| Naoko Egi, corresponding author. e-mail: naokoegi@yahoo.co.jp phone: +81-568-61-2327; fax: +81-568-62-6823 Published online 25 May 2007 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.061008 |
The distribution of colobines is today limited to Southeast Asia, South Asia, and equatorial Africa, but colobines previously had a much wider geographical distribution. Mesopithecus and Dolichopithecus were widespread throughout Europe and West Asia during the late Miocene and the Pliocene (Figure 1) (Delson, 1994; Fortelius, 2003). In East Asia, Parapresbytis is known from the middle Pliocene of Transbaikalia, Siberia (Borisoglebskaya, 1981; Kalmykov and Maschenko, 1992, 1995), a fossil from the late Pliocene of Japan has been assigned to Dolichopithecus (Delson, 1994; Iwamoto et al., 2005), and Rhinopithecus existed in central China during the Pleistocene (Figure 1) (Jablonski, 2002). The adaptability of cercopithecid monkeys in general, and colobines in particular, has been briefly discussed in Jablonski (2005). Parapresbytis from Siberia has the most northern distribution of all the colobines, and its occurrence is peculiarly isolated from the other Neogene primate localities in Eurasia (Jablonski, 2002; Fortelius, 2003). The phylogenetic position of the genus and the nature of its adaptation to such high latitudes are still the subject of debate (Delson, 1988, 1994; Kalmykov and Maschenko, 1992, 1995; Jablonski, 2002; Kalmykov et al., 2005; Maschenko, 2005).
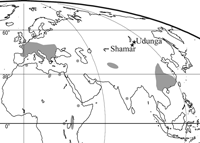 View Details | Figure 1. Locations of Udunga and Shamar, where Parapresbytis eohanuman has been discovered. Other fossil colobine localities are indicated in grey (from Delson, 1994). |
The Siberian fossil colobine was first discovered at Shamar in Mongolia (50°06′14″N, 106°11′30″E: Fortelius, 2003). Based on a mandibular specimen, a new species, Presbytis eohanuman Borisoglebskaya, 1981, was established. Later, maxillary and fragmentary frontal specimens were found at Udunga in Russia (51°8′23″N, 105°58′55″E: Erbajeva et al., 2003). The complete dentition is known (i.e. I1/–M3/ for the Udunga materials and i/1–m/3 for the Shamar materials), and based on their morphology and size, the materials from the two localities are considered to belong to the same species (Kalmykov and Maschenko, 1992, 1995; Maschenko, 2005). Kalmykov and Maschenko (1992) erected a new genus, Parapresbytis, for the species. Maschenko (2005) and Kalmykov et al. (2005) suggested that Parapresbytis is a member of Rhinopithecomorpha. On the other hand, Jablonski (2002) noted that Parapresbytis has dental and postcranial similarities to Semnopithecus, Nasalis, Pygathrix, and Rhinopithecus. Delson (1988, 1994) proposed, in contradistinction to the above, that the P. eohanuman materials are not significantly different from those of Dolichopithecus and that the species should therefore be placed in Dolichopithecus.
The known materials of Parapresbytis also include postcranial elements: a distal humerus and an incomplete ulna lacking its distal portion and the olecranon process (Figure 2). A range of inferences on the morphologies of these materials have been drawn by different researchers. In addition to the dentognathic materials, Delson (1988, 1994) described the morphology of the postcranial materials to be quite similar to those of Dolichopithecus, which is the most terrestrially adapted colobine known. Jablonski (2002) assigned the substrate preference of Parapresbytis as “?terrestrial”. Maschenko (2005) suggested that Parapresbytis is arboreally adapted based on postcranial comparisons with Macaca and Papio.
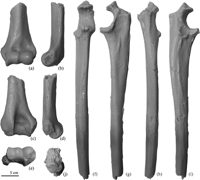 View Details | Figure 2. Postcranial specimens of Parapresbytis eohanuman. Left distal humerus (PIN 3381-210) in anterior (a), medial (b), posterior (c), lateral (d), and distal (e) views. Right ulna (PIN 3381-211) in anterior (f), medial (g), posterior (h), lateral (i), and proximal (j) views. |
This study aims to clarify the characteristics of the elbow morphology of Parapresbytis in comparison with those of other arboreal and terrestrial colobines. Then, implications for the phylogenetic position of Parapresbytis and the correspondence between the reconstructed locomotor adaptation and the paleoenvironmental evidence are discussed.
