| |
| Collected Papers in Honor of Professor Emeritus Banri Endo: Commemoration of His Seventieth Birthday Changde Shi, corresponding author. e-mail: j-shi@dokkyomed.ac.jp phone: +81-282-87-2123; fax: +81-282-86-6229 Published online 8 August 2006 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.050308 |
|
Since erect bipedality in humans is an important topic in physical anthropology, the adaptability to bipedalism in other animals has been analyzed in terms of comparative morphology, kinetics and the kinematics of primate species from prosimians to humans (Kondo, 1985). In addition to the above methodologies, experimental morphological studies in mammals may help to further our understanding of the mechanisms of human locomotion.
Fuld (1901) pioneered the experimental study of bipedal animals using forelimb-amputated dogs. Subsequent researchers have adopted the same type of experimental models to search for morphological evidence of bipedal posture and locomotion in hindlimb bones, vertebral columns, and the skulls of laboratory animals (Colton, 1929; Goff and Landmesser, 1957; Riesenfeld, 1966; Matsumura, 1991). Goff and Landmesser (1957) pointed out that the lumbar spinous processes of bipedal rats tended to be angulated more toward the head when compared with their quadrupedal controls. This experimental rat model has been used extensively in orthopedics (Sato, 1959; Rodriguez et al., 1965; Riesenfeld, 1966). Diseases of the vertebral column, caused by increased load on the lumbar vertebrae due to bipedal standing, have been examined in rats, in which intervertebral disc herniations (Sakamoto, 1959; Sato, 1959; Ushikubo, 1959; Yamada et al., 1960; Yamada, 1962; Higuchi et al., 1983), osteophytes (Gloobe and Nathan, 1973), and spinal canal stenosis (Nasu, 1981) were present.
Meanwhile, with respect to the shape of vertebral bodies, two experimental studies concerning the cervical through lumbar vertebrae have been reported, one by Slijper (1946) and the other by Shi et al. (2000). However, both articles were case reports that commented upon only a single subject, which was analyzed from a mechanical viewpoint. In order to analyze bipedal influences on vertebral morphology statistically, experiments involving a number of genetically homogenous animals are required. Generally, rats have been regarded as a suitable animal for such studies. While maintaining bipedal posture, they can stretch their hip and knee joints almost to the maximum (Matsumura, 1991). Unlike forelimb-amputated rats, both lordosis and kyphosis of the vertebral column have been detected in bipedally conditioned rats, because the former group could not maintain complete bipedal posture (Matsumura and Okada, 1990; Matsumura, 1991; Bailey et al., 2001). Since the burdening of operant-conditioned bipedal rats could be evaluated in terms of the duration of bipedal standing posture, the latter model is more appropriate for quantitative experimental study on skeletal adaptation in animals.
In the present study, we used genetically homogenous rats that had performed bipedal standing exercises and examined changes in their vertebrae from the cervical through lumbar levels. Our goal was to provide morphological evidence for bipedal effects on the vertebral bodies of laboratory rats, using statistical analysis of the various morphological measurements.
The skeletal materials that we used in the present study were the vertebrae of Sprague Dawley rats (Jcl: SD, CLEA Japan Inc.) that had been trained and sacrificed by Matsumura and Okada (1990), who had examined the effects of erect bipedal standing on the femur. The experimental apparatus was a bipedal training box, in which a rat was reinforced positively with bipedal standing behavior using operant conditioning (Matsumura and Okada, 1990; Matsumura, 1995). From 64 to 140 days of age, rats in the exercise group were set to perform bipedal standing and lever pushing exercises twice daily, 10–30 minutes in the morning and 30 minutes in the evening. The rats maintained an erect bipedal posture for about 6 seconds, on average, to reach each pellet of food. They pushed a lever with a weight equal to their own body weight (500 g maximum) from age 80 days onwards. The total number of exercise sessions was 136–138, and the rats in the exercise group stood with a bipedal posture for a cumulative mean of 171 minutes during the experimental period (Matsumura and Okada, 1990). No exercise was imposed on the control group. At the age of 140 days, all the rats were sacrificed using ether anesthesia.
After all the soft tissue and the intervertebral discs had been removed, the rats’ vertebrae were dried at room temperature to allow for measurement (Figure 1). Seventeen male rats were used for the osteometry, comprising an exercise group (n = 9) and a control group (n = 8). The mean amount of food consumed and the mean body weight during the period of breeding was similar in both groups.
