| R. F. Kay, corresponding author. e-mail: Rich_kay@baa.mc.duke.edu phone: +1-919-684-2143; fax: +1-919-684-8542 Published online 11 August 2004 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.04S005 |
Several clades of primate taxa are known from the middle and late Eocene of South Asia of which the Amphipithecidae is among the most diverse, including Pondaungia, Amphipithecus, Siamopithecus, and Myanmarpithecus. Most of these taxa are known from teeth and jaws although several specimens from Myanmar, assignable to Amphipithecus and Pondaungia, are known from parts of the humerus, ulna, calcaneus, talus, face, and circumorbital region. In this paper I review the phylogenetic position of amphipithecids and, in somewhat more detail, comment on the paleobiology of this interesting clade.
Myanmar amphipithecid specimens come from the Pondaung Formation. A fission-track age of ~37 Ma (late middle Eocene) is reported from one level in the Pondaung Formation (Tsubamoto et al., 2002), concordant with the formation being overlain by marine beds with foraminifera of late Eocene age (Aung, 1999; Mon, 1999). Thailand amphipithecids come from coal deposits at Krabi. The magnetostratigraphy and paleofaunas with which it is associated suggest a somewhat younger age than the Myanmar beds—31 to 34 Ma (Benammi et al., 2001)—making it late Eocene to early Oligocene (Berggren et al., 1995). Thus, in total, these extinct primates overlap and encompass the age distribution of anthropoids described from Fayum province, Egypt, and elsewhere in northern Africa and Arabia.
Amphipithecids are known from three or perhaps four species. From Thailand comes Siamopithecus eocaenus. From Myanmar come Amphipithecus mogaungensis and Myanmarpithecus yarshensis. A third Myanmar taxon, Pondaungia, seems to be represented by two species (P. cotteri and P. savagei), although these could also represent a single sexually dimorphic species. One argument for the former interpretation is that the ratio of the size of the lower canine (or its root socket) to the dimensions of the molars is similar in the larger and smaller specimens. In contrast, among extant sexually dimorphic primates, there is more size difference between the canines than between the molars of the two sexes.
All amphipithecid species are known from the maxilla, mandible, and teeth, but cranial and postcranial evidence is sparser. Frontal bones of Amphipithecus have been described (Ciochon and Gunnell, 2002; Gunnell et al., 2002; Takai et al., 2003; Shigehara and Takai, 2004). Three associated postcranial fragments (humerus, ulna, and calcaneus) belonging to a large-bodied species from Myanmar could be either Amphipithecus or Pondaungia (Ciochon et al., 2001; Ciochon and Gunnell, 2004). A talus from the Pondaung Formation also is of a size appropriate to go with Amphipithecus (Marivaux et al., 2003).
The phylogenetic position of amphipithecids is debated. A cladistic analysis of dental, cranial, and postcranial anatomy by Kay et al. (2004c) examined the possibilities. Considering the collective evidence, Kay et al. (2004c) found it to be slightly more parsimonious (owing to dental characters) to root amphipithecids within the anthropoid clade, especially with Propliopithecidae, the earliest catarrhine anthropoids from the African Oligocene. Dental evidence provides the only support for a crown anthropoid hypothesis. Some or all amphipithecids bear a strong resemblance to propliopithecids, sharing with them deep jaws, spatulate and enlarged upper central incisors, robust canines with a rounded oval cross-section, P3 with a convex distal margin, p4 with a metaconid but lacking a paraconid, low crowned molars without paraconids, with little disparity between trigonid and talonid heights, and the presence of wear facet X on the molars (Ducrocq, 1998, 1999; Jaeger et al., 1998a, b; Chaimanee et al., 2000; Shigehara et al., 2002; Kay et al., 2004c). However, a careful evaluation of the total available anatomical evidence leads to the conclusion that a linkage with catarrhine anthropoids is unlikely because it would require many convergences in the orbital anatomy (lack of postorbital closure, a feature of stem anthropoids). Furthermore, the humerus and calcaneus lack the shared-derived features characteristic of late Eocene and early Oligocene African oligopithecids, parapithecids, and propliopithecids (Kay and Williams, 1994; Ciochon and Gunnell, 2004; Seiffert et al., 2004). This interpretation is reinforced by a recently described amphipithecid talus which, while more diagnostically anthropoid, does not associate exclusively with crown anthropoids (Marivaux et al., 2003). Thus, this postcranial evidence in total indicates that the amphipithecids might be stem anthropoids outside the clade of African Eocene-to-Recent crown anthropoids (Kay et al., 2004c).
