| * Correspondence to: Kathryn B.H. Clancy, Department of Anthropology, University of Illinois, Urbana-Champaign, 607 S. Mathews Ave., Urbana, IL 61801, USA. E-mail: kclancy@illinois.edu Published online 30 April 2009 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.090130 |
The endometrium is an important component of women’s reproduction; its receptivity helps determine the fecundability of a given cycle (Psychoyos, 1986; Hegele-Hartung et al., 1992; Yaron et al., 1993; Lessey et al., 1996), and its thickness, differentiation, and molecular structure are important to implantation, placentation, and gestation (Gonen and Casper, 1990; Noyes et al., 1995; Rinaldi et al., 1996; Bassil, 2001; Burton et al., 2002; Cavagna and Mantese, 2003; Dey et al., 2004; Hempstock et al., 2004; Zhang et al., 2005; McWilliams and Frattarelli, 2007; Richter et al., 2007). The endometrium is the site of implantation of the fertilized egg, it is where placentation occurs, and while the placenta is being formed, the endometrium provides nourishment to the growing fetus. The thickness of the endometrium has been implicated as an important component of pregnancy success in spontaneous and assisted cycles (Noyes et al., 2001). Further, while the ovaries are the source of ovulation and contribute to conception, the endometrium evokes more downstream mechanisms of fertility, including implantation and gestation. The endometrium’s thickness, differentiation, and maintenance through the luteal phase are important to fecundity, fertility, and reproductive success.
While many assisted reproduction studies have sought to determine a threshold endometrial thickness (ET) or degree of endometrial receptivity under which pregnancy is unlikely to occur (Bassil, 2001; Amir et al., 2007; McWilliams and Frattarelli, 2007), there appears to be a great degree of inter-individual variation in ET and physiological activity that leads to implantation success and pregnancy, thereby confounding the search for a reliable threshold value. For instance, while most research supports the hypothesis that a thicker endometrium can better support pregnancy (e.g. McWilliams and Frattarelli, 2007), others show no effect or even a detrimental effect on pregnancy and implantation rates (Dietterich et al., 2002; Weissman et al., 1999). Nonetheless, most forms of assisted reproduction require the administration of hormones at several times the normal physiological level (Chen et al., 2003). Studies of spontaneous menstrual cycles are necessary to determine not only the full range of variation in normal endometrial function, but eventually the proximate determinants of implantation and pregnancy success.
One main concern with most current studies of endometrial morphology is that endometrial measurements are usually made only once per cycle, near ovulation. The assumption seems to be that while endometrial proliferation may vary, ET during the luteal phase does not change in a significant way, or, alternatively, that ET measured prior to ovulation is a sufficient index of endometrial quality. However, this assumption has never been directly tested.
We tested the hypothesis of a constant ET in the luteal phase in samples of urban US (New Haven, CT) and rural Polish women (Mogielica Human Ecology Study Site) by taking daily saliva samples to measure ovarian steroid hormones and one luteal ET measurement; we also collected urine twice a week to measure C-peptide concentrations, a biomarker of insulin in order to assess within- and between-population variation in energetic status. Many components of energetics, including factors such as energy expenditure and intake, are important determinants of ovarian function variation (Ellison, 1990; Panter-Brick et al., 1993; De Souza, 2003; Jasienska, 2003; Furberg et al., 2005). Thus energetic variables may be relevant in the study of endometrial function variation within and between these populations.
We found that the hypothesis was supported in US women but rejected in Polish women. We also detected significant demographic differences between these two populations and suggest that these differences may explain why ET is not independent of luteal phase day in Polish women. A hypothesis of partial endometrial resorption is proposed to explain this relationship in Polish women, but requires more testing. Finally, we review the literature for support for our finding of population variation in the changes of ET in the luteal phase.
The Polish population of Mogielica and the US population of New Haven, CT were chosen because their different subsistence patterns and known variation in ovarian function made it likely that significant population differences in endometrial function exist. The Polish population is a rural, family-owned farming population that utilizes traditional farming techniques where women participate fully (with the exception of horse handling) (Jasienska and Ellison, 1998, 2004). The steep slopes of the Beskid Wyspowy mountain range where they live and farm prohibit modern farming technology, and most work is done by hand. The Polish harvest season has a moderate to high workload: even those relatives, neighbors, and friends who do not own farms themselves are usually involved in farmwork in July and August. In contrast, the US population sampled is urban, professional, and largely sedentary.
