses were compared with 3D AET, while basal thickness did not show any trend, cuspal thickness changed proportionally with 3D AET. This indicates that the average thickness of the entire tooth crown is influenced predominantly by the amount of enamel around the cuspal region, and the contribution of the basal enamel is relatively small. As for occlusal and lateral thicknesses, the two thickness measures were comparable in Pan molars, while, in the molars of the other three species, occlusal thickness was greater than lateral thickness.
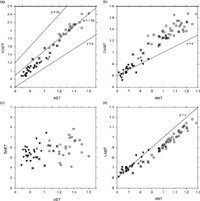 View Details | Figure 8. Regional average enamel thickness values plotted against average values of the entire crown. The lines are arbitrarily drawn for reference. Circles, Homo; filled triangles, Pan; open triangles, Gorilla; crosses, Pongo. |
Comparison between tooth type of the average thicknesses of the partitioned crown (Appendix 2) showed that, in Homo, enamel of the cervical region was thinner in the second molars than in the first molars in both jaws, while occlusal enamel was thicker in the second molars (although this difference was not statistically significant in the upper molars). In contrast, no significant difference was seen between tooth type in Pan. The difference between occlusal and lateral enamel thicknesses, when both are plotted against AMT, was greater in the second molars of Homo (Figure 9), but no such pattern was seen in the sample of Pan (data not shown). In Homo, basal enamel was thicker relative to 3D AET in the first molars (Figure 9).
 View Details | Figure 9. Regional average enamel thickness values in human molars plotted against average values of the entire crown. The dotted lines are the least squares regression lines for each molar type. Filled circles, M1; open circles, M2. |
In order to analyze enamel distribution patterns in more detail, average minimum thickness of the occlusal fovea, and maximum, median, and average minimum thicknesses of the lateral crown surfaces were calculated respectively for each cusp (Appendix 3, Figure 10 and Figure 11). In Pan, only molars with no wear on the occlusal fovea were included in the analysis of occlusal thickness, assuming that slight wear in this region may considerably affect the average value. In Homo, because of the differences seen between tooth type, the first and second molars were analyzed separately in the following comparisons.
 View Details | Figure 10. Cusp-by-cusp enamel distribution patterns in the mandibular molars. Filled circles, Homo M1; open circles, Homo M2; filled triangles, Pan; open triangles, Gorilla; crosses, Pongo. |
 View Details | Figure 11. Cusp-by-cusp enamel distribution patterns in the maxillary molars. Filled circles, Homo M1; open circles, Homo M2; filled triangles, Pan; open triangles, Gorilla; crosses, Pongo. |
In the mandibular molars (Figure 10), average thickness of the occlusal surface and maximum thickness of the lateral surface tended to be thick and thin at the hypoconid and metaconid, respectively. This was especially prominent in the Homo molars. In Pongo molars, median and average thicknesses of the lateral surface were smaller in the buccal cusps than in the lingual cusps. In comparison with the apes, Homo had relatively thicker enamel at the hypoconulid. Also, the average thickness of the occlusal fovea in Homo molars was greater in the more distal cusps. Between tooth type of Homo, it was shown that occlusal and lateral maximum thicknesses were greater in the second molars than in the first, except with the metaconid. All other variables of the metaconid were also relatively thinner in the second molar. On the other hand, occlusal thickness of the protoconid of the first molar was confirmed to be distinctively thin (Kono et al., 2002).
In the maxillary molars (Figure 11), the overall pattern can be summarized as thick enamel occurring at the hypocone and protocone, and thin enamel at the paracone. The difference between Homo and the great apes was not as striking as that seen in the mandibular molars. Pongo molars were again unique in that, in median and average lateral thicknesses, the difference between buccal and lingual cusps was far greater than that seen in any other taxa. In Homo, there was a tendency for occlusal average thickness to increase posteriorly, as seen in the mandibular molars.
Finally, thickness variables were rearranged bucco-lingually to depict enamel thickness pattern in that direction (Figure 12 and Figure 13). The overall pattern of enamel thickness transition was common in all the species regardless of difference of absolute thickness. The buccal half of the crown is characterized by thicker enamel than the lingual half in the lower molars (Figure 12), while the opposite is true in the upper molars (Figure 13). The contrast between buccal and lingual crown portions was most prominent in maximum thickness of the lateral crown wall.