A major challenge for the anthropoid status of amphipithecids comes from orbital and olfactory anatomy. The earliest primates had a postorbital bar but there was no bony partition between the orbit and the muscles of mastication (Martin, 1990). Adapoids, omomyids, and crown strepsirrhines share this condition. In haplorhines, a bony partition separates the orbital contents from the muscles of mastication (postorbital closure). Amphipithecus appears to have had the plesiomorphic condition in having a postorbital bar but lacking postorbital closure (Shigehara and Takai, 2004; Takai and Shigehara, 2004). Many authors argue that postorbital closure evolved just once at the base of the Tarsius + Anthropoid clade (e.g. Ross, 1994, 1996). If so, amphipithecids would be placed outside the crown haplorhine clade, or postorbital closure was secondarily lost in amphipithecids. An alternative possibility is that postorbital closure evolved convergently in Tarsius and Anthropoidea as argued by Simons, Rasmussen, Beard, and others (Rasmussen and Simons, 1992; Beard and MacPhee, 1994; Simons and Rasmussen, 1996). A resolution of this problem awaits recovery of new material establishing the orbital structure of eosimiid anthropoids.
One of the amphipithecid frontal fragments mentioned above preserves the dorsal endocranial surface of the olfactory fossa and frontal pole of the cerebrum (Takai et al., 2003). The olfactory bulbs apparently were quite large—within the range of extant strepsirrhines, and comparable to those of Eocene omomyids and adapoids, but distinctly larger than Fayum anthropoids like Apidium and Aegyptopithecus. Again, this reinforces the point of view that amphipithecids are more primitive than Fayum and more recent anthropoids but does not rule out a haplorhine status.
In summary, the dental, cranial, and postcranial evidence point in very different directions: the dental evidence suggests a link to crown anthropoids; the postcranial evidence suggests that amphipithecids could be anthropoids but are outside the more inclusive clade of crown and African stem anthropoids; the orbital and olfactory anatomy suggests that the group, while possibly haplorhine is outside the crown haplorhine clade.
A very different phylogenetic placement of the amphipithecid clade is as a sister group to one or another group of adapoid primates. The case for a relationship to notharctine adapoids from North America was made by Gunnell, Ciochon, and colleagues (Ciochon et al., 2001; Gunnell et al., 2002). Such an argument was based mainly on overall large size and postcranial primitiveness. Additionally, synapomorphies of the dentition were proposed, especially the presence of a ‘pseudohypocone’ in amphipithecids and some advanced notharctines. Ducrocq (1999) and later Shigehara et al. (2002) reviewed the latter proposed similarity and discounted it.
A possible phyletic link has been suggested between amphipithecids and non-notharctine adapoids of Europe like Adapis, Leptadapis, Pronycticebus, and their relatives on other continents such as Mahgarita (North America) and Aframonius (Africa). Kay et al. (2004c) reported that such a link is less parsimonious than the anthropoid hypothesis but has the advantage of not requiring parallel evolution of postorbital closure. At that time, we indicated our preference for this second, admittedly less parsimonious, interpretation that amphipithecids are adapoids with dental and gnathic convergences toward later larger-bodied Oligocene African anthropoids. However, as noted above, the recent discovery that the amphipithecid talus is distinctly anthropoid-like (Marivaux et al., 2003) now leads me to conclude that amphipithecids, like eosimiids, may well be stem anthropoids or at least stem haplorhines.
Published estimates of body size in amphipithecids are based on molar size (Table 1) (see also Egi et al., 2004) as well as estimates from humerus length (Ciochon et al., 2001) and talus size (Marivaux et al., 2003). The larger specimens of Pondaungia (specimens referred by some to the species Pondaungia savagei) had a mass of between 7.1 and 8.2 kg. Smaller specimens (for some P. cotteri sensu stricto) were between 4.6 and 5.0 kg. If the two taxa prove to be sexual morphs of one species, males would on average have been as much as 60% larger than females. Such a high level of dimorphism does occur among anthropoids, and might count as an added symplesiomorphy with anthropoids.