The distal humerus (PIN 3381-210) and the ulna (PIN 3381-211) of Parapresbytis from the Shamar site are stored in the Paleontological Institute, Russian Academy of Sciences, Moscow. Because taking the fossils outside Russia is difficult, the measurements and morphological observations were carried out using casts of the specimens.
In order to evaluate the morphological characteristics of the Parapresbytis elbow, it was compared with those of extant and extinct colobines (Table 1). All the compared specimens are adults, with closed epiphyses at the distal humerus and the proximal ulna. The extant colobine sample includes a total of nine genera with 13 species and 43 individuals. The smallest species in the sample is Procolobus verus, which has a female mean body mass of 4.2 kg (Smith and Jungers, 1997). The largest species is Semnopithecus entellus, which has a male body mass of up to 23.6 kg (Macdonald, 2001), and Nasalis larvatus, which has a male mean body mass of 20.4 kg (Smith and Jungers, 1997). The fossil colobine sample includes Dolichopithecus and Mesopithecus from the Mio-Pliocene of Europe. No well-preserved ulnae from Dolichopithecus were available for this study.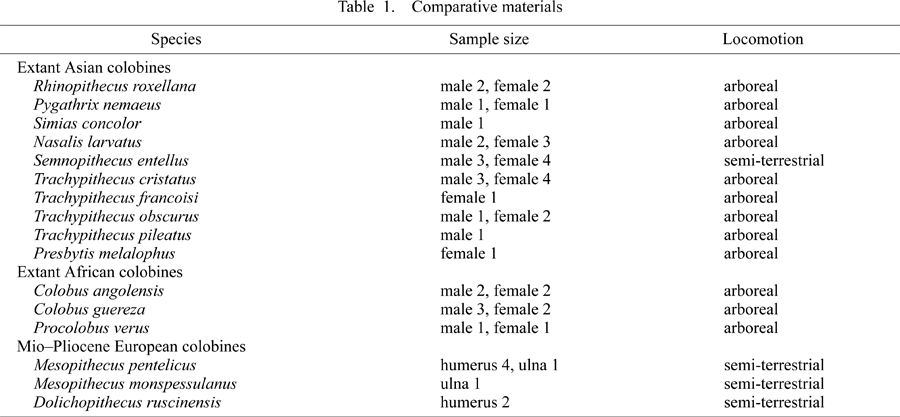
Among the comparative taxa, Dolichopithecus, Mesopithecus, and Semnopithecus have been suggested to be adapted to semi-terrestrial locomotion (Jablonski, 2002), while the rest are primarily arboreal. We first examined whether or not these two groups of colobines, arboreal and semi-terrestrial, can be distinguished one from the other. We then evaluated the similarities of Parapresbytis to these two groups.
The extant species were examined at the Japan Monkey Centre (Inuyama, Aichi, Japan) and Department of Mammalogy, United States National Museum of Natural History (Washington, DC). The fossil colobines were examined at the Département Histoire de la Terre, Muséum National d’Histoire Naturalle (Paris, France) and the Laboratoire de Paléntologie, Université Montpellier II (Montpellier, France).
The humeral and ulnar specimens of Parapresbytis (Figure 2) are incomplete; the preserved part of the humerus is limited to the distal 30–40%, the olecranon of the ulna is broken, and the ulnar shaft is broken at a point proximal to the pronator crest. Because the entire lengths of the two elements are unknown, morphological comparisons among colobines were carried out using linear measurements of the distal humerus, the proximal ulna, and the ulnar shaft.