 View Details | Figure 12. Enamel thickness transition within the mandibular molar crown. Variables are aligned in relative buccolingual position across the mesial (top) and distal (bottom) two cusps. Buccal side to the left. Filled circles, Homo M1; open circles, Homo M2; filled triangles, Pan; open triangles, Gorilla; crosses, Pongo. |
 View Details | Figure 13. Enamel thickness transition within the maxillary molar crown. Variables are aligned in relative buccolingual position across the mesial (top) and distal (bottom) two cusps. Buccal side to the left. Filled circles, Homo M1; open circles, Homo M2; filled triangles, Pan; open triangles, Gorilla; crosses, Pongo. |
The several noticeable patterns already described above were confirmed by Figure 12 and Figure 13. For example, the thin lateral thickness of the buccal cusp in Pongo molars, the markedly thin occlusal enamel in Pan molars relative to lateral thickness, and the difference between first and second molars in Homo, are all well expressed.
Martin (1983) suggested that the ideal measure of the amount of enamel of a tooth is the volume of enamel, and that, theoretically, average thickness of the enamel of the entire crown can be derived by dividing enamel tissue volume with the surface area of EDJ. Because EDJ area is proportional to the number of ameloblasts producing enamel matrix, provided that ameloblast size differs little between species, average enamel thickness is equivalent to the average amount of enamel tissue produced by a single ameloblast cell. In the 1980s, however, it was technically difficult to measure enamel volume or EDJ surface area of the entire tooth crown. Thus, as a substitute, 2-dimensional average enamel thickness was introduced; enamel area was divided by EDJ contour length measured on a specifically-positioned section running through the two mesial cusps (Martin, 1983, 1985).
The present study is the first to achieve, with substantial accuracy, an assessment of average enamel thickness in 3-dimensions, derived from size variables of the tooth crown such as enamel tissue volume (Suwa and Kono, 2000; Kono, 2002). Earlier, Kimura et al. (1977) attempted 3D reconstructions of the molar crown, and made rough estimates of enamel volume and EDJ surface area by stacking traced outlines of nine to 14 serially cut buccolingual sections (Kimura et al., 1977). The resolution of their data was less than one-tenth of that of our study, and was not sufficient to discuss enamel thickness or the exact amount of enamel tissue. Yet, it is interesting to note that average thickness values can be calculated from their reported measurements, and its value in two upper first molars (1.38 and 1.62 mm) do fall within the range of variation shown in the present study.
The results of the present study, based on accurate 3-dimensional average thickness, provide some new insights. First, the relationship between 3D AET and conventional 2D AET is not simple, and may differ among species. It was shown that Pan and Pongo are comparable in terms of 3D AET relative to tooth size, in contrast to previous notions based on 2D AET. Additionally, 3D AET appears to vary independently of tooth size within species. These points are discussed more fully in the following paragraphs.
Studies of enamel thickness are always met with the difficulty of obtaining a sufficiently large sample size, because of the usual necessity to analyze unworn or, at most, only slightly worn teeth. It is especially difficult to obtain specimens of great ape species. Therefore, most previous studies dealt with limited sample sizes and included molars with some wear (e.g., Molnar and Gantt, 1977; Martin, 1983; Shellis et al., 1998). The present study is not an exception, in that only a limited number of teeth were available for Gorilla and Pongo. In order to examine the possibility that our results were biased by small sample size, conventional 2D AET of the present sample was compared with those reported in previous studies (Figure 14). The comparative data were taken from Martin (1983) and from part of Shellis et al. (1998; provided by D. Reid), and thus include almost all the data published so far for the great apes. The results of the present study are confirmed not to be biased, although there are some differences seen among the studies. When inspecting Martin’s data (1983) in more detail, it is apparent that he often included two or more teeth from the same individual. This probably biased the data in terms of variation. Also, wear must have been heavier in the anterior molars of those individuals. In the case of Pan, especially, it is likely that these effects lowered the species mean of 2D AET in his study.
