| H. Ishida, corresponding author. e-mail: ishidaha@med.u-ryukyu.ac.jp phone: +81-98-895-1100; fax: +81-98-895-1400 Published online 13 July 2004 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.03102 |
The discussion of Ainu origins, migrations, and affinities has been important in the field of physical anthropology not only in Japan but also in Europe (e.g. Busk, 1867; Tsuboi, 1887; Koganei, 1893). It has been recognized that the prehistoric Jomon in the Japanese islands were the probable ancestors of the Ainu, their close affinities being indicated by studies of cranial and dental morphology and even by ancient mitochondrial DNA analysis (e.g. Turner, 1976; Yamaguchi, 1982; Dodo, 1986; Ossenberg, 1986; Ishida, 1990, 1992, 1999; Horai et al., 1991; Ishida and Kida, 1991; Kozintsev, 1992). However, few paleopathological findings of Ainu people have been reported (Suzuki, 1985).
Between 1991 and 1997, the Department of Archaeology and the Tokoro Research Laboratory of the Graduate School of Humanities and Sociology, The University of Tokyo excavated the Tokoro Chashi site, Tokoro, Hokkaido, and published a monograph of their findings (Utagawa et al., 2001). A burial was found in the ‘chashi’, a structure surrounded by a moat built in the Ainu period. In the burial, a human skeleton was recovered in good condition with the head oriented toward the southeast. The skeleton was accompanied by several ornaments of the Ainu culture, including a sword, copper parts of a quiver, a knife, fiber material, and joint shafts for harpoons. It is very rare to find a burial within the precinct of a chashi. Because the construction of this chashi was radiocarbon dated to about 300 years ago, the buried human may date to approximately the same era (Utagawa et al., 2001). In this paper, we present and discuss the vertebral pathology found in these remains, which is of interest because vertebral assimilation (the joining of two vertebral bodies) is fairly rare.
The morphology of the cranium, teeth, and postcranial bones has been fully described in a report by Saso et al. (2001). The skeleton is well preserved except for the vomer, ethmoid, inferior conchae, and a part of the sternum and ribs. Using standard methods, this individual was estimated to be an adult male (Miles, 1963; Brothwell, 1981; Knussman, 1988). Although high-faced and relatively tall in stature (160.8 cm), this individual has typical Ainu characteristics. He had no dental caries or periodontal disease, although dental calculus was deposited on all teeth. No cribra orbitalia or dental enamel hypoplasia was observed. In addition, no injuries or pathological findings, other than that reported here, were recognized in the cranial and postcranial skeleton.
The vertebrae were well preserved from the 1st cervical to the 5th lumbar vertebra. Osteophytes on the vertebral bodies were detected from the 10th thoracic to 2nd lumbar vertebrae. In addition, the vertebral bodies and right zygapophyseal joint between the 12th thoracic and 1st lumbar vertebrae were completely united by osseous tissue (Figure 1). Table 1 shows direct and estimated measurements and indices of the 12th thoracic and 1st lumbar vertebral bodies using the methods of Knussman (1988). Because an anterior wedge shape associated with about 42% loss of anterior vertebral body height was seen in both the 12th thoracic and 1st lumbar vertebrae, the normal convex-forward curve of the lower thoracic and lumbar region had disappeared. The radiograph shows that the intervertebral space had completely disappeared and spontaneous bony fusion was fully achieved between the 12th thoracic and 1st lumbar vertebrae, whose bodies were not otherwise damaged. Slight osteoporosis was seen in the body, while new bone formation was not observed. Other vertebrae and lower limb bones, including the os coxae, showed no evidence of inflammation or abscess.
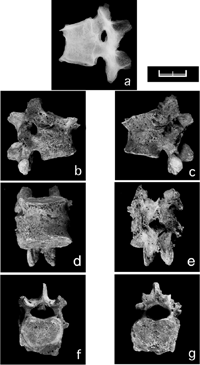 View Details | Figure 1. Vertebral assimilation of the 12th thoracic and 1st lumbar vertebrae, probably caused by pyogenic spondylitis. (a) Lateral view radiograph showing spontaneous bony fusion and slight osteoporosis, (b) right lateral, (c) left lateral, (d) anterior, (e) posterior, (f) superior, (g) inferior views (scale = 20 mm). |

The bony fusion of the two vertebral bodies suggests several causative diseases, including congenital anomaly (or block vertebrae), osteoblastic bone tumor, compression fracture due to osteoporosis, and pyogenic or tuberculous spondylitis (Resnick and Niwayama, 1981).
Congenital block vertebra is caused by the abnormal rearrangement of segmental sclerotomes (Williams, 1995). However, this congenital anomaly can be dismissed because the left zygapophyseal joint is intact and this condition is rare.
Benign or malignant tumors generally show no significant loss of intervertebral disc space. Osteolytic, osteoblastic, or osteosclerotic lesions of the body combined with intact intervertebral space are much more common in tumors than in infections (Ortner, 2003). Thus, we can eliminate the possibility of a bone tumor due to the bony fusion of two vertebral bodies and the loss of intervertebral space combined with absence of osteolytic or osteoblastic lesions.