A total of 18, 11, and 2 measurements (Table 2 and Figure 3) were taken on the distal humerus, the proximal ulna, and the ulnar mid-shaft, respectively. These measurements were modified after Birchette (1982) and Harrison (1982). Measurement values for Parapresbytis are shown in Table 2. These measurements were taken using calipers to the nearest tenth of a millimeter. The measurements of each specimen were transformed into the natural logarithmic scale. Comparisons were carried out by means of principal components analysis (PCA) performed on the covariance matrices (Wilkinson, 2002). Because the humeri and the ulnae are not associated in the fossil colobine specimens, the PCA was conducted separately for the humeral and ulnar measurements.
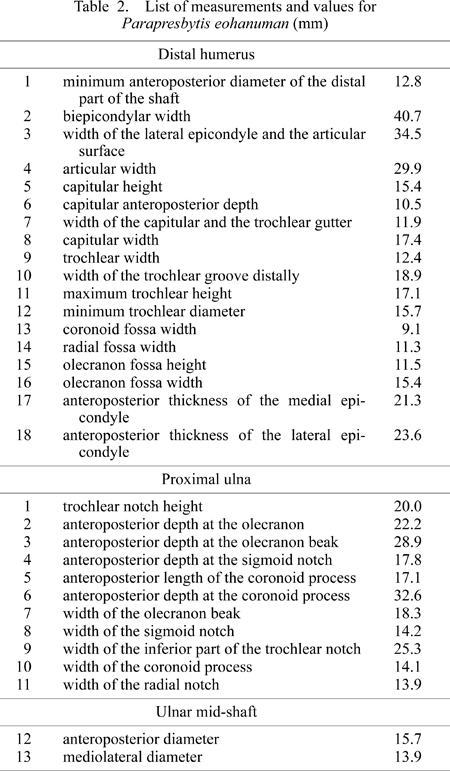 View Details | Table2 |
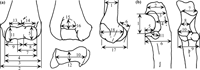 View Details | Figure 3. Schematic explanation of measurements on the distal humerus (a) and the proximal ulna (b). See Table 2 for the list of variables. |
In the PCA of colobine distal humeri and ulnae, the first three principal components (PCs) contribute more than 90% of the total variance. The loading values, eigenvalues (variances), and percentage variances of the first, second, and third PCs are shown in Table 3.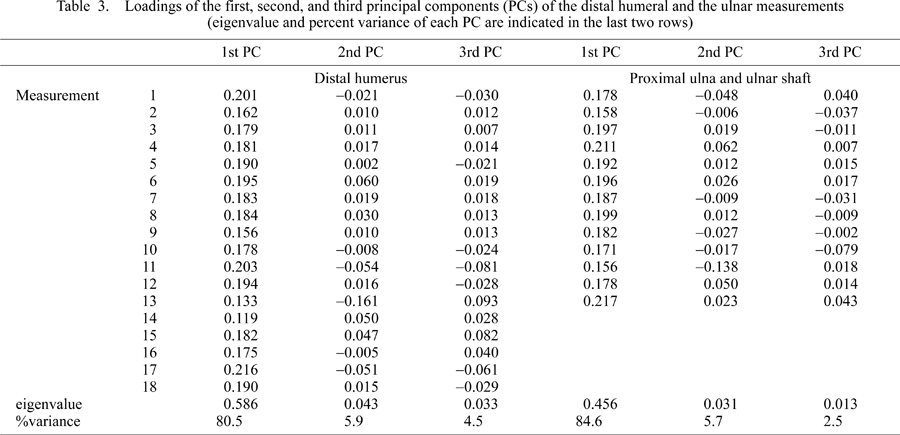
The first PC in an analysis based on covariances between linear measurements is generally considered as an indicator of size. The overall sizes of the distal humerus and the ulna were evaluated using the first PC scores (Figure 4). Percentage variances of the first PC are 80% and 85% for the distal humeral measurements and for the ulnar measurements, respectively (Table 3). In terms of the size of the distal humerus (Figure 4a), Parapresbytis is similar to a larger male Semnopithecus and Dolichopithecus. It is slightly larger than the largest male Nasalis (associated body mass, 22 kg) and Rhinopithecus (male average body mass, 17.9 kg: Smith and Jungers, 1997). In terms of ulna size (Figure 4b), Parapresbytis is larger than any extant colobine. The size of the proximal ulna of Parapresbytis is similar to that of male Papio ursinus (species mean male body mass = 29.8 kg: Smith and Jungers, 1997) although the latter species is not included in the quantitative comparison (Figure 5).