The body size of Amphipithecus mogaungensis was between 5.8 to 6.3 kg and that of Siamopithecus eocaenus was approximately 4.9 kg. These estimates place Pondaungia, Amphipithecus, and Siamopithecus among the largest Eocene primates. The only other taxon that approaches them in size is Leptadapis magnus from the late Eocene of Europe; its size range was up to 8 kg (Gingerich, 1980). No late Eocene African primate approaches these amphipithecids in size (Kirk and Simons, 2000).
The body size of Myanmarpithecus is estimated to be about 1.2 kg. This animal was comparable in size to Adapis parisiensis (late Eocene, Europe). The body sizes of known amphipithecids exceed those of extant insectivorous primates (Kay, 1975; Gingerich, 1980; Kay and Covert, 1984). On this basis we can rule out a primarily insectivorous diet for all known amphipithecids.
The orbits of amphipithecids are known only in Amphipithecus, and even in that taxon, not sufficiently preserved to estimate the orbital diameter. Therefore, we hazard no inference about the activity pattern of Amphipithecus. The absence of postorbital closure in Amphipithecus (see above) allows some inferences to be made about visual acuity. A functional link has been proposed between postorbital closure, a retinal fovea, and the absence of a tapetum lucidum (Cartmill, 1980; Ross and Hylander, 1996; Kirk and Kay, 2004), all adaptations for more acute vision in anthropoids than in extant strepsirrhines whose visual acuity, while excellent by mammalian standards, is less than in anthropoids (Kay and Kirk, 2000; Kirk and Kay, 2004). Therefore the absence of postorbital closure in Amphipithecus suggests this animal did not possess the acute vision present in modern anthropoids (Kay et al., 2004b). Indeed, the preserved parts of the orbits of Amphipithecus resemble those of Leptadapis, an Eocene adapoid that apparently did not possess acute vision (Kay and Kirk, 2000).
On the ventral surface of one Amphipithecus frontal, the olfactory fossa, which housed the olfactory bulbs, is preserved (Takai et al., 2003; Shigehara and Takai, 2004). The fossa appears to be larger than in living or fossil anthropoids but similar in relative size to those of living and fossil strepsirrhines including Leptadapis (Kay and Cartmill, 1977; for discussion see Radinsky, 1977; Takai et al., 2003). The implication is that the sense of smell may have been more important as a mode of perception and communication in amphipithecids than is the case for living anthropoids and of comparable importance to living strepsirrhines (Kay et al., 2004a, d).
The upper central incisor of Amphipithecus is proportionately large and spatulate and has a buccolingually broad root, suggesting that it was optimized to resist powerful buccolingual stresses engendered when it was used to separate a bite of food (Shigehara et al., 2002). Apparently, Pondaungia used its incisors for powerful incision, as do fruit-husking anthropoids.
In large-bodied amphipithecids [Amphipithecus, Pondaungia (Figure 1A), and Siamopithecus], the development of the shearing crests on the molars, expressed as shearing quotients (SQs, see explanation in Table 2, Table 3) are poorly developed. The molar shear development among these amphipithecids is similar to that of living strepsirrhines and platyrrhines that have low-fiber diets (fruit- and seed-eaters) and far less than molar SQs of living species that have higher-fiber diets. Shearing development in large-bodied amphipithecids is especially similar to that of extant seed-eating platyrrhines (Figure 2).
 View Details | Figure 1. Box and whisker plots of the distribution of shearing quotients (SQs) of extant strepsirrhines, extant platyrrhines, and amphipithecids based on the frugivorous strepsirrhine model (after Kay et al., 2004b). See Table 2, Table 3 for data and details. |


 View Details | Figure 2. Pondaungia dentition. (A) Occlusolateral view of Pondaungia cotteri (NMMP 1), showing the poorly developed shearing crests and crenulated enamel characteristic of the species; (B) Pondaungia cotteri (NMMP-KU0003), medial view of M1 and broken M2; and (C) traced outline of the occlusal surface and exposed dentine-enamel junction along the natural break of M2 (after Kay et al., 2004b). |
Myanmarpithecus has better developed shearing than other amphipithecids; nevertheless, it exhibits far less shearing than do extant folivorous strepsirrhines and platyrrhines. For this taxon, a mixed diet is inferred, consisting primarily of fruit with substantial components of leaves or insects as a protein source.
