| J. Sawada, corresponding author. e-mail: jsawada@mail.tains.tohoku.ac.jp phone: +81-22-717-8029; fax: +81-22-717-8030 Published online 11 August 2004 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.00094 |
Since Neanderthal children had large skulls and robust limb bones compared with modern children, Neanderthals must have grown more rapidly (Dean et al., 1986; Stringer et al., 1990; Stringer and Gamble, 1993). However, in addition to gross morphological studies, studies of dynamic bone modeling and remodeling at the microscopic level are needed to verify this hypothesis. Bone histomorphology provides information important for the clarification of microscopic features of bone development.
Bone histomorphology of Late Pleistocene hominids, including the Neanderthals, has been studied since the 1980s because of an increased interest in various issues such as longevity, bone turnover, and skeletal robusticity (Thompson and Trinkaus, 1981; Trinkaus and Thompson, 1987; Abbott et al., 1996; Pfeiffer and Zehr, 1996; Schultz, 1999). However, samples analyzed to date have all been adult, and the early stages of Neanderthal growth have not been described. In this study, we prepared a thin section of the femur of the Dederiyeh 1 Neanderthal child discovered in Syria, and studied its histomorphology to clarify bone formation and growth of Neanderthals.
Dederiyeh 1 is the fossilized skeleton of a Neanderthal child unearthed by the joint Japan-Syria expedition team in 1993 from the Middle Paleolithic layer of Dederiyeh Cave, northern Syria (Akazawa et al., 1993, 1995a, b; Akazawa and Muhesen, 2003). The skeleton is exceptionally well preserved and nearly complete.
Concerning the age of Dederiyeh 1, Dodo et al. (2003) used the morphology of its lower incisors, canines, and first molars to estimate the child’s age at about 2 years by Smith’s (1991) method. On the other hand, Sasaki et al. (2003) examined enamel cross striations of its left upper first molar and estimated its age at 1 year and 7 months. Although the ages estimated by the two investigators differ slightly, this difference is within the error range of estimation in light of individual variation of tooth formation. In this study, the age of Dederiyeh 1 was assumed to be about 2 years on the basis of these estimates.
The cranial and postcranial anatomical features of Dederiyeh 1 clearly showed that this skeleton was that of a Neanderthal, and that its limb bones were generally more robust than those of a modern 2-year-old child (Dodo et al., 2003; Kondo and Dodo, 2003).
Femurs of 32 modern humans aged 0–6 years (three probable South Asians, 25 Japanese, and two Jomon, one Yayoi and one Okhotsk prehistoric Japanese; see Table 1) were prepared for comparison. The ages of the modern humans were estimated predominantly by the method of Smith (1991) using dental formation of the lower permanent teeth. Each individual was classified into age-groups of 0, 1, 2, 3–4, and 5–6 years. One South Asian and six Japanese individuals could be examined only for eruption of teeth or deciduous teeth formation, so their ages were estimated by the method of Ubelaker (1989). Since teeth were not available for examination in 12 Japanese individuals, their ages were estimated from the maximum length of femoral diaphysis. This was done by reference to femoral length of specimens in which the age could be estimated from dental formation stages. Femoral length data of modern Japanese of known age, reported by Kondo and Dodo (2003), were also used. Sex was unknown except in three Japanese females (Japanese individuals 1, 2, and 7), whose records at death were available.
The histological sample of Dederiyeh 1 was prepared from the left femur. Since the distal quarter of its shaft was not recovered, the maximum length of its diaphysis could not be determined. However, the position 80 mm from the proximal diaphyseal end was regarded as midshaft by comparison with the complete right femur (maximum length of diaphysis: 159.7 mm). Bone was removed from the full thickness of the anterior cortex 75–80 mm from the proximal end of the diaphysis. The comparative samples of modern children were obtained from the anterior cortices of the femoral midshaft in the same way as in Dederiyeh 1. The left femurs were used whenever possible.
These blocks were embedded in resin prepared by mixing Rigolac-2004 and Rigolac-70F (NISSIN EM) at a ratio of 7:3. After polymerization of the resin, 50 μm thick transverse sections were made with a microtome (SP-1600, Leica). These sections were examined under ordinary and polarized light with an optical microscope (Biophot, Nikon).