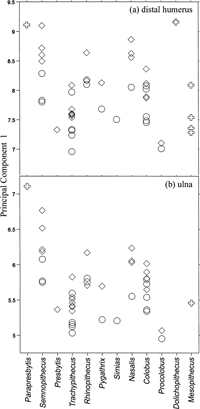 View Details | Figure 4. Comparison of distal humeral and ulnar sizes based on the first principal component scores. Genera are indicated along the horizontal axis. Symbols: square, male; circle, female; cross, fossil. Trachypithecus, Colobus, and Mesopithecus include multiple species. |
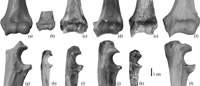 View Details | Figure 5. Comparison of Parapresbytis with other colobines and Papio. Left distal humeri in anterior view (a–f) and right proximal ulnae in lateral view (g–l). Parapresbytis (a, g), Trachypithecus (b, h), Rhinopithecus (c, i), Semnopithecus (d, j), Dolichopithecus (e), Mesopithecus (k), and Papio (f, l). All to the same scale. |
The size difference between Parapresbytis and extant arboreal colobines is more obvious in the ulna than in the distal humerus. The humeral specimen (PIN 3381-210) of Parapresbytis was previously thought to associate with the ulnar specimen (PIN 3381-211), but the comparisons conducted here suggest that these two elements belonged to different individuals. The humerus belonged to an individual with a body size of a male Nasalis, while the ulna belonged to another individual with a body size of a male Papio. This size difference can be regarded as evidence of intraspecific variation of body size in Parapresbytis as in extant colobine species.
The second and subsequent PCs are considered to reflect variation in shape after removing the size factor. The comparison of distal humeral shapes among colobines is summarized in a graph of the third PC score versus the second (Figure 6). Along the second PC, large positive loadings are found for capitular anteroposterior depth, radial fossa width, olecranon fossa height, and capitular width, while large negative loadings are found for coronoid fossa width, trochlear height, anteroposterior depth of the medial epicondyle, and anteroposterior diameter at the distal shaft (Table 3). The loadings of the third PC are positive and large for coronoid fossa width, olecranon fossa height, and radial fossa width, and negative and large for trochlear height, medial epicondyle depth, anteroposterior diameter at distal shaft, lateral epicondyle depth, minimum trochlear diameter, trochlear width at the distal aspect, and capitular height (Table 3).
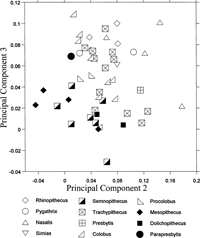 View Details | Figure 6. Projection of colobine distal humeral morphology onto the second and third principal component shape plane. |
The three terrestrial colobines, Semnopithecus, Mesopithecus, and Dolichopithecus, have low scores along both second and third PCs (Figure 6). Their distal humerus is characterized by a high trochlea and capitulum, anteroposteriorly thick medial and lateral condyles, an anteroposteriorly elongated cross-section at the distal shaft, and a wide trochlear groove relative to the articular width. These terrestrial colobines are mostly separated from the other colobines (selected species are shown in Figure 5b–e). The arboreal taxa overlap with one another except that the Nasalis individuals tend to exhibit higher scores along the second and third PCs. The arboreal colobines generally have a mediolaterally wide distal humeral articulation. Their capitulum is wide relative to the whole articulation, and its shape is round. The olecranon fossa is superoinferiorly large, so that a long olecranon of the ulna can fit. In Nasalis, the trochlear rim is low, and the relatively large capitulum expands laterally.
A humeral trochlea with well-developed lateral and medial rims and a wide trochlear groove are suggested to increase stability, especially in a semi-flexed posture (Rose, 1988, 1993; Harrison, 1989; Nakatsukasa, 1994). Thus, the trochlea of the terrestrial colobines is more efficient in parasagittal movement of the elbow than that of the arboreal colobines. In contrast, development of the trochlear rim is weak, and the capitulum is round and expands laterally in the arboreal colobines, particularly in Nasalis. These morphologies allow rotational movements of the radius during elbow flexion (Rose, 1988, 1993; Harrison, 1989; Nakatsukasa, 1994).