 View Details | Figure 1. Two typical lumbar vertebrae (L2) prepared for measurement: one (right) from the exercise group, and the other (left) from the control group. In the cranial (superior) view, arrows indicate CrDV. In the lateral view, dashed lines denote the gradient of the cranial surface of the vertebral body. |
The vertebral level for each vertebra was identified with reference to Hebel and Stromberg (1976). Along the vertebral column, from the third cervical (C3 level) to the last lumbar (L6 level) vertebrae, measurements of each vertebral body were recorded to the nearest hundredth of a millimeter by C.S. in a blind fashion using a digital sliding caliper (NTD15-15C, Mitsutoyo Co.), and the raw data were rounded to the nearest tenth of a millimeter in the subsequent numerical processing.
Six linear dimensions were selected from Martin’s measurements (Martin and Knußmann, 1988): ventral height (VH) and dorsal height (DH) in the median plane; and cranial dorsoventral diameter (CrDV), caudal dorsoventral diameter (CaDV), cranial transverse diameter (CrTR) and caudal transverse diameter (CaTR) across the intervertebral surfaces (Figure 2).
 View Details | Figure 2. Linear dimensions measured on the vertebral body: VH, ventral height; DH, dorsal height; CrDV, cranial dorsoventral diameter; CaDV, caudal dorsoventral diameter; CrTR, cranial transverse diameter; CaTR, caudal transverse diameter. (n), Martin’s number (Martin and Knußmann, 1988). |
From the above dimensions, five ratios were calculated: dorsal height/ventral height (DH/VH); dorsoventral diameter/transverse diameter at the cranial surface (CrDV/TR); dorsoventral diameter/transverse diameter at the caudal surface (CaDV/TR); mean height/mean dorsoventral diameter (MH/DV) [where mean height = (VH + DH)/2 and mean dorsoventral diameter = (CrDV + CaDV)/2]; and mean height/mean transverse diameter (MH/TR) [where mean transverse diameter = (CrTR + CaTR)/2]. Wedging of the vertebral body was defined in accordance with guidelines published by Martin and Knußmann (1988): DH/VH ≥ 1.02, ventral wedging (responsible for kyphosis in the vertebral column); DH/VH < 0.98, dorsal wedging (responsible for lordosis in the vertebral column). Statistical differences in the nine variables between the two groups were tested by means of Fisher’s least significant difference test, as performed using STATISTICA Version 5.1J software (StatSoft, Inc.), with the level of significance set at P < 0.05.
VH demonstrated no significant differences between the exercise and control groups (Figure 3). The DH dimensions of the exercise group, in the caudal thoracic and lumbar vertebrae, appeared to be smaller than those of the control group, the differences being statistically significant at T12 (P < 0.05), L1 (P < 0.01), and L3–L5 (P < 0.05) (Figure 4).
 View Details | Figure 3. Ventral height (VH) plotted from C3 to L6. Closed squares: means for the exercise group; open squares: means for the control group. Vertical bars attached to the squares represent ± one standard deviation. |
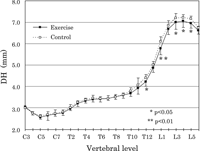 View Details | Figure 4. Dorsal height (DH) plotted from C3 to L6. The level of significance is indicated by asterisks (*, P < 0.05; **, P < 0.01). For other symbols, see Figure 3. |
The CrDV diameters of the exercise group generally were larger than those of the control group in the mid-thoracic to lumbar region, but only significantly so at T13, L1 (P < 0.05), and L2 (P < 0.01) (Figure 5). CaDV tended to be larger in the exercise group, but this difference was significant only at L6 (P < 0.05) (Figure 6). The CrTR and CaTR diameters of the exercise group did not differ significantly from those of the control group throughout the vertebral column (Figure 7, Figure 8).
 View Details | Figure 5. Cranial dorsoventral diameter (CrDV) plotted from C3 to L6. The CrDV is smaller in the exercise group at the T13–L2 levels. For symbols, see Figure 3 and Figure 4. |
 View Details | Figure 6. Caudal dorsoventral diameter (CaDV) plotted from C3 to L6. For symbols, see Figure 3 and Figure 4. |
 View Details | Figure 7. Cranial transverse diameter (CrTR) plotted from C3 to L6. No significant differences were observed between the two groups. For symbols, see Figure 3. |
 View Details | Figure 8. Caudal transverse diameter (CaTR) plotted from C3 to L6. No significant differences were observed between the two groups. For symbols, see Figure 3. |
The DH/VH ratios of the exercise group appeared to be smaller than those of the control group at the cervical, cranial-most thoracic, and lumbar levels; however, significant differences were detected only at L3 and L5 (P < 0.05) (Figure 9). The means of the DH/VH ratios along the vertebral column were larger than 0.98 in both groups, indicating no wedging (0.98 ≤ DH/VH < 1.02) at T1–T5, and ventral wedging (DH/VH ≥ 1.02) at the other levels, although some individual cervical and thoracic vertebrae in the exercise group were smaller than 0.98, consistent with dorsal wedging (Figure 9).