Features of the thin sections were digitally captured with a CCD camera (Digital Microscope Camera, Polaroid) attached to a microscope, and histomorphometrically assessed on a Macintosh computer (G4, Apple Computer) using the public domain NIH Image program (US National Institutes of Health, available at http://rsb.info.nih.gov/nih-image/).
Bone histomorphometry was performed in the following area of the cortical bone (Figure 1). First, the median line of the femoral midshaft, determined with the naked eye, was marked with water ink on the periosteal surface of the sample (line A in Figure 1). Next, two points, each at an interval of 1/16 of the midshaft circumference from line A, were inscribed on the periosteal surface of the photomicrograph of the section. Then, from the two points, lines B and C were drawn to the endocortical surface perpendicular to the periosteal surface. The entire area of cortical bone surrounded by the periosteal surface, the endocortical surface, and these two lines, i.e. the anterior cortex that accounts for 1/8 of the total circumference, was determined as the area in which bone histomorphometry would be performed. This area is referred to as the cortical area (CA).
 View Details | Figure 1. Schematic illustration of cortical area (gray area surrounded by the periosteal surface, the endocortical surface, line B, line C, and line H). Line A: median line of the femoral midshaft. Lines B and C: lines perpendicular to the periosteal surface, drawn from points on the periosteal surface at a distant of 1/16 of the midshaft circumference from line A. Trabeculae of cancellous bone are not included in the area of histomorphometry. Line H: borderline between cortical and cancellous bone. This borderline is made by connecting two points on either sides of the trabecular bone, where the perpendiculars from the periosteal (lines D and E) and endocortical surfaces (lines F and G) converge at an angle of 30 degrees. |
Trabeculae of cancellous bone were not included in the area of histomorphometry. Where trabeculae extended from the endocortical surface, a line H was drawn to define the borderline between cortical and cancellous bone. As shown in Figure 1, this was done by connecting two points on either sides of the trabecular bone, where the perpendiculars from the periosteal (lines D and E) and endocortical surfaces (lines F and G) converged at an angle of 30 degrees.
The following variables ware measured: cortical width, percent osteonal bone, osteon population density, non-Haversian canal population density, osteon area, and Haversian canal area. The terminology used here is basically the same as that of Parfitt et al. (1987).
Cortical width (Ct.Wi)
This is the thickness of cortical bone in the median plane of the femoral midshaft (mm).
Percent osteonal bone (POB)
This is the percentage of the total area of secondary bone relative to CA. Here, ‘secondary bone’ means secondary osteons, osteon fragments, and resorption cavities. Resorption cavities are basic multicellular units observed in an early stage of remodeling (Frost, 1985; Stout, 1989), and are present in large numbers in young individuals, so that the addition of resorption cavities as a parameter was considered appropriate for evaluation of remodeling in a child.
Osteon population density (OPD)
This is the value obtained by dividing the number of secondary bone features (secondary osteons, osteon fragments, and resorption cavities) in the CA by the area of CA (number/mm2). Concerning secondary bone features present at the border of CA, only those that lay more than half within the CA were counted.
Non-Haversian canal population density (nCaPD)
This is the value obtained by dividing the number of non-Haversian canals in the CA by the area of CA (number/mm2). Non-Haversian canals are vascular channels formed in primary bone. As for non-Haversian canals at the border of CA, the same procedures as outlined above were followed.
Osteon area (On.Ar)
This is the mean area of complete secondary osteons in the CA (μm2). To eliminate irregular-shaped osteons and osteons that were cut obliquely, those in which the maximum diameter was greater than twice the minimum diameter were excluded, according to the method of Pfeiffer (1998).
Haversian canal area (H.Ar)
This is the mean area of complete Haversian canals in the CA (μm2). Similarly to secondary osteons, those in which the maximum diameter was greater than twice the minimum diameter were excluded.
Cortical width was measured in the femurs of Dederiyeh 1 and all 32 modern children. The other histomorphometric variables were measured in Dederiyeh 1 and 18 modern individuals aged 1–6 years (Japanese individuals 1–14, South Asian individuals 1–3, and Jomon individual 1) in which the histological details could be clearly observed, while these were not available for the other 14 modern individuals (Japanese individuals 15–25, Jomon individual 2, Yayoi individual 1, and Okhotsk individual 1) because of damaged microstructure.