Parapresbytis is within the range of the arboreal rather than of the terrestrial colobines. The medial rim of the trochlea is clear in Parapresbytis, but this morphology is probably related to its large body size (Rose, 1993). The trochlear groove and the capitular tail can increase the contact area with the rims of the radial head, and will enhance the stability between the large round capitulum and the radial head during the rotation of the radius (Rose, 1993). It is concluded that Parapresbytis shares a distal humeral morphology with arboreal colobines such as Colobus, Procolobus, Trachypithecus, Pygathrix, Simias, and Rhinopithecus.
Ulnar shape does not clearly differentiate the terrestrial from the arboreal colobines (Figure 7). The loadings of the second PC are positively large for anteroposterior depths at the sigmoid notch, at the coronoid process, and at the olecranon beak and for the anteroposterior and mediolateral diameters of the ulnar shaft. The loadings of the second PC are negative and large for the widths of the radial notch, the coronoid process, and the inferior part of the trochlear notch, and for the height of the trochlear notch (Table 3). Along the third PC, loadings are positive and large for the mediolateral diameter of the ulnar shaft and height of the trochlear notch, while loadings are negative and large for the widths of the sigmoid notch and the olecranon beak and for the anteroposterior depth at the olecranon (Table 3).
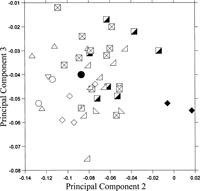 View Details | Figure 7. Projection of colobine ulnar morphology onto the second and third principal component shape plane. See Figure 6 for legends. |
Mesopithecus is distinguished from the other colobines in having higher second PC scores. The other terrestrial colobine, Semnopithecus, also tends to indicate high second PC scores, although the range overlaps with that of the arboreal colobines. In the ulna of these terrestrial colobines, the medial surface of the proximal trochlea and the lateral surface of the distal trochlea are inclined. The diameter of the trochlear notch is small. The anteroposterior depths are large at the olecranon beak and the sigmoid notch. The ulnar shaft is robust. Among the arboreal colobines, Nasalis, Simias, Pygathrix, and Rhinopithecus tend to have lower second PC scores than Trachypithecus, Colobus, and Procolobus, with a large radial notch, a wide distal trochlea, and a large trochlear notch. Mesopithecus, Pygathrix, Rhinopithecus, Procolobus, and Presbytis have relatively low third PC scores, which seem to be associated with a wide sigmoid notch and a wide olecranon beak relative to other measurements.
There appear to be some relationships between the above summarized ulnar and distal humerus morphologies. The medial and lateral rims of the humeral trochlea are well-developed in terrestrial colobines, and these surfaces fit with the inclined medial surface of the proximal trochlea and the inclined lateral surface of the distal trochlea of the ulna, respectively (Rose, 1993). The ulnar trochlear notch diameter is small so that the ulnar trochlea fits to the deep humeral trochlear groove, enhancing stability during parasagittal movement. Load transmission through the ulna increases in the forearm of the terrestrial forms, so the ulna is robust in the terrestrial colobines. In arboreal colobines, the capitulum and the radial head are large (Rose, 1993), which is reflected in the large radial notch of the proximal ulna. The wide ulnar trochlea contacts with the cylindrical humeral trochlea, allowing some rotational movement in the elbow.
The orientation and relative length of the olecranon process, the insertion for m. triceps brachi, are one of the features that distinguish terrestrial from arboreal animals (Jolly, 1967; Birchette, 1982; Rose, 1993). Terrestrial species generally have a posteriorly flexed and shorter olecranon process, allowing a greater range of elbow extension, while arboreal species tend to have a straight and long olecranon process, gaining a longer lever arm for the main elbow extensor (Jolly, 1967; Birchette, 1982; Rose, 1993). The olecranon process in the Parapresbytis specimen is incomplete, so that we could not include measurements of this skeletal part in the comparison. If comparisons of the olecranon measurements had been available, differentiation between the terrestrial and arboreal species may have become clearer than it currently is. The relatively large anteroposterior depths at the olecranon beak and at the sigmoid notch in Mesopithecus and Semnopithecus seem to reflect their posteriorly flexed olecranon process.
