| AYUMI YAMAMOTO, corresponding author. e-mail: yamamoto@pri.kyoto-u.ac.jp phone: +81-568-63-0524; fax: +81-568-61-5775 Published online 8 March 2006 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.050811 |
In the extant human atlas, the superior aspect of the posterior arch is characterized by a shallow groove on each side for the vertebral artery. It is well known that bony bridges, known as atlas bridges, are sometimes observed over this groove (Williams, 1995). There are two kinds of atlas bridge, the ‘posterior bridge’ and the ‘lateral bridge’. The posterior bridge is an osseous bridge that is formed between the posterior margin of the superior articular facet and the posterior arch of the atlas. The lateral bridge is an ossification that bridges the lateral margin of the superior articular facet and the transverse process of the atlas (Figure 1).
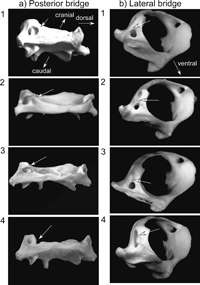 View Details | Figure 1. Categories of the atlas bridge. a) The posterior bridge: 1, complete; 2, incomplete; 3, incipient; 4, absent. Viewed from the dorsal and slightly lateral direction. b) The lateral bridge: 1, complete; 2, incomplete; 3 incipient; 4, absent. Viewed from the cranial and lateral direction. |
Data on the prevalence of atlas bridging in humans have been accumulated for years. Geographical variation of atlas bridging has been examined in various human populations (Selby et al., 1955; Shibata et al., 1965; Sato and Noriyasu, 1978; Saunders and Popovich, 1978; Dugdale, 1981). However, the effects of ontogeny on atlas bridging in humans are still being debated. Some authors consider that bridge formation is affected by development (Nathan, 1962; Taitz and Nathan, 1986), while other authors recognize no age effect (Selby et al., 1955; Kendrick and Biggs, 1963; Dugdale, 1981). It is difficult to clarify this problem because atlas bridging is a relatively rare variation in extant humans, usually present in less than 20% of individuals.
As for atlas bridging in extant nonhuman primates, there are only a few studies (Loth-Niemerycz, 1916; Sato and Noriyasu, 1978; Yamamoto and Kunimatsu, 2002a, b; Le Minor and Trost, 2004), most of which were based on analysis at the genus or family levels. These studies showed that prevalence of atlas bridges in humans is much lower than in the other primate taxa. Le Minor and Trost (2004) studied atlas bridges in 41 genera of extant nonhuman primates and discussed possible evolutionary trends. In their study, however, the material lacked data such as age and sex, and, except for the hominoids, the data were analyzed at the generic level. Consequently, the effects of ontogenetic change, sexual differences, or intraspecific (and in most cases, even interspecific) variation were not considered. At present, there is little information regarding age effects, sexual differences, and intraspecific variation of atlas bridging in nonhuman primates. We need to confirm these aspects of the trait before conducting reliable interspecific comparisons.
The aim of this study is to examine the intraspecific stability of atlas bridges. We examined the effects of age on atlas bridging by using skeletal specimens of Japanese macaques (Macaca fuscata) of known ages. We also examined sex differences and geographical variation in the Japanese macaques to reveal the degree of variation in the prevalence of atlas bridging that may occur within a single nonhuman primate species. Additionally, since the fossil record does not always follow the evolutionary trends suggested by Le Minor [e.g. Nacholapithecus kerioi (Ishida et al., 2004)], we discuss the validity of such interpretations.
In most previous studies, two (complete/incomplete) or three (complete/incomplete/absent) categories have been used to describe the degree of development of the posterior and lateral atlas bridges. Following Hauser and De Stefano (1989), we used the following four categories to better examine the relationship between age and development of the atlas bridges (Figure 1).
(1) Complete: The bony bridge is completely formed.
(2) Incomplete: The bony bridge is incomplete, but more than half of the bridge is formed.
(3) Incipient: Less than half of the bony bridge is formed.
(4) Absent: No trace of a bony bridge is developed.