 View Details | Figure 14. Comparison of 2D AET values. Diamonds, from Martin (1983); circles, this study; crosses, from Shellis et al. (1998; provided by D. Reid). Worn specimens are placed slightly to the right. For the molars included in the study of Martin (1983), presence of wear follows his own notes. |
Body weight is often used as the measure of body size when relative size is to be considered. However, it is not so simple in the case of enamel thickness, since it is necessary to think of the correspondence between body size and tooth size before considering the relationship between body size and enamel thickness. If the tooth is larger than expected from body size, enamel thickness may appear thicker in relation to body size, even if it is proportional to tooth size (Shellis et al., 1998). Therefore it is more reasonable to use some kind of dental measure to standardize enamel thickness, instead of using a direct measure of body size (Shellis et al., 1998). While Martin and his colleagues standardized average enamel thickness with dentine area measured on the MCS (Martin, 1983, 1985; Grine and Martin, 1988; Andrews and Martin, 1991), Shellis et al. (1998) plotted average enamel thickness against the dentine area. In the present study, both approaches were tried, and it was shown that 3D AET does not differ significantly between Pan and Pongo in relation to tooth size. This differs from what Martin (1983, 1985) suggested, and also from the results obtained for 2D AET in the present study (which is in agreement with Martin’s results). Although it should be noted that 3D AET is not the only way to represent an “average thickness”, the present results have meaningful implications.
Martin (1983) explained that the reason for using the MCS in “estimating” average thickness of enamel was because the MCS should be the most “radial” section, and not “oblique”. The assumption is that an “oblique” section would not cut the enamel perpendicular to the EDJ or OES, and thus would inflate enamel thickness values. Our own 3D datasets enable an evaluation of this assumption. We find that the 3D topography of the EDJ and OES is sufficiently complex, that even with the relatively simply shaped lateral crown face the MCS does not necessarily produce a more “radial” section relative to other crown sections. Indeed a true “radial” section would take the form of an undulating “ribbon”-like non-planar section (Kono and Suwa, 2000). We therefore reject the notion that the MCS is superior to other sections in evaluating overall enamel thickness.
How then would one interpret the differences observed between 2D AET taken at the MCS and whole-crown 3D AET? It is suggested by this study that local differences in tooth crown shape may be emphasized on specific cross sections, such as the MCS. For example, when cusp steepness differs, EDJ length at the MCS would differ proportionately more so than would total EDJ surface area. Therefore, it is possible to say that 2D AET is not necessarily a good estimator nor an adequate substitute of 3D AET, even though it may have its own significance. In as much as 3D AET of the present study is considered an ideal overall measure of enamel thickness (e.g., Martin, 1983; Conroy, 1991), both Pan and Pongo molars are characterized by comparable overall enamel thickness relative to tooth size.
Previous studies suggest that developmental mechanisms that determine tooth size and enamel thickness are not necessarily correlated or dependent upon each other (e.g., Butler, 1956; Harris et al., 2001). In the present study, no significant correlation was seen between tooth (dentine) size and AET, within each of the Homo and Pan molar samples. Also, no correlation was seen between enamel thickness and tooth size as represented by the horizontal areas of the tooth crown. During the course of odontogenesis, the topography of EDJ is the first to be formed, and apposition of dentine and enamel starts at each side of the EDJ proceeding in opposite directions. Therefore the size of the tooth crown is intimately related to EDJ formation, while enamel thickness is determined by the extent to which the ameloblasts continue to deposit enamel matrix. Thus, it is quite reasonable that these two heterochronological processes are controlled independently (Hlusko et al., in press).
Total enamel volume relative to occlusal view size of a molar can be considered as a rough measure of durability or longevity of the tooth. The present results show that the above measure increases almost isometrically among taxa except with Homo, which conspicuously deviates towards a greater amount of enamel for a given tooth size. This could be interpreted as revealing that human molars are much more durable against wear compared to those of great apes. Possibly, human molars might have adapted to different functional demands from that of the great ape species. One must keep these possibilities in mind when comparing enamel distribution patterns within the tooth crown.
It is noteworthy that Gorilla molars, from the same criteria, are as durable as the relatively thicker enameled Pan and Pongo molars, in terms of potential resistance to wear. While an evaluation of 3D AET shows Gorilla molar enamel to be relatively thinner than that of the other species, enamel volume per horizontal section area of the crown is absolutely greater than, and comparable in relation to tooth size with, molars of the other ape species. Hypsodonty is well known in herbivorous mammals such as horses, as an adaptation to high wear rates (Janis and Fortelius, 1988). In Gorilla molars, even though enamel is thinly distributed, the total amount is kept sufficient by means of their high cusps and greater occlusal relief.