Mogielica Human Ecology Study Site, Poland
Subjects were healthy premenopausal women (n = 26) living in the Mogielica Human Ecology Study Site in Poland (Jasienska and Ellison, 2004), between the ages of 20 and 40 (mean 29.12 [SD 5.32] years). Women were screened for any major health problems, particularly reproductive or endocrine disorders. Subjects had not used hormonal contraceptives for at least three months, had not been pregnant or breastfeeding for at least six months, and were nonsmokers. Nineteen subjects had children (73%; mean 1.73 [SD 1.31] children for the whole group) and were employed outside the home (73%), with typical jobs being hairdressers, cashiers, and secretaries. Many subjects lived on farms, but even those subjects that were not farm owners tended to spend time in the harvest months (July–August) helping family or neighbors with their crop. Two subjects were excluded because their endometrial measurements were pre-ovulatory, and one was excluded due to a potentially anovulatory cycle, for a final subject pool of 23.
The agricultural Polish population was observed during the harvest season, which is their period of highest physical activity and has been documented to have the greatest degree of ovarian suppression of the year (Jasienska and Ellison, 2004). Subjects were recruited over the summer of 2005 through word of mouth and home visits, as well as through the approval and help of the local doctor and priest. Subjects provided written informed consent, were financially compensated for their involvement, and were provided with copies of their ultrasound results for their own records. The Yale University (New Haven, CT, US) Human Subjects Committee approved all protocols for this investigation.
New Haven, CT USA
Subjects were healthy premenopausal American women (n = 11) living in New Haven, CT, between the ages of 23 and 35 (mean 28.6 [SD 3.6] years). Women were screened for any major health problems, particularly reproductive or endocrine disorders. Subjects were not using hormonal contraceptives, had not been pregnant or breastfeeding for at least six months, and were nonsmokers. Four subjects had been pregnant before (23.5%), but all subjects were nulliparous. Subjects all worked outside the home and had professional careers (e.g. administrative worker, lab technician), or were graduate students. Two subjects were excluded for possible anovulatory cycles, for a final pool of nine.
Subjects were recruited in June–August 2004 through emails on listservs in the Yale community, posters throughout New Haven, and newspaper ads in New Haven and Yale periodicals. Subjects provided written informed consent and were monetarily compensated. The Yale University Human Subjects Committee approved all protocols in this investigation.
Anthropometric measurements were taken on the day the subject entered the study, and on the day they returned their samples at the conclusion of the investigation. Height was measured to the nearest hundredth of a centimeter using a stediometer, with weight to the nearest tenth of a kilogram and body fat to the nearest percent measured using a Tanita Body Fat Monitor/Scale (Jebb et al., 2000). Medical measuring tape was used to measure bust, ribs, waist, and hip circumferences to determine energy distribution using the protocol of Jasienska et al. (2004).
Subjects underwent a transvaginal ultrasound by a gynecologist trained in ultrasonography; every effort was made to make measurements in the luteal phase by waiting until 16 days after menses. Post-hoc analysis of the menstrual cycles using the E2 mid-cycle drop day method revealed five US and two Polish subjects were measured in the follicular phase (Lipson and Ellison, 1996); these individuals’ data were removed from the analysis. US subjects were measured at the Yale Reproductive Endocrinology and Infertility Clinic. In Poland, the first four subjects had their ultrasounds performed at the Limanowa Hospital, and the other 22 at a private practice in Limanowa. The sonographer calculated the endometrial double thickness measurement on the midsagittal plane using standard clinical methods. Previous studies have shown that inter- and intra-observer variation is low in the measure of ET via transvaginal ultrasound, provided the sonographer is experienced (Delisle et al., 1998; Epstein and Valentin, 2002).
US subjects completed five to seven 24 hour recall surveys on physical activity over the course of one reproductive cycle. Polish subjects completed daily epidemiological physical activity surveys that documented the number of minutes and degree of strenuousness of basic daily activities (traveling to and from work, work inside and outside the home, recreational activity). Energy expenditure was calculated by multiplying total time spent in each activity by the energy cost of that activity (expressed as the multiple of the predicted basal metabolic rate (BMR) (FAO/WHO/UNU, 1985)) after Jasienska et al. (2006).