In the comparison of the ulna, Parapresbytis is plotted in the middle of the range of the arboreal colobines; thus, the ulnar shape of Parapresbytis is equally similar to those of the arboreal colobines and differs from that of the terrestrial colobines such as Semnopithecus and particularly Mesopithecus (selected species are shown in Figure 5h–k).
In sum, the major difference between the distal humerus and ulna of Parapresbytis and those of the other colobines is in terms of size. The smaller individuals of Parapresbytis probably had a body mass similar to that of the largest extant colobines, while the larger individual of Parapresbytis was much larger. The distal humeral and ulnar morphologies of Parapresbytis are distinct from those of the terrestrial colobines and similar to those of the arboreal colobines, suggesting that Parapresbytis was as arboreally adapted as are extant arboreal colobines. The elbow morphologies of extant arboreal colobines are similar, with the exception of Nasalis. Parapresbytis is equally similar to the extant arboreal colobines except Nasalis.
Elbow morphology adaptations (arboreal and terrestrial) were overplotted on colobine cladograms in order to clarify their distributions in an evolutionary context (Figure 8). The phylogenetic relationships within Colobinae are poorly resolved, but at least many researchers agree with the dichotomy between extant Asian and African genera (e.g. Strasser and Delson, 1987; Delson, 1994; Disotell, 2000). Molecular data suggest four old lineages among the extant Asian colobines (i.e. Semnopithecus, Trachypithecus, Rhinopithecus/Pygathrix, and Nasalis), leaving an unsolved multitomy at the base of the Asian colobines (Disotell and Stewart, data shown in Fleagle, 1999; Disotell, 2000). The grouping of Rhinopithecus and Pygathrix has been supported by some molecular studies (Disotell, 2000 and references therein). Presbytis has been suggested to be close to either Semnopithecus or Trachypithecus, while Simias is traditionally classified with Nasalis based on morphological studies (Strasser and Delson, 1987). Because most of the extant colobines have the arboreal type of distal humerus and ulna, the arboreal type (indicated as black branches in Figure 8) is the likely primitive condition for the crown colobines.
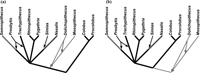 View Details | Figure 8. Distribution of elbow morphology types in the colobine phylogenetic tree. The European fossil colobines are placed next to the Asian colobines (a) or as sister groups of the crown colobines (b). Elbow types are: terrestrial (grey), arboreal (black; except Nasalis), and Nasalis (dark grey). Parapresbytis, having an arboreal type elbow, can be inserted onto any branch indicated in black without increasing the number of steps of the tree. Thick branches have been supported by the molecular studies, and the thin branches connecting with the thick branches are based on morphological studies. |
Extinct European colobines, Mesopithecus and Dolichopithecus, have been suggested to be closer to the Asian than to the African colobines (Figure 8a) (Delson, 1994; Jablonski, 1998). In this case, the likely primitive condition for all colobines is also the arboreal type. On the other hand, a recent molecular study estimated the time of the Asian–African colobine split as 10.9 Ma (with a 95% confidence interval of 12.3–9.6 Ma) (Raaum et al., 2005). This is in the chronological range of Mesopithecus, the fossil record of which begins in MN 9 (11.2–9.5 Ma) (Jablonski, 2002; Fortelius, 2003), suggesting that Mesopithecus may be too old to be a member of the Asian clade. Alternatively, the European fossil genera are placed as sister groups of the crown colobines (Figure 8b). Given this tree topology, the terrestrial type becomes the likely primitive condition for all colobines, because the outgroup (cercopithecines) is generally thought to be more terrestrially adapted than colobines (Fleagle, 1999).