The cheek teeth of living primates and other mammals that specialize in eating hard seeds or in splitting open tough, hard fruits often have thick enamel (Kay, 1981). While precise measurement of enamel thickness is not possible for any amphipithecid, one broken specimen of Pondaungia (NMMP 12) reveals that its enamel is very thick (Figure 2B, C). This suggests that Pondaungia incorporated substantial quantities of very hard objects such as seeds encased in hard shells in its diet, a conclusion that is consistent with inferences about powerful incisal biting and poorly developed molar shearing.
The mandibular symphyses of Siamopithecus, Amphipithecus, and Pondaungia are not fused, but the symphyseal surface is rugose with substantial relief (Figure 3). In spite of the lack of fusion, this arrangement would have allowed the efficient transfer of muscle forces from the balancing to the working side of the mandible. In light of the large spatulate upper incisors, poorly developed molar shearing, and (in Pondaungia) thick molar enamel, this symphyseal construction suggests hard or tough-object feeding.
 View Details | Figure 3. Mandibular symphysis of Amphipithecus mogaungensis in medial view showing the rugose unfused symphyseal surface (after Kay et al., 2004b). |
Humeral, ulnar, and calcaneal remains of a single individual (NMMP 20) and a talus of another are referable to Amphipithecidae. NMMP 20 was a slow-moving arboreal quadruped like a modern lorisid and the late Eocene European adapoids Adapis and Leptadapis (Dagosto, 1983), but quite unlike the middle Eocene North American adapoids Notharctus or Smilodectes (Dagosto, 1993; Schmitt, 1996). In the proximal humerus the tuberosities are positioned well below the summit of the articular surface of the humeral head. In active arboreal and terrestrial quadrupedal primates (AAC), and primates that engage in vertical clinging behaviors (in association with leaping) (hereafter VCL; Figure 4C), the tuberosities for insertion of the rotator cuff muscles rise to the same level as, or are superior to, the summit of the glenohumeral articular surface. In contrast, living slow-moving arboreal quadrupeds (Figure 4A) have the tuberosities positioned below the summit, that is to say, the humeral head projects distinctly superior to the tuberosities (Jolly, 1967; Gebo, 1988; Rose, 1988; Harrison, 1989).
 View Details | Figure 4. Medial views of the proximal humeri of (A) Nycticebus coucang, a slow-moving arboreal quadruped; (B) NMMP 20; and (C) Propithecus verreauxi, a vertical clinging and leaping species. The dashed line illustrates the position of the tuberosities. The median orientation of the glenohumeral articular surface is depicted by the solid line (after Kay et al., 2004b). |
As illustrated in Figure 4, the proximal articular surface of the humeral head of NMMP 20 is oriented cranially in a manner most like that of a loris or Alouatta, the howler monkey (Schon-Ybarra, 1998). This goes along with the positioning of the tuberosities below the articular summit to indicate that the shoulder joint was optimized for overhead reaching and bridging (Jolly, 1967; Walker, 1974; Gebo, 1988) and had reduced stability in protracted positions (Rose, 1989; Schmitt, 1996). In contrast, the articular surface is oriented more acutely to the long axis of the shaft (i.e. more cranially) than in most AAQ and VCL primates (Schmitt, 1996).
NMMP 20 has a nearly equal mediolateral breadth relative to its proximodistal length and is relatively flat when its depth is compared either with its proximodistal length or mediolateral length. Primates that engage in habitual VCL are separated from AAQ species and extant cautious arboreal quadrupeds as well (Loris, Nycticebus, Perodicticus, and Alouatta) (Schmitt, 1996; Kay et al., 2004b). In indices of humeral head shape, NMMP 20 falls among the slow-moving arboreal quadrupeds (Figure 5).