Subjects collected between five and seven morning void urine samples in 4 oz. polystyrene containers over the course of one reproductive cycle for radioimmunoassay analysis of C-peptide concentrations. Subjects froze samples within 2 hours of collection. Radioimmunoassays were performed in the Yale Reproductive Ecology Laboratory, following the long protocol for urine using an 125I radioimmunoassay kit (Diagnostic System Laboratories, Webster, TX).
Subjects collected saliva samples every day in tubes pretreated with sodium azide (as a preservative) over the course of one menstrual cycle for radioimmunoassay analysis of sex steroid hormones; samples were kept at room temperature until the subject’s participation had ended and samples were then frozen. Radioimmunoassays were performed in the Yale Reproductive Ecology Laboratory according to previously established methods (O’Rourke, personal communication). Estradiol (E2) and progesterone (P) were measured from saliva samples using an 125I radioimmunoassay kit (Diagnostic System Laboratories), modified for saliva. Estradiol concentrations were used to determine whether the cycles during which ET was measured were ovulatory and whether the ET measurements had been taken in the luteal phase.
Alignment of menstrual cycles for analysis and determination of ovulation was based on the mid-cycle drop of estradiol, defined as the second of the two consecutive days (following estradiol peak) between which the greatest decrease in estradiol occurred (see Lipson and Ellison, 1996, for a full description of the method). Quality control, curve, and nonspecific binding values were within appropriate limits. US and Polish estradiol results were run one year apart with different pools so their values are reported separately; US and Polish progesterone and C-peptide assays were run together so their values are reported together. Estradiol: interassay high quality control Poland = 11%, US = 17%; low quality control Poland = 16%, US = 14%; intra-assay variation Poland = 13%, US = 13%. Progesterone: inter-assay high quality control = 7%; low quality control = 12%; intra-assay variation = 7%. C-peptide: high quality control = 16%; low quality control = 10%; intra-assay variation = 10%. Values were calculated using RIA AID radioimmunoassay analysis software for PC (Robert Maciel Associates, Arlington, MA, USA).
Multiple regressions were run separately in the Polish and US samples with ET as the dependent variable and the following independent variables: USG day (the day ET was measured by ultrasound), age, age at menarche, EE (energy expenditure in METs), C-peptide concentrations, midfollicular E2, and midluteal P. For the US sample, age, EE and midluteal P had to be removed from the analysis due to multicollinearity.
Unpaired t-tests were used to calculate differences between US and Polish study participants as well as differences between Polish groups sampled early and late in their luteal phase. For the comparisons between the US and Polish groups, several sampling and data issues had to be resolved. Standard deviations were significantly different when comparing the following variables: weight and C-peptide, and EE was almost significant (P = 0.056). Further, the following variables did not have normal distributions: Polish age at menarche, Polish and US C-peptide, and Polish midfollicular. Thus, comparisons between groups regarding weight and EE were run as unpaired t-tests with Welch corrections, and Mann–Whitney nonparametric tests were performed on comparisons regarding age at menarche, C-peptide concentrations, and midfollicular E2.
The Polish participants were divided into early and late luteal phase sampling two ways in order to better examine the effects of phase day measurement on ET: first, we divided participants by whether they were measured in the first or second half of the luteal phase. Second, we divided participants by whether they were sampled before or after day –6, which appeared to be a day after which there was a drop in ET.
The InStat analysis package for Windows was used to perform all analyses (GraphPad Software, La Jolla, CA). A P-value less than 0.05 was considered statistically significant. Post-hoc power analysis was also performed to test the power (1–beta) of the mean comparisons.
The change in ET through the luteal phase in each population was qualitatively different between populations. While ET and the luteal phase day upon which it was measured were independent of each other in US subjects (P = 0.10, r = 0.56), ET was negatively correlated with luteal phase day in Polish subjects (P = 0.02, r = −0.44; Figure 1). Luteal phase length did not correlate with ET in any subject grouping (Polish, US, or All), but luteal phase length and measurement day did negatively correlate across all three groupings (Poland: P = 0.03, US: P = 0.002, All: P = 0.001). Of the other variables examined, only age at menarche was significantly correlated with ET, and only in the US sample (P = 0.04, r = −0.41, Table 1 and Table 2). Further, in unpaired t-test comparisons of Polish ET in the early and late luteal phase, ET was significantly greater in the early luteal phase; early and late luteal phase groups were calculated using the mid-cycle point (day –8.8) (F1,31 = 2.96; P = 0.03) and the day ET appeared to drop (day –6) (F1,31 = 3.91; P = 0.01) (Table 3). Sample size limitations prohibited a similar test in the US sample. We tested the hypothesis that ET is independent of the luteal phase day upon which it was measured. This hypothesis was rejected in the Polish sample: instead, it appears that ET is negatively correlated with luteal phase day in this participant group of women.