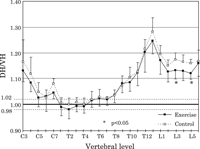 View Details | Figure 9. Dorsal/ventral height index (DH/VH) plotted from C3 to L6. DH/VH ≥ 1.02: ventral wedging, DH/VH < 0.98: dorsal wedging. For symbols, see Figure 3 and Figure 4. |
The mean CrDV/TR and CaDV/TR ratios were below 1.00 at all levels in both groups; thus, the intervertebral surfaces of rats were elongated transversely, rather than dorsoventrally (Figure 10, Figure 11). The CrDV/TR ratios of the exercise group tended to exceed those of the control group in the middle to caudal thoracic and cranial lumbar vertebrae, but they were significantly different only at T5 and L2 (P < 0.05), where the means of CrDV/TR in the exercise group were close to 1.00 (Figure 10). The CaDV/TR ratios of the exercise group tended to be larger in the caudal thoracic to mid-lumbar region than those of the control group; however, no significant differences were revealed (Figure 11). Between the opposite surfaces of the vertebral body, from T3 to L6, CrDV/TR surpassed CaDV/TR in both groups (Figure 10, Figure 11).
 View Details | Figure 10. Dorsoventral/transverse diameter index (CrDV/TR) plotted from C3 to L6. For symbols, see Figure 3 and Figure 4. |
 View Details | Figure 11. Caudal dorsoventral/transverse diameter index (CaDV/TR) plotted from C3 to L6. No significant differences were observed between the two groups. For symbols, see Figure 3. |
The MH/DV ratios in both groups were larger than 1.00 at all levels, implying that the vertebral body was longer craniocaudally than dorsoventrally (Figure 12). The MH/DV ratios of the exercise group appeared smaller than that of the control group at all levels except C3 and C4, with statistically significant differences being obtained at T5, T10, L3, and L4 (P < 0.05), and most notably at T11–L2 (P < 0.01). At levels other than C3–T1, MH/TR exceeded 1.00, corresponding to craniocaudal, rather than transverse, elongation of the vertebral bodies (Figure 13). The MH/TR ratios of the exercise group seemingly were smaller than those of the control group along the caudal half of the vertebral column; however, only the differences at T11 and L1 were significant (P < 0.01).
 View Details | Figure 12. Mean height/mean dorsoventral diameter index (MH/DV) plotted from C3 to L6. The MH/DV is smaller in the exercise group at the T5 and T10–L4 levels. For symbols, see Figure 3 and Figure 4. |
 View Details | Figure 13. Mean height/mean transverse diameter index (MH/TR) plotted from C3 to L6. The MH/TR is smaller in the exercise group at the T11 and L1 levels. For symbols, see Figure 3 and Figure 4. |
The spinal lordosis observed in humans is formed by a combination of dorsally wedged intervertebral discs and vertebral bodies (Warwick and Williams, 1973). Since we removed cartilaginous discs prior to all vertebral measurements, our interest was restricted to bone measurements. The absolute and relative dimensions among experimental rat vertebrae were analyzed to prove or disprove bony accommodation in the development of spinal curvature due to conditionally reinforced bipedal standing posture.
Comparative studies of mammalian vertebrae (Shi et al., 1995, 1999) have revealed that all vertebral bodies are ventrally wedged in quadruped mammals. In humans, however, the bodies are dorsally wedged at the lumbar levels (L4 and L5) of the vertebral column, where the spine is anteriorly convex to form the lower part of its sigmoid curvature (Shi et al., 1995, 1999). In our bipedally conditioned rats (Matsumura and Okada, 1990), the vertebral body exhibited obvious diminution in ventral wedging at the lumbar level; that is to say, transformation in a direction toward dorsal wedging, which is a human characteristic (Figure 1, Figure 9).
According to Nakatsukasa et al. (1995) and Shi et al. (2000), among bipedal Japanese macaques that have been trained for a long time in Sarumawashi (a monkey show), dorsal wedging can be detected in the cervical vertebrae, and lumbar vertebrae appear to be less wedged ventrally than among untrained control macaques. Among the controls, no vertebral body (C3–L6) exhibited any trace of dorsal wedging. In bipedal rats, meanwhile, exercise effects were observed only at the lumbar level (Figure 9). The inter-species difference in wedging at the cervical level may be related to how vertebrae support the head. In the case of a Japanese macaque assuming a quadrupedal standing posture, the head is supported inferiorly by cervical vertebrae (Hayama, 1986; Hayama et al., 1992), whereas the head of a rat is held posteriorly by the vertebrae (Greene, 1935). Additionally, careful inspection of X-ray films of both bipedally trained rats (Matsumura and Okada, 1990) and macaques (Hayama et al., 1992) demonstrates that lordosis in the cervical column is more prominent in macaques than in rats.