 View Details | Figure 5. Comparative morphology of the proximal humerus. Means and ranges of (A) humeral head shape, and (B) humeral head inflation in slow climbers (SC), active arboreal quadrupeds (AAQ), and vertical clingers and leapers (VL). Humeral head shape measurements are the length of the base (cord) of a contour along the central proximodistal (superior-inferior) surface of the humeral head (PD), the same for a central mediolateral contour (ML), and the central height of the ML contour from its base (AH). The sample includes five species of active arboreal quadrupeds, six species of vertical clingers and leapers, and four species of slow moving quadrupeds (for details see Kay et al., 2004b). |
The cortical walls of the humeral shaft of NMMP 20 (Figure 6) were exceptionally thick compared with most living strepsirrhines and most closely approximate those of slow-moving arboreal quadrupeds (Kay et al., 2004b). The internal and external dimensions of the humeral shaft give an estimate of cortical area (CA); the ratio of internal diameter to external diameter (K) can also be estimated (Table 4). Values for CA and K in NMMP 20 are larger for this specimen than for any leaper or active arboreal quadruped of similar body size (Kay et al., 2004b). CA of NMMP 20 is most similar to Adapis and Leptadapis (Runestad, 1994) and extant slow-climbing lorises (Runestad, 1997). The values for K show that this specimen has extraordinarily thick cortical bone, approached only by lorises and Daubentonia.
 View Details | Figure 6. Morphology of the NMMP 20 humerus. (A) Humeral reconstruction after Ciochon et al. (2001) and the outline of the natural mid-shaft cross-section showing the distribution of cortical bone and medular cavity. (B) Anteroposterior values for the quantity K (relative cortical thickness, described in the text and Table 4) for samples of active arboreal quadrupeds, slow climbers, and vertical clingers and leapers (data from Runestad, 1994). |

The elbow joint of NMMP 20 shows additional functional similarities to lorises. In particular, the morphology of the radiohumeral and ulnohumeral joints are well suited to provide stability in habitually flexed postures and shows the greatest functional similarity to extant lorises and howler monkeys (Alouatta). The capitulum is large and rounded, and distinctly separated from the trochlea by a narrow, shallow zona conoidea. The capitulum has a well-developed anterolateral flange (‘tail’) with a distinct and deep ridge (Figure 7). This design provides stability for the radius in flexed postures and is best developed in slow climbers like Nycticebus and Perodicticus (Szalay and Dagosto, 1980; Rose, 1993). A distinct capitular ‘tail’ with a deep ridge is also found in Alouatta (Schon-Ybarra, 1998). Taken as a whole, the large, round capitulum, truncated cone-like trochlea, and the strong capitular tail suggest cautious arboreal quadrupedalism. This is consistent with the clear signal from the proximal humerus and humeral shaft.
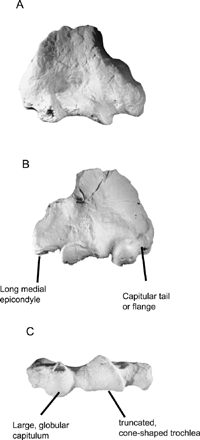 View Details | Figure 7. Distal humeral fragment of NMMP 20, posterior (A), anterior (B) and distal (C) views. |
The NMMP 20 calcaneus does not exhibit the extreme distal elongation of Tarsius. Its proportions are more reminiscent of African late Eocene to early Oligocene anthropoids and Eocene North American and European adapoids. These findings are not especially informative as to locomotion. Extant primates exhibiting a similar degree of foot elongation have a wide spectrum of locomotion.
The talus of a large-bodied amphipithecid (NMMP 39) not associated with dental remains has recently been described by Marivaux et al. (2003). Using size, Marivaux et al. allocate this specimen to Amphipithecus. However, given the size overlap between Amphipithecus and smaller specimens of Pondaungia, allocation of NMMP 39 to either taxon would appear plausible.
Marivaux et al. (2003) reported that NMMP-39 exhibits a straight and moderately long talar neck, a fairly high talar body, and parallel-sided medial and lateral trochlear facets (not wedged-shaped) with circular rims, all of which features might be indicative of leaping. However, they note that (1) the trochlea is quite flattened rather than deeply grooved as in most leapers, where only one primary plane of movement is needed at the talocrural joint; (2) the medial trochlear rim has a long radius of curvature; and (3) the posterior trochlear shelf is absent. These three features suggest much more mobility at the tibiotalar joint than typically seen in specialized leaping primates. They suggest that NMMP-39 might have belonged to an arboreal quadruped, not an extreme leaper.