Parapresbytis has been suggested to be phylogenetically close to Dolichopithecus (Delson, 1988, 1994). However, the results of the present study indicate that the elbow morphology of Parapresbytis lacks the terrestrial adaptations seen in Dolichopithecus. The fossil record of Dolichopithecus begins in the late Miocene (Fortelius, 2003). If Dolichopithecus is the closest relative of Parapresbytis, Mesopithecus and Dolichopithecus must have acquired their terrestrial adaptations independently (Figure 8a), and Parapresbytis must have split from Dolichopithecus at the very beginning of this lineage. If Dolichopithecus and Mesopithecus are considered as sister groups of the crown colobines (Figure 8b), the arboreally adapted elbow is considered to be an apomorphy that appeared at the root of the crown colobines. If Dolichipithecus is the closest relative of Parapresbytis, and if this clade is a sister group of the crown colobines, Parapresbytis must have acquired arboreal adaptations independently from the crown colobines. Thus, the comparison of the elbow morphologies suggests that grouping Parapresbytis with Dolichopithecus increases the homoplasies of arboreal-terrestrial transitions, thereby lending support to the notion that Dolichopithecus is unrelated to Parapresbytis.
Parapresbytis has been also suggested to have some similarities to Semnopithecus (Borisoglebskaya, 1981; Jablonski, 2002). The oldest fossil record for Semnopithecus comprises dentognathic and talar specimens from the Plio–Pleistocene deposits of the Siwaliks (Jablonski, 2002). If we assume that Parapresbytis split from Semnopithecus before the latter acquired terrestrial adaptations, Semnopithecus becomes a candidate for the closest relative of Parapresbytis.
The elbow morphology examined in the present study was demonstrated to be similar among the extant arboreal colobines, with the exception of Nasalis. Likewise, Parapresbytis was found to be equally similar to all the extant arboreal colobines examined except Nasalis. Thus, Parapresbytis can be inserted in any parts of the arboreal branches of the colobine cladogram (black branches of Figure 8) without increasing the number of homoplasies of the phylogenetic tree. Although Parapresbytis has been claimed to be closer to some arboreal taxa such as Presbytis (Borisoglebskaya, 1981), Rhinopithecus (Jablonski. 2002; Maschenko, 2005; Kalmykov et al., 2005), and Pygathrix (Jablonski. 2002), based on dentognathic morphologies and the robustness of the skeletal materials, the elbow morphology does not suggest phylogenetic closeness between Parapresbytis and any particular arboreal colobine.
The results of this study suggested that the elbow of Parapresbytis is adapted to arboreal locomotion. This implies that Parapresbytis lived in a forested environment. Thus, it is worth examining the paleoenvironmental data to evaluate whether or not the presumed locomotor habit of Parapresbytis is congruent with its surroundings.
Table 4 lists the mammalian taxa of the Udunga and Shamar faunas (Devyatkin and Zazhigin, 1974; Zazhigin, 1989; Kalmykov, 1992; Erbajeva et al., 2003 and cited therein; Fortelius, 2003). The Udunga fauna is divided into three units, but only the mammalian species from the middle unit, where Parapresbytis was recovered, are listed. The mammals co-occuring with Parapresbytis include 33 genera and 38 species from Udunga and 34 genera and 37 species from Shamar. Simpson’s index of faunal resemblance between the two faunas is 42% at the generic level and 27% at the specific level. Both faunas yield lagomorphs, rodents, carnivorans, artiodactyls, perissodactyls, and a primate. The major taxonomic differences between the two faunas are in insectivores and ungulates; insectivores are known only from Shamar and ungulates are more abundant in Udunga than in Shamar. The former fauna also includes large ungulates such as a proboscidean and a rhinoceros.
Among herbivorous mammals, hypsodont grazers are generally considered to be associated with an open habitat, while mesodont or brachyodont herbivores live in more closed habitats (e.g. Jernvall and Fortelius, 1996; Mendoza et al., 2002; Fortelius, 2003). In the Udunga and Shamar faunas, Ovis and Hipparion are hypsodont grazers, Gazella, Axis, Orchoceros, and Stephanorhinus are mesodont, and odocoileine cervids and Zygolophodon are brachyodont browsers (based on data from Fortelius, 2003); thus, both grassland-living and forest-living ungulates are known in these faunas.