Below the lower thoracic level in bipedal rats, the larger CrDV causes an increase in the area of the cranial surface and, consequently, a gain in its moment of inertia in the dorsoventral direction; from a mechanical point of view, the former resists craniocaudal compression and the latter withstands dorsoventral bending (Figure 5). On the other hand, the vertebral level (L6), where the significantly larger CaDV was detected in the exercise group, does not correspond to the levels (T13–L2) of greater CrDV (Figure 5, Figure 6). This regional disagreement in DV difference between the corresponding (cranial and caudal) intervertebral surfaces within a bone may occur because they are not parallel to each other (Figure 1); or it may be related to the way specific contractile muscle forces and joint reaction forces are exerted in each of the vertebrae and intervertebral discs. According to Hongo et al. (1999), several biomechanical investigations have provided no information regarding differences in the tendency of injuries between superior and inferior sites in the vertebral body. In human lumbar vertebrae under axial compression, these authors proved, for the first time, that the tensile strain measured at the cranial vertebral rim is greater than that at the caudal rim (Hongo et al., 1999). The mechanics behind this difference in strain, however, remain unknown.
In bipedal rats, the cranial surface proportion of vertebral bodies, defined by CrDV/TR, was more elongated dorsoventrally at T5 and L2 (Figure 10), levels that correspond approximately to the dorsal and ventral maximum curvatures of the vertebral column, respectively (Matsumura and Okada, 1990; Matsumura, 1994). In human children, after two years of age, anteroposterior growth of lumbar vertebral bodies may depend upon activities associated with weight-bearing in the erect posture (Taylor, 1975). The increase in the APD/LD (anteroposterior diameter/lateral diameter) index (Taylor, 1975) of the vertebral body during human growth, while developing bipedal locomotion, coincides with the dorsoventral elongation (CrDV/TR) we detected in the experiment involving rats. Meanwhile, the difference in CaDV/TR, which should be in parallel with the result in CrDV/TR, was not significant at all along the column. The reason for this is not clear, as stated above for the dimensions CrDV and CaDV.
The ‘robusticity’ (craniocaudally short and dorsoventrally and/or transversely thick proportion) of the rat vertebral body, which was defined by MH/DV in the median plane and by MH/TR in the horizontal plane (or the frontal plane in bipedal posture), increased significantly in the caudal third of the vertebral column (Figure 12, Figure 13). This regional bias in robusticity gain suggests mechanical accommodation in rats, since the lower vertebrae in bipedal posture are subjected to a greater load resulting from body weight. By comparative morphometry, the lumbar vertebral column or bodies in humans are more robust than in other quadruped mammals (Rose, 1975; Shi et al., 1995, 1999). In a bipedally conditioned Sarumawashi macaque, vertebral bodies were more robust than in normal macaques (Shi et al., 2000). Theoretically, the load should peak in the last lumbar vertebra in humans assuming upright posture, but the robusticity increment in L5 and L6 was not significant in our bipedal rats. Comparative anatomy of the junction between the last lumbar vertebra and the pelvis may provide a hint for this non-significance in robusticity increment.
The size and shape of vertebral bodies (C3–L6) were examined using operant-conditioned bipedal rats. In the caudal third (thoracolumbar and/or lumbar levels) of the vertebral column, three osteological changes were responsive to bipedal standing exercises:
(1) From the lateral view, an obvious diminution in ventral wedging was detected at the lumbar level, indicating transformation in a direction toward dorsal wedging, which is a human characteristic.
(2) From either a cranial or caudal view, dorsoventral elongation was detected at the thoracolumbar level, in both dimension and proportion; it was more pronounced across the cranial surface than the caudal surface.
(3) The ‘robusticity’ (craniocaudally short and dorsoventrally and/or transversely thick proportion) increased with exercise in the caudal thoracic to mid-lumbar region but no significant increment was found between L5 and L6.
We wish to express our sincere gratitude to Professor emeritus Banri Endo, the University of Tokyo, and Professor Kenjiro Matsuno, Dokkyo Medical University School of Medicine, for their advice and support, and also to Mr Hideo Sakurai, Dokkyo Medical University School of Medicine, for his technical support in preparation of the specimens. We are also indebted to Dr Masato Nakatsukasa (the editor-in-charge) and two anonymous reviewers for a number of helpful comments.
|