To study ontogenetic change, we used a total of 193 atlases (102 males and 91 females) of Japanese macaques coming from individuals of known age, and spanning the various life stages. These specimens are stored in the Primate Research Institute (PRI), Kyoto University. All the specimens belonged to the Takahama group, one of the captive groups of Japanese macaques of the PRI. The original stock of the Takahama group was derived from Takahama town in Fukui prefecture (Figure 2). Their original bloodline has been preserved under captivity. The age of death of the specimens used in this study ranged from 0 to 8667 days (Matsubayashi et al., 2000).
In the study of geographical variation, we used a total of 328 atlases (155 males and 173 females) of Japanese macaques stored in the PRI, the Laboratory of Physical Anthropology of Kyoto University, the Japan Monkey Center, and the Research Group of Monkeys in Miyagi Prefecture. All the specimens were adult, i.e. all permanent teeth were erupted. In Japanese macaques (our Takahama material), the permanent dentition is completed at an age of around 7.2–8.3 years (2633–3023 days).
The specimens for the geographical variation study come from seven local areas in Japan (Figure 2, Table 1). Except for the Takahama group, almost all specimens are those of wild-ranging monkeys. Shoudoshima, Kinkazan, and Yakushima are small islands, while all the other sample areas are on Honshu, the main island of Japan. The Yakushima group is usually assigned to a different subspecies, Macaca fuscata yakui, while the other six groups are classified as Macaca fuscata fuscata. However, in genetic aspects, the Yakushima population is not particularly different from the mainland populations (Kawamoto, 2002).
 View Details | Figure 2. Geographic distribution of the sample groups. |
Because there was no clear difference between the right and left sides, we show, in Figure 3a-1 and Figure 3b-1, the scatter diagrams for the posterior and lateral bridges of the right side. Sex differences were not apparent in either of the two types of atlas bridging (Figure 3a-1, Figure 3b-1). In clear contrast to the case of Homo sapiens, adult Japanese macaques commonly have both posterior and lateral bridges. We show the presence of obvious ontogenetic change, from 0 to 2500 days, in the frequencies of both types of bridge using frequency bar graphs (Figure 3a-2, Figure 3b-2).
 View Details | Figure 3a. Posterior bridge formation (right side). 1) Category distribution by age. 2) Percentage bar graph of the posterior bridge. Categories are the same as in Figure 1, age in days, white circles are females, black triangles are males. |
 View Details | Figure 3b. Lateral bridge formation (right side). 1) Category distribution by age. 2) Percentage bar graph of the lateral bridge. Categories are the same as in Figure 1, age in days, white circles are females, black triangles are males. |
However, there are some differences in the timing of development between the posterior and lateral bridges. The posterior bridge is already formed to some extent at birth. For example, the youngest two individuals (PRISK 5819 and 5243, 0 days old) show an incipient development of the posterior bridge on both sides (category 3 in Figure 1a). The posterior bridge appears to develop rapidly after birth (Figure 3a-1, Figure 3a-2). At around the age of 400 days, some individuals have a complete posterior bridge, with the youngest record of bridge formation being 142 days old on the left side (PRISK 3740) and 370 days old (PRISK 4081) on the right side. All individuals older than 830 days in the present sample have a complete posterior bridge on both sides (Figure 3a-1). These results suggest that formation of the posterior bridge is completed at around 400–830 days in Japanese macaques. In Figure 3a-2, the frequency of category 1 is seen to increase at around 1000 days, in both males and females.
The formation of the lateral bridge starts later and the rate of development appears to be considerably slower relative to the posterior bridge. A complete lateral bridge starts to be observed at around 700 days; the youngest individual with a complete lateral bridge is 469 days old (PRISK 4336). In Figure 3b-2, the frequency of category 1 is seen to increase gradually from around 1000 to 2500 days in both males and females. The completion of the lateral bridge formation appears to occur by the age of ca. 2500 days. The majority of adult individuals possess a complete lateral bridge, although in a few individuals the lateral bridge is incomplete even in adulthood.
In Table 2 and Table 3, the prevalence of category 1 (‘complete’) of the posterior and lateral bridges is given. Sex difference is not obvious in any group in relation to the posterior bridge. As for the lateral bridge, the Shimane group shows a relatively greater sex difference (a difference of approximately 15–25%), which was statistically significant (P = 0.028, Mann–Whitney U-test).
Because a significant sex difference was found only in the lateral bridge of the Shimane group, we combined the data of males and females for comparisons of geographic variation.