Among the small herbivores of the two faunas, all lagomorphs are hypsodont grazers (data from Fortelius, 2003). Among the rodents, possible grazers with hypsodont teeth include a dipodine dipodid (Allactaga), alvicoline murids (except Mimomys cf. minor), and a myospalacine murid (Prosiphneus). Earlier forms of Micromys (alvicoline murid) had not developed strong hypsondonty (Lindsay, 2003), and Mimomys cf. minor from Udunga is brachyodont (data from Fortelius, 2003). Erbajeva et al. (2003) mentioned that cricetine murids from Udunga, such as Cricetinus, Gromovia, and Kowalskia, do not exhibit high hypsodonty. Sicista (sicistine dipodid), Cricetulus (cricetine murid), and Micromys (murine murid) from Shamar are also brachyodont browsers (data from Fortelius, 2003). Although castorids have hypsodont teeth, they are browsers with a semi-aquatic locomotion (Fortelius, 2003) that live in damp environments (Macdonald, 2001). Similar to the ungulates, the small herbivores of the Udunga and Shamar faunas include both hypsodont and brachyodont species.
The paleoenvironments of Udunga and Shamar can also be inferred from locomotor adaptations seen in the insectivores and carnivorans. The Shamar insectivores include two semi-aquatic species, Neomys and Nectogalini (Beremendia) (Macdonald, 2001), indicating that some water systems are represented at Shamar. Among the carnivorans, Acinonyx and hyaenids are fast-running taxa that are presently distributed in open environments (Nowak, 1991; Macdonald, 2001). Ailurids, Lynx, Gulo, and Ursus are scansorial (VanValkenburgh, 1987); thus, they require a forested environment. Overall, insectivores and carnivorans contain elements of both forested/humid and dry, open environments.
In sum, the presence of forested environments at Udunga and Shamar is supported by the co-occurrence of browsing herbivores, semi-aquatic mammals, and scansorial carnivorans, although the environment probably also contained some patches of grassland. Thus, the arboreal adaptation recognized in the postcrania of Parapresbytis is congruent with the paleoenvironment of the localities.
Müller et al. (2001) estimated climate changes in the Lake Baikal area using mineralogical contents and the relative amounts of diatoms in drilling cores from sediments of the lake. They concluded that a major climatic change occurred at 2.65 Ma and that the climate of the area was warmer and more humid before the change. A forested habitat where colobines could live was probably maintained in northern East Asia until 2.65 Ma. The ages of the Udunga and Shamar faunas have been suggested to be 3.4–3.0 Ma (early MN 16a) (Erbajeva et al., 2003; Fortelius, 2003; Vislobokova et al., 2003) and 3.16–2.68 Ma (approximately MN 16b) (Fortelius, 2003), respectively—i.e. before the forests retreated from the area.
It has been suggested that the European colobines accomplished their northern invasion with their terrestrial adaptations. In contrast, as indicated in this study, Parapresbytis lacks terrestrial adaptations. It is reasonable to consider that the northern distribution of Paprapresbytis was achieved via a widespread forested habitat in East Asia during the middle Pliocene and that the genus kept its arboreal adaptations from the ancestor of the crown colobines.
Access to the specimens was provided by the following personnel: Linda Gordon at the Department of Mammalogy, United States National Museum of Natural History (Washington, DC), Tomo Takano at the Japan Monkey Centre (Inuyama, Aichi, Japan), Pascual Tassy, Claire Sagne, and Brigitte Senut at the Département Histoire de la Terre, Muséum National d’Histoire Naturalle (Paris, France), and Jean-Jacque Jaeger and Bernard Marandat at the Laboratoire de Paléntologie, Université Montpellier II (Montpellier, France). Financial support was provided by the MEXT Grant-in-Aid for JSPS Fellows (No. 15004748 to Egi), Scientific Research B (No. 16370103 to Prof. N. Shigehara, Kyoto University), Overseas Scientific Research (No. 09041161 to Prof. N. Shigehara, Kyoto University; No. 14405019 to Takai), and the 21st Century COE Program (A14 to Kyoto University), and the JSPS Bilateral Joint Projects with RFBR (to M. Takai).
|