In the femur of Dederiyeh 1, the histological structure was preserved exceptionally well; lamellae, cement lines, and vascular canals of the cortical bone could be clearly distinguished. The cortical bone of Dederiyeh 1 consisted of secondary osteons which developed in the endosteal region, and primary bone which filled the periosteal region and gaps of secondary osteons (Figure 2). New secondary osteons encroached on old osteons at various places, indicating active bone turnover. Primary bone was composed of woven bone and primary osteons, but no inner and outer circumferential lamellae were observed. No morphological abnormalities suggestive of bone disease were noted.
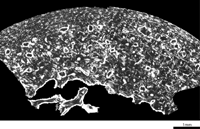 View Details | Figure 2. Femoral cross-section of the Dederiyeh 1 Neanderthal child (ca. 2 years of age). |
In modern humans, cortical bone generally consists of primary osteons and woven bone immediately after birth, and remodeling starts after the age of about 1 year to form secondary bone (Enlow, 1962; Jee, 1983). Primary bone, mainly composed of woven bone, is replaced by lamellar bone by the age of about 5 years as a result of remodeling (Frost, 1987a). These features were observed in the femoral cortical bone of the modern children examined in this study (Figure 3, Figure 4, Figure 5).
 View Details | Figure 3. Femoral cross-sections of modern humans (1 year of age). |
 View Details | Figure 4. Femoral cross-sections of modern humans (2 years of age). |
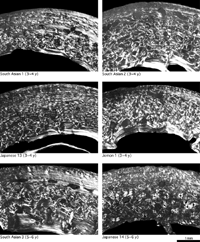 View Details | Figure 5. Femoral cross-sections of modern humans (3–6 years of age). |
Age-associated changes in circumferential lamellae formation in early childhood have not been described in detail. Therefore, we examined for the formation of circumferential lamellae, which extend over half of the endosteal and periosteal circumferences of CA, in the bones of all 32 modern children. We found that circumferential lamellae were infrequent in those aged 1–2 years, but inner or outer circumferential lamellae were formed in more than half the children aged 3–6 years (Table 2).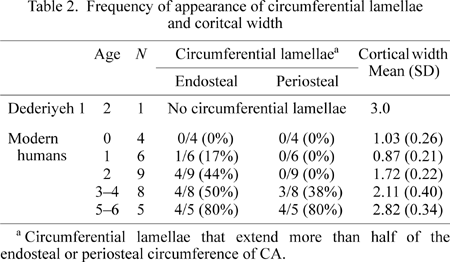
The cortical width (Ct.Wi) of Dederiyeh 1 was about twice that of the mean of 2-year-old modern children (Table 2, Figure 6). In the modern children examined in this study, cortical bone mass tended to increase with age, and the Ct.Wi of Dederiyeh 1 was close to the mean of modern children aged 5–6 years.
 View Details | Figure 6. Cortical width (Ct.Wi) plotted against age for Dederiyeh 1 and modern humans. Solid lines connect the mean values of modern children of the respective age groups. |
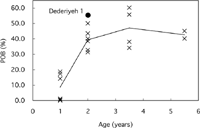
Percent osteonal bone (POB) and osteon population density (OPD), which represent the development of secondary bone formation, were greater in Dederiyeh 1 than in 2-year-old modern children, by 2.3 standard deviations (SD) in POB and 2.0 SD in OPD (Table 3, Figure 7, Figure 8). The POB and OPD of modern children increased with age although they decreased slightly from the age of 3–4 to 5–6 years in this study. This was roughly consistent with the findings of Kerley (1965) and Thompson (1979), which showed that OPD increased with age. In Dederiyeh 1, the POB and OPD were higher than the means of 3- to 6-year-old modern children, and therefore the development of secondary bone was more advanced than in modern children.
 View Details | Figure 7. Percent osteonal bone (POB) plotted against age for Dederiyeh 1 and modern humans. Solid lines connect the mean values of modern children of the respective age groups. |
 View Details | Figure 8. Osteon population density (OPD) plotted against age for Dederiyeh 1 and modern humans. Solid lines connect the mean values of modern children of the respective age groups. |
On the other hand, the non-Haversian canal population density (nCaPD) of Dederiyeh 1 was within 1 SD of 2-year-old modern children (Table 3, Figure 9). Variable nCaPD tended to decrease with age in modern children, and that of Dederiyeh 1 was smaller than the mean of 1-year-old modern children and clearly greater than the mean of 3- to 6-year-old modern children.