Macho and Berner (1993, 1994) suggested that enamel was thicker in the posterior molars than in the first molar of the human maxillary dentition. They interpreted this as showing that posterior molars resist greater loads during mastication. On the other hand, if enamel thickness in humans is primarily an adaptation to resist tooth wear, the first molars are expected to have thicker enamel than the posterior molars, since the first molars erupt earlier and must function the longest. The present results show that in measures such as total enamel volume, 3D AET, and thickness relative to horizontal crown areas, the first molars do tend to exhibit greater values than the second molars, despite its thinner enamel near at certain locations of the crown, as reported by Macho and Berner (1993, 1994).
The functional interpretation of Macho and Berner (1993, 1994) was based on the notion that bite force becomes greater posteriorly, although this has not yet been conclusively shown. The opposite condition was suggested in two in vivo studies, that bite force is actually greater at the first molar than at the second molar position (Mansour and Reynick, 1975; Pruim et al., 1980). In several papers, bite force was calculated, using a biomechanical model, to be greater at the posterior tooth positions (Osborn and Barager, 1985; Koolstra et al., 1988). These simulation studies were based on the assumption that the chewing muscles are potentially equally active regardless of biting position. Spencer (1998) studied the relationship between chewing position and activity levels of the temporal and masseter muscles, and showed that the two muscles are most active when chewing at the first molar position. This does not directly suggest a greater bite force at the first molar, but does point out that some of the assumptions of the simulation studies may not be realistic. Macho and Spears (1999) have recently noted that crown topography, rather than enamel thickness, is more important in a molar’s ability to resist mechanical loads.
Burak et al. (1999) reported that the speed of abrasion differs between enamel and dentine, when each tissue is rubbed against each other. Their results showed that dentine wears faster in general. This means that a greater amount of enamel for a given crown size would allow the tooth to last longer in function, supporting the view that enamel thickness could be an adaptation to resist abrasion. On the other hand, it was further suggested by Burak et al. (1999) that enamel also wears as fast as dentine when rubbed under certain magnitudes of load. It is possible that, not only with tooth-tooth contact but also with tooth-food contact, enamel wears faster under greater loads (discussed in Maas, 1994). Supposing that the greater the load, the faster the enamel wears, it can be said that thickening of enamel does increase a molar’s “resistance” to mechanical load (e.g., Butler, 2000), although in a way different from mechanical strengthening.
The present study is the first to compare enamel distribution patterns of the whole molar crown, as far as we are aware. In this study, local measures of enamel thickness were not taken on certain sections but defined so as to represent regions, such as the average thickness of the occlusal surface enamel of the protoconid, or maximum thickness of the entire lateral surface of the metacone. Linear measurements on particular sections are more prone to be affected by the minor and variable local details of EDJ and OES topography. Slight displacements of section position and/or small amount of wear may affect linear measures considerably, especially those of the occlusal surface. On the other hand, parameters defined to represent thickness of crown regions are less susceptible to such problems, and were used in the present study to assess within tooth distribution patterns of enamel thickness.
In his comparisons of molar enamel thickness of great apes and humans, Schwartz (1997, 2000a) proposed that it is possible to discriminate species from enamel thickness patterns on the MCS of the maxillary molars. His data of great apes were taken from Martin (1983), and that of humans from Macho and Berner (1993, 1994). Since the definitions of the measurements are partly different between these studies, it is doubtful if the results based on the mixed data-set are reliable. In addition, according to Schwartz’s interpretation, enamel thickness measured at the protocone tip is suggested to be a key variable in discriminating Pan and Gorilla molars. The sample of Pan molars used in Martin’s (1983) study, however, includes considerably worn molars (judging from his photographs), which renders his thickness values at the protocone tip unreliable. Because of these uncertainties, it is not clear if the discrimination made by Schwartz was genuine, or an artifact of methodological limitations.
Although it is not possible to compare the present results directly with previously published 2D based measurements, it is still worthwhile to see enamel distribution patterns in a similar manner. When the regional variables of thickness of each cusp are aligned in buccolingual direction for the two mesial and two distal cusps (Figure 12 and Figure 13), a general pattern common to all four species is seen, despite the considerable differences in absolute thickness. In the “functional” sides of the crown, that is the buccal side in mandibular molars and the lingual side in maxillary molars, enamel is thicker than it is in the other side in all the species. There is no evidence that Pan and Gorilla molars differ markedly in terms of occlusal thickness of the protocone (contra Schwartz, 1997, 2000a).