As shown in Table 2, adult Japanese macaques, as a whole, show an extremely high prevalence of a completely formed posterior bridge (over 98%). All local groups have similarly high prevalences. In five local groups (Shimane, Shoudoshima, Bousou, Kinkazan, and Takahama), prevalence of the complete posterior bridge was 100%. The lowest prevalence obtained was still high: 92% in the Yakushima sample. These results suggest that the complete posterior bridge is a normal structure of adult Japanese macaques. According to the Kruskal–Wallis test, there was no statistically significant difference in the prevalence of the posterior bridge among these local groups.
The lateral bridge tends to show relatively lower prevalence than the posterior bridge, especially in the Shimane group (Table 3). In most groups, however, the observed prevalence was over 90%. In the Shimane group, the ‘cranial’ prevalences were 94.6% (total), 84.6% (male), and 100% (female); ‘side’ prevalences were 86.5% (total), 73.1% (male), and 93.8% (female); and ‘bilateral’ prevalences were 78.4% (total), 61.5% (male), and 87.5% (female). In contrast to the posterior bridge, only the Kinkazan group exhibited a 100% prevalence of the completely formed lateral bridge. The Kruskal–Wallis test showed statistically significant differences in the ‘side’ and ‘bilateral’ prevalences of the lateral bridge (P = 0.0007 and P = 0.0015, respectively), but this was not the case with the ‘cranial’ prevalence. Bonferroni’s multiple comparison tests were conducted with the following results. ‘Side’ prevalence of the lateral bridge in the Shimane group was significantly different from that of the Yakushima (P = 0.0027), Nagano (P = 0.0018), Bousou (P = 0.0005), and Kinkazan (P = 0.0056) groups. As for the ‘bilateral’ prevalence, the Shimane group differed from the Yakushima (P = 0.0031), Nagano (P = 0.0041), and Bousou (P = 0.0024) groups. Therefore, although the lateral bridge is a normal structure in Japanese macaques, its prevalence is significantly lower in the Shimane group.
In extant humans, the low prevalence of atlas bridging makes it difficult to determine whether or not this is affected by ontogeny. The present study brings new insight to this matter. Ontogenetic change of atlas bridging is obvious in the Japanese macaques, the development of which is demonstrated to be a normal process of growth. Judging from the cross sectional data of the present study, atlas bridging starts to develop early in life and becomes common by early adulthood. This indicates that atlas bridges are not abnormal ossifications related to aging. The lateral bridge starts to form later and more gradually than the posterior bridge. We infer that this may be associated with the slightly lower prevalence of the lateral bridge observed in adults.
Interestingly, extant hominoids show a relatively low prevalence of atlas bridging, especially lateral bridging, compared to most other primate taxa (Loth-Niemerycz, 1916; Yamamoto and Kunimatsu, 2002b; Le Minor and Trost, 2004). It is likely that the occasional appearance of atlas bridging in extant humans may be a remnant condition of an ancestral trait that was once a normal process of growth. Le Double (1912) reported that the posterior bridge occurs in various mammals (Chiroptera, Carnivora, Artiodactyla, Cetacea, Pinnipedia, Edentata). Since the posterior bridge is a common structure occurring widely throughout mammals, the evolutionary history of atlas bridging may go back to the age of primitive primates.
The low prevalence of atlas bridging in hominoids may relate to their posture. In non-hominoid primates, the prevalence of both types of bridges is very high. The prevalence in hominoids is lower than that in non-hominoids, and the prevalence in humans is even lower than in hominoids. This trend appears to correspond to postural change in primates, from quadrupedalism to bipedalism, which may have affected the path of the vertebral artery that passes under the atlas bridge. In the evolutionary history in primates, the foramen magnum has moved towards the center of the cranial base. Corresponding to the movement of the foramen magnum, the shape of the atlas has changed (Gommery, 1996). The vertebral artery enters the transverse foramen of the 6th cervical vertebrae and then changes its direction to run along the atlas, and finally, enters the foramen magnum (Sato and Noriyasu, 1978). Therefore, a change in the angular relationship of the head and neck, and change in the shape of the atlas itself, results in a change in the course of the vertebral artery. These changes may affect the development of atlas bridges. Further studies, however, are needed to provide an answer to this problem.