 View Details | Figure 1. Endometrial thickness in the luteal phase in Polish women. Endometrial thickness negatively correlated with measurement day (r = −0.44, P = 0.02, n = 23). |



Several differences between the two population samples should be noted. The Polish group had a significantly older age at menarche (P = 0.002), higher energy expenditure (P = 0.007), lower C-peptide concentrations (P = 0.0001), and lower midluteal progesterone (F1,31 = 2.29; P = 0.0006). Mean ET, including all measurements and subjects across the luteal phase, was not different between populations (F1,31 = 2.84; P = 0.28, Table 4). However, in this preliminary study the number of subjects was not large enough to have the kind of predictive power necessary to determine differences between populations in ET (Table 5).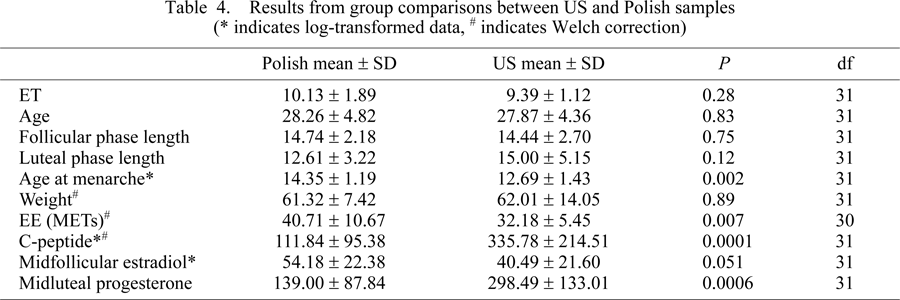

In the Polish population, a negative association between ET and measurement day was seen that is suggestive of luteal decline and, as one possibility, partial resorption of the endometrium. The US subjects had a positive but nonsignificant trend (r = 0.32, P = 0.11). Luteal phase length did not correlate with ET in any subject grouping (Polish, US, or All), but luteal phase length and measurement day did negatively correlate across all three groupings (Poland: P = 0.03, US: P = 0.002, All: P = 0.001). This suggests that luteal phase length did not play a role in ET in these subjects, but that it did appear to affect the timing of the endometrial measurement taken. Subjects were measured generally after day 16 of the cycle in order to obtain a luteal phase measurement (three of the US subjects, and one of the Polish subjects were still found to have been measured in the follicular phase in post-hoc estradiol concentration analysis, and thus were removed from further analysis). However, the most logical (and most cited in the literature, e.g. Jasienska and Ellison (1998)) way to document progesterone concentrations across the luteal phase for statistical comparisons is to align individuals’ cycles from the end of the cycle; this does make it more likely that those women with longer luteal phases would have relatively earlier measurement days. Yet women with longer luteal phases do not appear to have thicker endometria in these preliminary results.
Our hypothesis that ET is independent of luteal phase day was rejected in a rural Polish sample; instead ET and luteal phase day were negatively correlated. While the positive relationship was not statistically significant in the urban US sample (P = 0.11), the results did not provide particularly strong support for the hypothesis. This cross-sectional data suggests that ET varies during the luteal phase; this means that even if maximal ET is measured around the time of ovulation, investigators may be missing important changes in ET in subjects during the window of implantation, which would be a more physiologically relevant time to document endometrial function variation.
Even in empirical articles that report little luteal variation in ET, a clear, though qualitative, change in ET can be observed (Figure 2, Table 6). Research measuring ET serially is infrequent, but we have found articles that document ET via transvaginal ultrasound in Canadian (Baerwald and Pierson, 2004), Scottish (Randall et al., 1989), English (Raine-Fenning et al., 2004), and Swedish populations (Bakos et al., 1994). There are a few confounding problems with the serial measurements from the literature when it comes to assessing luteal endometrial function. Aligning cycles at ovulation rather than at the end of the cycle (and in one case, normalizing cycle length) provides information about ET early in the luteal phase, which can supply important evidence about the influence of estradiol on ET. But as women may have different luteal phase lengths, this method ends up removing any variation that may occur in the way their ET varies through the end of the cycle. Thus, any variation between women from the window of implantation on is largely lost. This variation would be relevant to understanding the impact of the endometrium on fecundity, as the degree of maintenance of the endometrium may indicate degree of energy constraint. A better method to examine the implantation window is to align cycles by reverse cycle day, or number of days before menses.