Although the wedge shape of the body might indicate a compression fracture due to osteoporosis and forward flexion or axial compression (Leventhal, 1992), the X-ray showed neither severe osteoporosis nor intact intervertebral space. Instead, the right zygapophyseal joint was also fused. Intervertebral disc space narrowing also suggests intervertebral osteochondrosis. However, this involves reactive sclerosis of the body and generally shows a well-defined vertebral end-plate.
Table 2 shows the differential diagnosis among several vertebral pathologies on the basis of many reports (Kulowski, 1936; Wiley and Trueta, 1959; Griffiths and Jones, 1971; Kemp et al., 1973; Eismont et al., 1983; Fang et al., 1994; Carragee, 1997a, b; Aufderheide and Rodríguez-Martín, 1998; Kashimoto, 1998; Leitao et al., 1999; Hadjipavlou et al., 2000; Magnus and Hoffman, 2000; Ortner, 2003). Intervertebral disc involvement, or fusion caused by discitis, clearly differentiates between a simple vertebral compression fracture, metastatic tumor, and infectious spondylitis (Kashimoto, 1998; Ortner, 2003).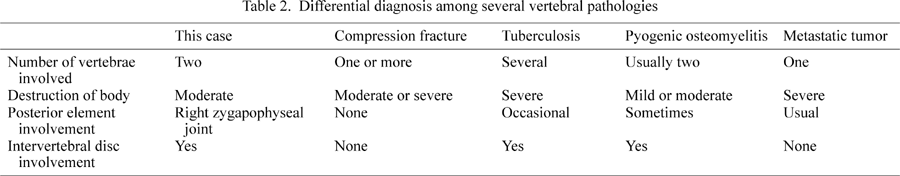
As a result of the differential diagnosis, we can consider that this pathological change was caused by infectious spondylitis or osteomyelitis of the spine. However, we should distinguish between pyogenic spondylitis and tuberculous spondylitis. Tuberculous spondylitis is a well-known chronic infectious disease caused by Mycobacterium tuberculosis (Morse, 1967). Pyogenic infection of the spine has been investigated for about one hundred years (e.g. Kulowski, 1936; Griffiths and Jones, 1971; Leitao et al., 1999; Magnus and Hoffman, 2000). Tuberculous spondylitis usually occurs during childhood, and tends to involve not two but several vertebrae. The vertebral body is destroyed and the lesion spreads to adjacent intervertebral discs. The vertebrae are subject to collapse, producing blockage vertebrae and angular kyphosis (Anderson and Scotti, 1976). On the other hand, pyogenic spondylitis usually occurs during adulthood and mainly involves two adjacent vertebrae and the intervertebral disc. The intervertebral disc is fully destroyed and the space disappears while collapse of the body may be less pronounced. When pyogenic spondylitis heals, the two adjacent vertebrae usually unite spontaneously.
Prior to the development of antibiotics, Kulowski (1936) reported that pyogenic spondylitis is mainly caused by Staphylococcus and Streptococcus. Precursor infections, including urinary, respiratory, and dermal, were sometimes present (32–46%). Pyogenic spondylitis caused by trauma might also occur. The average age of patients was 31 years, and males were more frequently affected (Kulowski, 1936). Lumbar vertebrae were mostly affected, followed by thoracic and cervical vertebrae. Not only the vertebral body but also the arch and processes were sometimes affected (Table 2). Most patients had spondylitis that involved only one or two vertebrae with the intervertebral disc (75–90%), while involvement of several vertebrae was rare (Carragee, 1997a; Ortner, 2003). The fatality rate was about 25% or more before the introduction of antibiotics (Makins and Abbott, 1896; Kulowski, 1936).
In order to definitively diagnose an infectious disease, the microorganism must be identified. However, it is difficult to apply such an analysis to archaeological materials due to contamination by exogenous DNA and fragmentation, although microbiologists have tried to amplify the DNA of ancient microorganisms by the polymerase chain reaction (Mays et al., 2002). As a result, we diagnosed this case as probable pyogenic spondylitis according to the macroscopic and radiographic findings, although the clinical sensitivity was not very high.
Macroscopic and radiographic findings of this study suggested that the fusion of the 12th thoracic and 1st lumbar vertebrae shows a healed state of pyogenic spondylitis in this male Ainu skeleton from Hokkaido. Because this case of pyogenic spondylitis had healed, the cause of death is uncertain. However, in addition to his tall stature, the absence of any complications from pyogenic spondylitis, enamel hypoplasia, or cribra orbitalia suggested that both this male and his enviroment were in good condition (e.g. Larsen, 1997). Recent stable isotope analyses by Yoneda (2002) showed that more recent Ainu people consumed not only marine mammals but also fish and several terrestrial animal and plant resources. This would suggest that in this individual nutritional status might not have been a dominant factor relating with the pathology.
We wish to thank Professor H. Utagawa, The University of Tokyo for offering us a chance to study the material. This study was supported in part by a Grant-in-aid for Scientific Research of Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology (No. 15068210).
|