The present results reveal, on the other hand, some distinct aspects of enamel distribution pattern among the four species analyzed (Figure 15). Pan molars have relatively thin enamel in their occlusal fovea, whereas, in Pongo molars, enamel is notably thick in the occlusal fovea but thins towards the basal cervical region. In Homo molars, the cuspal region shows thickly distributed enamel while the basal cervical region is comparable to the condition seen in Pan. In Gorilla molars, enamel is relatively thin throughout the crown. Beynon et al. (1991) reported that enamel deposition rate increases from EDJ to the outer surface in great ape and human molars, but that the amount of such increase is less in Pongo and Homo molars cervically. The above outlined differences in lateral crown enamel might be based on such differences of enamel deposition speed.
 View Details | Figure 15. Thickness maps of mandibular first molars of the four species. Thickness values are shown in color grading. Top left, human; top right, orangutan; bottom left, chimpanzee; bottom right, gorilla. |
Previously, Pan and Gorilla were both treated as “thin enameled” species. The present results distinguish these two species in terms of enamel distribution pattern. Enamel of the occlusal fovea is relatively thin in the molars of both species, but that of the lateral crown face is relatively thicker in Pan than in Gorilla. Pongo was assigned to the “intermediately thick” category and regarded as expressing the condition closest to that of Homo, but the present study shows that, on average, its enamel thickness is not different from that of Pan. Rather, the distribution pattern in Pongo molars is unique, as described above, and thus differs from either Homo or Pan conditions. Interestingly, average or median thickness of the lateral surfaces of the buccal cusps was found to be notably thin in both upper and lower molars of Pongo. Since the functional significance along the buccolingual direction is reversed between upper and lower molars, this common pattern of thickness involving the same side in molars of both jaws is difficult to interpret in terms of function. It is likely that this is a unique aspect of the Pongo molar, perhaps related to developmental pattern of its cervical enamel.
Gorilla is known to be dependent on a relatively folivorous diet, with high intake of leaves and stems, than any other great ape species (Fleagle, 1999). Teeth of herbivorous animals, in general, are characterized by thin, vertically-oriented blade-like enamel ridges on their worn occlusal surfaces. Dietary items consisting of cellulose-rich plant parts, such as grass, are mechanically broken down by the shearing force exerted when these blades run across each other (Lucas and Luke, 1984; Janis and Fortelius, 1988). Thus, the effective length of shearing blades governs the efficiency of mastication. It has been shown that among primates, leaf-eating species tend to have higher cusps and longer shearing crests than fruit-eating species (Kay, 1975, 1977, 1981).
The present results show that the Gorilla molar has relatively thin enamel throughout its crown. Cusps of the Gorilla molar are relatively high compared to those of the other species (Kobayashi, 2001). The combination of tall cusps and thin enamel is likely to be an evolutionary and/or developmental correlate, as part of an adaptation to folivorous diet. It may be a way to make shearing efficient, at the same time retaining the functional longevity of the tooth.
Pongo is known as a frugivore, as with Pan, but differs from the latter in including substantial amounts of hard-shelled seeds (Fleagle, 1999). It is arguable that an occlusal surface covered with enamel, rather than the more elastic dentine, is advantageous when eating hard and brittle objects, such as nuts or some fruits. Cusps are less steep in Pongo molars compared to Gorilla and Pan (Hartman, 1988, 1989), and this could also be interpreted as an adaptation to the crushing of hard and brittle food stuffs (Spears and Crompton, 1996).
Pan is highly dependent on softer ripe fruits, but with a significant omnivorous component (Fleagle, 1999). The distribution pattern of enamel in Pan molars is characterized by the distinctly thin occlusal fovea enamel. Considering the fact that the outer surface morphology of the tooth crown is determined by a combination of the original EDJ topography and the amount of enamel deposited onto it, it is reasonable to think that selective pressures would act on enamel thickness to regulate crown surface morphology. The occlusal fovea of Pan molars is larger than it is in the other three species in relation to total crown area or molar length, and is relatively deep compared to that of Pongo (Kobayashi, 2001). The observed pattern of enamel distribution in Pan might have been achieved by thinning the occlusal enamel in order to acquire a deep and wide occlusal basin. In this way, molars of Pan might have increased their crushing capacities, while at the same time maintaining some degree of shearing efficiency.