In the case of the posterior bridge, almost all Japanese macaques have a bilaterally complete posterior bridge. Extremely high frequencies were observed even in small local groups. The high prevalence observed in the present study is similar to that previously reported for non-hominoid primates (Table 4). The exception was the relatively low values reported by Loth-Niemerycz (1916). However, that author did not provide details on the method used to calculate prevalence.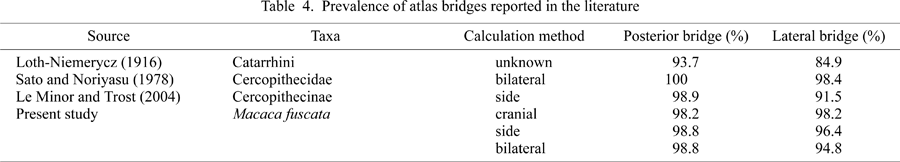
In the case of the lateral bridge, although the prevalence of a completely formed bridge was mostly high (over 90%), a considerably lower prevalence (e.g. 61.5% bilateral presence of the lateral bridge) was observed in the male group from Shimane. Because the formation of the atlas bridge is related to growth, if relatively young individuals had dominated the sample, then the prevalence would be relatively low. Therefore, we checked the sphenooccipital synchondrosis (which fuses after third molar eruption) of the Shimane specimens to ascertain the relative ages of those individuals that lack a complete lateral atlas bridge. Most of the specimens had a fused synchondrosis, suggesting that they were not particularly younger than the other adult individuals. Another possibility is that they may have come from a small genetic pool. Previous authors (Selby et al., 1955; Saunders and Popovich, 1978) have revealed that the posterior bridge is a familial trait in humans. It is possible that genetic variation causing hypoplasia of the atlas bridge had increased by genetic drift, including founder effect. Unfortunately, there are few morphological or genetic studies on Japanese macaques from Shimane prefecture (e.g. Mouri and Nishimura, 2002).
The present study shows that, in the Japanese macaque, ontogenetic change in atlas bridging is seen only during the early stages of growth. We also showed that atlas bridges are stable in adults. Sex differences and geographical variation are not significant in general, although the case of the Shimane population indicates that there can sometimes be a certain degree of intraspecific variation.
From the results of previous studies, atlas bridges may be considered as one of the traits that distinguish high-level taxa in primates. Well-preserved atlases have been recently reported in two Miocene hominoids, Otavipithecus namibiensis and Nacholapithecus kerioi (Conroy et al., 1996; Gommey, 2000; Ishida et al, 2004). Otavipithecus namibiensis from the Middle Miocene of Namibia (ca. 13 Ma) has the posterior bridge on both right and left sides, while it lacks any trace of the lateral bridge bilaterally (Conroy et al., 1996; Gommery, 2000). This pattern is rare in extant hominoids except for chimpanzees. Nacholapithecus kerioi from the early Middle Miocene of Kenya (ca. 16 Ma) has well-developed posterior and lateral bridges (Ishida et al., 2004). This is the pattern widely observed in extant non-hominoid primates and is most likely a primitive trait. It is interesting that Nacholapithecus was an arboreal quadruped, not a suspensory animal like extant hominoids (Ishida et al., 2004). These examples suggest that atlas bridging may be used for a better understanding of fossil primates and that further detailed studies are needed in extant primates.
We appreciate all who contributed to collecting the skeletons studied in this article. We are grateful to the staff of the Primate Research Institute of Kyoto University, Laboratory of Physical Anthropology of Kyoto University, Japan Monkey Center, and Dr Kosei Izawa and the Research Group of Monkeys in Miyagi Prefecture for allowing us to examine the specimens under their care. Dr Martin Pickford (Chaire de Paléoanthropologie et de Préhistoire du Collège de France) and Dr Brigitte Senut (Département Histoire de la Terre du Muséum National d’Histoire Naturelle) kindly allowed us to examine the cast of Otavipithecus namibiensis. We would like to thank Mr Robert Schubert of the Ohio State University for improving our manuscript. We also would like to thank two anonymous reviewers for their valuable comments. This study was financially supported by Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, and Culture (#10CE2005, #15570193).
|