 View Details | Figure 2. Results of four studies of natural luteal phase endometrium (Sweden: Bakos et al., 1994; Canada: Baerwald and Pierson, 2004; England: Raine-Fenning et al., 2004; Scotland: Randall et al., 1989). Studies were aligned by ovulation day, with the knowledge that ovulation (as confirmed by ultrasound) is 24 hours after the luteinizing hormone surge. |

That said, these data from previous studies still suggest significant variation in ET through the luteal phase between populations, and suggest that ET changes over the course of the luteal phase rather than remaining constant. In particular, while the sample of Swedish women appears to have an increase in ET in the early part of the luteal phase, Canadian and English ET appears largely stable. As the cycle progresses, Canadian and Swedish ET drops, and throughout the luteal phase, Scottish ET appears to slowly increase. The significant variation in ET documented in these populations—from 6 to over 13 mm—suggests the capacity for variation even in normal, premenopausal ovulatory cycles in western women. Such qualitative comparisons should lead to research that is able to employ quantitative comparisons in larger samples of serial endometrial measurements.
Alternative hypotheses and further research can be suggested in the wake of these results. We suggest that some endometrial resorption helps explain luteal phase variation in endometrial maintenance, and that greater degrees of luteal failure may be correlated with a shorter luteal phase length. That is, individuals that experience some constraint in reproductive function may resorb endometrial tissue, but at a certain point it may be more efficient to shorten the luteal phase and move on to the menstrual phase in order to begin a new cycle. Resorption is a common physiological phenomenon in the uterus that occurs with embryos and placentas, and luteal variation in ovarian function has been documented across many populations and environments (Soules et al., 1989; Ellison et al., 1993; De Souza et al., 1998; Jasienska and Ellison, 1998, 2004). Endometrial resorption at or after the implantation window may cause subfecundity, which could lead to a reduction in the quality and invasiveness of implantation (Paulson et al., 1990; Bergh and Navot, 1992; Tabibzadeh and Babaknia, 1995; Xi et al., 1999), or the ability of the endometrium to nourish the fetus in the first trimester before placentation is complete (Jauniaux et al., 2000; Burton et al., 2002; Hempstock et al., 2004). Implantation that occurs more than ten days after ovulation is more likely to result in pregnancy loss (Wilcox et al., 1999). Further, the Polish population, where the hypothesis that ET is independent of luteal phase day was rejected, is the population that experiences more energy constraint: a later age at menarche is often associated with reduced nutritional status during childhood (Simondon et al., 1997; Pasquet et al., 1999), C-peptide concentrations are inversely associated with physical activity (Fung et al., 2000) and higher energy expenditure generally results in less resource allocation to reproductive processes (Jasienska and Ellison, 1998, 2004; Jasienska, 2003). Thus, failure to maintain ET could be an evolutionary rheostat to mediate fecundity.
It does appear that ET is related to the cycle day upon which it is measured in the Polish population sampled, even in the luteal phase where proliferating estradiol concentrations are reduced, which further reinforces the necessity of future research into possible ecological factors that may also affect ET. While our data is consistent with this resorption and luteal failure hypothesis, and an energetic explanation for endometrial variation, it is only indirectly supported, in part because we were only able to obtain a single luteal measurement per subject in this preliminary analysis.
Daily measurements of ET are necessary in future research regarding variation in endometrial function, complemented by ovarian hormone concentrations and other proxies of endometrial function such as endometrial pattern. Based on retrospective power analyses on ET in this study, over 100 subjects may be necessary to determine broad differences in mean ET; fewer are probably needed to simply assess differences in patterns of endometrial behavior through the luteal phase, but still more than the sample size included here. Finally, in order to begin to test causal or correlative hypotheses that try to answer whether there is an adaptive explanation for the existence of endometrial variation, demographic, anthropometric, and energetic data should also be collected, as an understanding of the mechanisms and gradation of function that occur in reproductive physiology is essential to forming new hypotheses regarding human adaptability and evolution.
Grant sponsorship: Polish Committee for Scientific Research (to G.J.), Center for Human and Primate Reproductive Ecology (CHaPRE)