Human molars are distinctive from those of great apes in the overall thickness of enamel. Interestingly, the variation of enamel thickness among cusps is greater than that seen in great ape molars. Buccolingual contrasts are most apparent in Homo molars, although it is not clear to what extent such patterns have functional meaning (Kono et al., 2002). Alternatively, relative magnitudes of within-tooth differences in enamel thickness might be exaggerated because the enamel is simply thicker on the whole. According to Macho and Spears (1999), human molars are generally less adapted to resist loads than those of Pan and Pongo. On the other hand, the present results indicate that human molars are more resistant to abrasion than those of great ape species. Taking these aspects into account, human molars are most likely adapted to increase their resistance to abrasion, but not necessarily to withstand high magnitude of mechanical load. Based on this assumption, certain portions of the crown with thicker enamel are expected to wear more quickly. The general pattern of molar wear is in accordance with this view, since the buccal side of lower molars and the lingual side of upper molars wear faster than the opposite sides (e.g., Murphy, 1959; Smith, 1984). It should be noted, however, that, in the lower molars, the protoconid is the first cusp to wear (Murphy, 1959), although its enamel is not thicker than that of the hypoconid which begins to wear later. Therefore, not all patterns seen in human molars are interpretable in terms of adaptation to wear resistance, and developmental constraints are surely an important part of this complex set of relationships.
Previous discussions concerning the evolutionary history of enamel thickness diversity seen within extant hominoid species focused mainly on the status of the last common ancestor of these taxa in terms of overall thickness, such as “thick” or “thin”, and the mode of enamel formation, such as “fast” or “slow” forming. Overall enamel thickness as judged by 2D AET is known to vary greatly, ranging from thin to very thick among extinct Miocene hominoid species (Nagatoshi, 1990; Andrews and Martin, 1991; Conroy et al., 1995; Beynon et al., 1998; Schwartz et al., 2003; Smith et al., 2003). We have elsewhere suggested that molar enamel thickness is potentially sensitive to selective pressures and thus prone to evolve in parallel (Hlusko et al., in press). Therefore it is difficult to determine the ancestral condition from the pattern seen among extant species. However, it is possible to propose a plausible hypothesis based on the present results. The overall thickness of molar enamel is shown to be comparable between Pan and Pongo, thicker in Homo, and thinner in Gorilla. Considering that the thin enamel of the Gorilla molar was likely acquired in order to increase its shearing efficiency, and the thick enamel of Homo to enhance wear resistance, they both may well represent derived conditions. If this is the case, then the overall enamel thickness in the last common ancestor is likely to have been intermediate.
The results of the present study further show that taxonomic differences are not only manifested in overall enamel thickness, but that the pattern of enamel distribution can be distinct. It can be said that Homo and Gorilla molars tend to have relatively thick or thin enamel throughout the crown, respectively, while the basal crown region in Pongo molars and the occlusal fovea in Pan molars show markedly thin enamel. It is likely that these latter conditions are derived (judging from published section images of a variety of Miocene hominoid material), in which case molar enamel of the last common ancestor can be hypothesized, in terms of pattern, to be relatively evenly distributed.
The above hypothesized sequence of enamel thickness evolution is schematically depicted in Figure 16. Each of the extant four species could be regarded as distinctly derived; that is, in Homo and Gorilla molars, largely in overall thickness, and in Pan and Pongo molars, largely in distribution pattern. Recent discoveries of hominid fossils from the Mio-Pliocene deposits of Africa (White et al., 1994, 1995; Leakey et al., 1995; Haile-Selassie, 2001; Senut et al., 2001; Brunet et al., 2002) are expected to shed light on such hypotheses of enamel thickness evolution.
 View Details | Figure 16. Enamel thickness and distribution pattern of the four extant species and the hypothetical last common ancestor (LCA). See the text for details. |
In Homo molars, enamel was shown to be distributed differently in the first and second molars. When occlusal and lateral crown enamel is compared, the difference of thickness in the two regions is less in the first molar than it is in the second; i.e., human second molars exhibit a relatively enhanced occlusal thickness. This resembles the condition seen between Pan and Pongo molars. Macho and Berner (1993, 1994) suggested that, in humans, enamel is thicker in the posterior molars than in the first molar. According to the present data, while the average thickness of the entire crown was not significantly different between first and second molars, occlusal thickness was found to be thicker in the second than in the first molar. This is in accord with their results, since the thickness variables they studied were defined at the occlusal and cuspal region on the MCS. Therefore, for the occlusal region, it can be said that enamel thickness increases posteriorly within the human molar row.