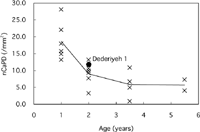 View Details | Figure 9. Non-Haversian canal population density (nCaPD) plotted against age for Dederiyeh 1 and modern humans. Solid lines connect the mean values of modern children of the respective age groups. |
Concerning osteon area (On.Ar), no clear age-related change was observed in modern children (Table 3, Figure 10). The Haversian canal area (H.Ar) was greater in 1-year-old modern children than in 2- to 6-year-old modern children, because the number of ‘forming secondary osteons’ that have large canals was greater in the 1-year-old children (Table 3, Figure 11). The On.Ar of Dederiyeh 1 was within about 1 SD of the means of 1- to 6-year-old modern children, and the H.Ar did not differ markedly from the range of variation in that of 2- to 6-year-old modern children.
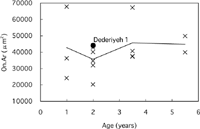 View Details | Figure 10. Osteon area (On.Ar) plotted against age for Dederiyeh 1 and modern humans. Solid lines connect the mean values of modern children of the respective age groups. |
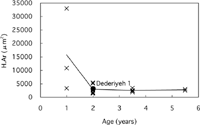 View Details | Figure 11. Haversian canal area (H.Ar) plotted against age for Dederiyeh 1 and modern humans. Solid lines connect the mean values of modern children of the respective age groups. |
The histological structure of cortical bone varies among sites (anterior or posterior, subperiosteal or endosteal, etc.) even in the same bone (Jowsey, 1966; Drusini, 1987; Pfeiffer et al., 1995). It is important to observe as wide an area as possible in histological studies of cortical bone (Robling and Stout, 2000), but limitations in sampling area are inevitable in rare fossil hominids such as Neanderthals. The bone sections of Dederiyeh 1 and modern children were all obtained from the anterior cortex of the femoral midshaft and thus were identical in location. The anterior cortex of the femoral midshaft was also used in the studies of Thompson (1979), Burr et al. (1990), Narasaki (1990), and Ericksen (1991), and is a standard sampling location in histological studies of regional cortical bone.
In this study, Japanese, prehistoric Japanese, and probable South Asians were regarded as a comparative series of modern humans. Therefore, the possibility of bias due to combining diverse human groups cannot be excluded. However, differences in bone histomorphometry among the groups of modern humans included in this study were generally not remarkable (Table 1, Table 3), so that it appeared reasonable to pool these groups for analysis of the Dederiyeh 1 child.
Whether the histomorphological characteristics of Dederiyeh 1 are common to Neanderthal children in general is unclear because of the lack of reports on other Neanderthal children. However, many of the gross anatomical features seen in Dederiyeh 1 are common among immature Neanderthals. For example, the robustness of the lower limb bones is a characteristic not only seen in adult but also in immature Neanderthals (Ruff et al., 1993); the robustness index of the femur (midshaft circumference/maximum length) of Dederiyeh 1 is similar to that of other Neanderthal children (Figure 12) (Kondo and Dodo, 2003). From these considerations, the microscopic features of the cortical bone of Dederiyeh 1 may not differ significantly from other Neanderthal children.
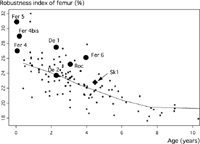 View Details | Figure 12. Robustness index of the femur (midshaft circumference/maximum length) plotted against age in Neanderthals (black circle), the early modern Skhul 1 (black diamond), and modern humans (asterisk). De, Dederiyeh; Fer, Ferrassie; Roc, Roc de Marsal; Sk1, Skhul 1. Modified from Figure VI-10 of Kondo and Dodo (2003). |
The development of secondary bone and cortical width of Dederiyeh 1 surpassed those of 2-year-old modern children. Meanwhile, primary bone mainly composed of woven bone and the nCaPD differed minimally between Dederiyeh 1 and 1- to 2-year-old modern children. Circumferential lamellae, which were observed in some 2- and many 3- to 6-year-old modern children, were not formed in Dederiyeh 1. Therefore, the histomorphology of the Dederiyeh 1 femur can be said to exhibit a complex of features seen in modern children of different ages.