Thus there is a discrepancy between the slower quadrupedalism inferred from the humerus (NMMP 20) and active above-branch quadrupedalism inferred from the talus (NMMP-39). A plausible but presently untestable possibility is that we might be dealing with the skeletal remains of two different amphipithecid genera.
Amphipithecidae is a phylogenetically enigmatic group that some place among Anthropoidea and others with Adapoidea. Dental evidence is often cited in support of the anthropoid status of amphipithecids. As noted above, amphipithecids bear a strong resemblance to propliopithecids of the African Oligocene, However, it appears that many of the apparent crown anthropoid dental synapomorphies seem to have evolved in several lines of anthropoids. Dissimilarities between the amphipithecid humerus and calcaneus and those of late Eocene and early Oligocene African oligopithecids, parapithecids, and propliopithecids suggest amphipithecids may instead lie outside a more encompassing clade of these stem anthropoids (see reviews by Ciochon and Gunnell, 2004; Seiffert et al., 2004). An amphipithecid talus shares a number of derived similarities with anthropoids from the late Eocene and early Oligocene of Africa but not exclusively with crown anthropoids. At the very least, this postcranial evidence indicates that the amphipithecids may be stem anthropoids but are outside the clade of the African Eocene-to-Recent anthropoids.
Postorbital closure is thought to be a key adaptive innovation related to visual acuity, often assumed to have evolved only once at the base of the Tarsius + Anthropoid clade (e.g. Ross, 1994, 1996). The frontal bone of Amphipithecus indicates that postorbital closure was absent or incomplete. Therefore, if amphipithecids are anthropoids, postorbital closure must have evolved independently twice (Beard and MacPhee, 1994), or evolved and then been lost in the anthropoid lineage.
The upper and lower teeth, mandibular structure, as well as humeral, talar, and calcaneal fragments and the talus provide detailed evidence for the adaptive profile of amphipithecids. The larger-bodied amphipithecids were as large as any known Eocene primates, and comparable in size to the largest extant platyrrhines and strepsirrhines. The mandibular corpora of these large-bodied amphipithecids suggest an ability to resist large chewing loads and to transfer muscle forces from the balancing side to the working side of the jaw, thus increasing the muscle force available for mastication. The robust, spatulate upper central incisor and projecting robust upper and lower canines show that they used the anterior teeth for powerful separation of food items, as occurs in fruit husking by living anthropoids. The molars have weak shearing crests, indicating a low-fiber diet of fruits or seeds. The presence of thick enamel in Pondaungia suggests a hard-object, low-fiber diet, possibly seed predation. The smaller and more primitive amphipithecid Myanmarpithecus weighed 1–2 kg. Its cheek teeth suggest a frugivorous diet.
The humerus, ulna, and calcaneus of one large amphipithecid species suggest that this animal was an above-branch quadruped, and most likely a slow, cautious one. The humeral structure resembles extant slow-moving primate quadrupedal species like the living lorises and Alouatta. The shoulder structure suggests a wide range of motion including overhead reaching. Midshaft bone strength was exceptional and elbow stability was enhanced in habitually flexed positions. No features suggest rapid above-branch locomotion nor a vertical clinging and leaping or simple leaping locomotion. A talus of another large amphipithecid of uncertain taxonomic assignment lacks certain features associated with vertical postures or springing. However, it was tightly articulated with the tibia making a stable ankle joint, features consistent with rapid arboreal quadrupedalism (Marivaux et al., 2003).
This paper draws from and summarizes the results of two investigations as they relate to the Amphipithecidae—that of Kay et al. (2004c) on the phylogeny of Anthropoidea and of Kay et al. (2004b) on the paleobiology of this interesting clade. I am indebted to the coauthors of those papers for many of the insights presented here: J. Perry, C. Ross, D. Schmitt, N. Shigehara, M. Takai, N. Egi, C. Vinyard, and B. Williams. I thank N. Shigehara, M. Takai, H. Hongo, and M. Hoffman of the Primate Research Institute of Kyoto University for their invitation to attend the conference held in Inuyama, Japan in 2003 at which I presented a version of this paper.
|