Occlusal enamel also tends to be thicker posteriorly within a human molar crown (Kono et al., 2002). Still, when comparing the first and second molars, enamel is not thicker in the mesial part of the second molar than it is in the distal part of the first molar. Such details of enamel thickness patterning, within the molar row, do not conform to the simple expectation of occlusal load, hypothesized to increase posteriorly along the molar row. Thus, within-tooth patterning of enamel thickness in human molars cannot entirely be interpreted as a simple functional adaptation to resist greater occlusal load that increases distally, such as was suggested by Macho and her colleagues.
Kono et al. (2002) reported a unique pattern of thickness in human first molars, in revealing enamel to be unexpectedly thin around the tips of the mesiobuccal cusps in both upper and lower first molars. This is not the case in human second molars, as visually judged by the occlusal T-map data (data not shown; see Kono et al., 2002 for methods). Numerical evaluations also show that average enamel thickness of the occlusal surfaces of the mesiobuccal cusps (paracone and protoconid) is thicker in the second molars than in the first. These results suggest that factors such as EDJ cusp saliency and topography may have direct developmental consequences to enamel thickness patterning, and should be a focus of future investigations.
In this study, molar enamel thickness and distribution patterns of human, chimpanzee, gorilla and orangutan permanent molars were investigated and presented, for the first time, for the entire tooth crown. It is demonstrated that thickness and/or distribution pattern of enamel is not a simple matter to evaluate. For example, 3D-based assessments of whole-crown features, such as enamel tissue volume and EDJ surface area, yield results different from those previously recognized from 2D-based studies. It is concluded that the conventional 2D average enamel thickness is not a reliable estimator of 3-dimensional whole-crown conditions.
The 3-dimensionally determined average thickness values show that human molars have absolutely and relatively thicker enamel than great ape molars. Enamel volume of human molars, in relation to horizontal section area of the molar crown, is also greater than in ape molars, both absolutely and relatively. Human molars are thus distinguished from ape molars by the greater amount of enamel, indicating greater durability against wear.
The 3-dimensionally determined average thickness values show that enamel thickness is comparable in chimpanzee and orangutan molars, in relation to tooth size, and thinner in gorilla molars. Volume of enamel relative to the horizontal section area of the molar crown does not differ significantly between the three species, in relation to tooth size. These results indicate that gorilla molars have relatively thin enamel but are still durable against wear.
The 3D whole-crown investigations of the present study also revealed taxon-specific characteristics of gross distribution patterns of molar enamel. Although the molars of Pan and Pongo are comparable in terms of average enamel thickness, they differ significantly with regard to enamel distribution patterns. Chimpanzee molars are characterized by markedly thin enamel of the occlusal fovea. Orangutan molars are characterized by thicker enamel occlusally and thinner enamel toward the basal cervical region. In gorilla and human molars, such distinctive thickness patterns were not seen.
Based on the above enamel thickness and distribution patterns seen among the extant hominoid species, molar enamel of the last common ancestor is hypothesized to have been intermediately thick and relatively evenly distributed. According to this view, each of the extant four species exhibits some distinctly derived condition. From the hypothesized condition of the last common ancestor, in human and gorilla molars, thickness would have changed, and in chimpanzee and orangutan molars, distribution pattern would have changed. Such hypotheses of enamel thickness evolution may be tested by future anatomical, developmental, and paleobiological studies.
I would like to thank the following individuals who kindly allowed access to the specimens: Gen Suwa, The University Museum, The University of Tokyo; Yuji Mizoguchi, National Science Museum; Tim D. White, University of California at Berkeley; Bruce Latimer, Cleveland Museum of Natural History. Don Reid kindly made original data available from previous studies. Special thanks go to Toyohisa Tanijiri of Medic Engineering Inc., for cooperating with us through the development of the Rugle series, and to Atsushi Murakoshi and people at TESCO Corporation for providing technical support with the CT scanner. Finally I thank Gen Suwa for his unlimited support, encouragement, and many invaluable comments, throughout this painstaking study.
|