What conditions might have formed the characteristic histomorphology of Dederiyeh 1? Factors that affect bone histomorphology are numerous and include mechanical effects, genes, metabolism, hormones, state of health, and pathological lesions (Frost, 1985, 1987a; Robling and Stout, 2000). Taking into account these factors, let us evaluate some alternative possibilities as an explanation of bone formation in Dederiyeh 1.
Frost (1987b) postulated that higher strain causes bone modeling to increase cortical bone mass. Bouvier and Hylander (1981, 1996) and Lieberman (1997) also reported that increased levels of mechanical loading from exercise promote remodeling. Then thick diaphyseal cortical bone and well-developed secondary bone of Dederiyeh 1 femur may suggest greater mechanical loading than occurs in modern humans. Longitudinal analyses of modern children indicate that femoral strength increases faster after adoption of bipedalism at about 1 year of age (Ruff, 2003). An early onset of bipedal locomotion or a longer duration of walking may be supposed as specific mechanical factors that might have affected femoral development of the 2-year-old Dederiyeh 1 individual. Alternatively, Neanderthals are estimated to have had a larger body weight than modern humans (Stringer and Gamble, 1993; Klein, 1999). If the tendency for a relatively large body weight is assumed to have appeared in early childhood, as suggested by the stout body build of Dederiyeh 1 (Kondo and Dodo, 2003), body weight might have been involved in enhanced mechanical effects.
However, the greater robustness index of the femur (midshaft circumference/maximum length) detected in 0- to 1-year-old Neanderthal infants (Figure 12) indicate that the Neanderthal cortical bone might have developed well before the acquisition of bipedal locomotion, and that it is probably difficult to attribute bone formation in Dederiyeh 1 only to mechanical factors. Independent of habitual higher strains, genetic factors that program bone growth may have been involved in the differences in bone formation compared with modern children.
Alternatively, if thick cortical bone and enhanced remodeling suggest abnormal bone metabolism, it is possible to assume that some environmental factors influenced calcium or vitamin metabolism of the Dederiyeh child. Although different from the findings of bone histomorphology in Dederiyeh 1, Walker et al. (1984) suggested that an excessive intake of vitamin A due to eating liver was a cause of the abnormal thickening of femoral cortical bone in Homo erectus from Koobi Fora. Abnormal bone formation also can result from general somatic infectious disease (Mensforth et al., 1978). Thus the possibility of nutritionally induced endocrinological disorders and those due to somatic infection may need evaluation.
Characteristics of bone formation in Dederiyeh 1 can be explained from the above factors; but it is at present difficult to determine which of these factors were dominant. However, we can at least come to the conclusion that the femoral histomorphological features of Dederiyeh 1 do not match those of modern children at any age level. In other words, the developmental pattern of cortical bone in Dederiyeh 1 was substantially different from that in modern children. As mentioned above, from gross anatomical evaluations, some have claimed that Neanderthals grew faster than modern humans; but the histomorphology of the femoral cortical bone in Dederiyeh 1 appears difficult to explain simply by rapid maturation. Clarifying this issue requires the accumulation of experimental data concerning bone formation and further histomorphological comparisons of Pleistocene hominids (including Neanderthals) and modern humans under influence of different environmental factors.
The osteon area of Dederiyeh 1 (44139 μm2) was comparable to that of the modern children examined in this study (mean, 40895 μm2; range, 20235–67740 μm2). In adult Middle/Late Pleistocene hominids, the size of secondary osteons was reported to be smaller than that of modern humans (Abott et al., 1996; Pfeiffer and Zehr, 1996). However, E. Trinkaus (personal communications) argues that there is indeed no apparent systematic difference in osteon size between Neanderthals, early modern humans, and living modern humans. Osteon size is generally considered not to change with age in modern humans (Jowsey, 1966; Pfeiffer, 1998; Robling and Stout, 2000). The factors that determine osteon size remain unclear (Pfeiffer, 1998, 2000), and further studies are required to elucidate these.
We thank Drs G. Murakami and H. Matsumura (Sapporo Medical University), Drs M. Kikuchi and T. Suzuki (Graduate School of Dentistry, Tohoku University), and Dr H. Ishida (Faculty of Medicine, University of the Ryukyus) for supplying the modern human samples. We also thank Dr E. Trinkaus for commenting on an early draft of the manuscript.
|