| * Correspondence to: Manuela Dittmar, Department of Human Biology, Zoological Institute, Christian Albrechts-University, Am Botanischen Garten 9, 24118 Kiel, Germany. E-mail: mdittmar@zoologie.uni-kiel.de Published online 28 August 2009 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase090402 |
More than 140 million humans live permanently at high altitude, i.e. above 2500 m (Moore et al., 1998). The highest living areas are the Andean (3000–4500 m) and Tibetan (4600–4900 m) plateaux (Heath and Williams, 1995; Rupert and Hochachka, 2001). Life at high altitude is characterized by extreme environmental conditions such as lowered barometric oxygen pressure, cold, aridity, daily temperature fluctuations, and high cosmic and solar radiation. The low oxygen partial pressure is the main factor restricting life at high altitude. At 3500 m, the oxygen pressure amounts to only 65% of that at sea level (Heath and Williams, 1995). This results in a lowered oxygen supply in the human body, known as hypoxia. Lowlanders are limited in their ability to adapt to high altitude, compensating the lowered oxygen supply by physiological reactions such as increased respiratory minute volume, increased basal metabolic rate, and erythropoiesis. By contrast, native highlanders are better adapted to high altitude. The indigenous populations of the South American Andean plateau show morphological and physiological adaptations to hypoxic stress (Rupert and Hochachka, 2001; Beall, 2007a). They have a larger thorax (Hurtado, 1932), higher lung capacity (Frisancho, 1969), higher lung diffusion rate (Jones et al., 1992), and higher hemoglobin levels than lowlanders (Faura et al., 1969).
The South American Andean plateau (altiplano) extends from Peru across Bolivia and Argentina to Chile. The actual indigenous high-altitude population comprises 1.6 million Aymara and 6.2 million Quechua (Caviedes and Knapp, 1995). Humans have lived there for 11000 years (Aldenderfer, 2003). It is likely that this time period is sufficient for genetic adaptation through natural selection to improve oxygen supply at high altitude. For more than 40 years, a genetic basis of adaptation to high altitude has been searched for (Beall, 2007b). The underlying hypothesis is that natural selection in humans resulted in genetic adaptation to high altitude. This can be analyzed by association studies, comparing highland and lowland populations with respect to genetic characteristics. Candidate genes are those which are connected with oxygen uptake, transport, and utilization (Rupert and Hochachka, 2001). If certain alleles could be associated with increased fitness at high altitude in a native highland population, compared with a native lowland population, this might indicate genetic adaptation.
In particular, the fetus is exposed to stronger intrauterine hypoxia at high altitude than at sea level (Ballew and Haas, 1986). This leads to low mean birth weight (McCullough et al., 1977) and increased infant mortality (Niermeyer et al., 1995). Neonatal and infant mortality are directly related to increasing altitude (McCullough et al., 1977). Nevertheless, native Andean highland populations are characterized by a lower perinatal mortality and higher birth weight than non-indigenous lowland populations living at high altitude (Gonzales, 2007). In addition to a better uteroplacental oxygen supply in the indigenous highland population (Moore et al., 1986, 2004), there might be genetic adaptations resulting in a better oxygen supply to the fetus. Since oxygen is reversibly bound to hemoglobin, hemoglobin genes are possible candidates for genetic adaptation to high altitude. The high heritability for hemoglobin concentration of 0.89 in Andean populations suggests a strong genetic influence (Beall et al., 1998).
Fetal hemoglobin (HbF) is of special interest, because it is the predominant hemoglobin in the fetus. It is produced after the eighth prenatal week and is responsible for the oxygen supply to the fetus. It consists of two α- and two γ-globin chains (α2γ2). There exist two different γ-globins, Aγ and Gγ, which differ by one single amino acid residue at position 136. At this position, the Gγ-chain contains glycine and the Aγ-chain alanine. Both γ-globin genes are expressed at the same time during fetal development as well as after birth. The ratio of Aγ- to Gγ-chains is 3 : 1 in the newborn and 2 : 3 in adults (Herrmann and Herrmann, 1980). HbF amounts to 60–80% of total hemoglobin at birth and drops to about 1% after two years through adult life (Karlsson and Nienhnis, 1985). In the adult, HbF is replaced by adult hemoglobin (HbA) (α2β2) which will be produced from birth on. Compared with HbA, prenatal HbF is characterized by increased oxygen affinity. This ensures the supply of oxygen to the fetus (Hennig, 2002). The increased oxygen affinity of HbF results from the two γ-globin chains. They have two positive charges less than adult HbA, and thus a lower affinity for 2,3-bisphosphoglycerate. This increases the oxygen affinity of HbF as compared with maternal hemoglobin (Berg et al., 2003).
The fetal globin genes HBG1 (Aγ) and HBG2 (Gγ) are part of the β-globin gene cluster on chromosome 11. They result from a tandem duplication of an approximately 5.5 kb DNA fragment (Shen et al., 1981). Approximately 16 kb upstream from the β-globin gene cluster, a locus control region (LCR) is located (Labie and Elion, 1996). LCRs are regulatory cis elements which increase the transcription of subsequent genes. The most important regions in the promoter regions of HBG1 and HBG2 are a duplicated CCAAT box (nucleotide positions −84 to −87, −111 to −114), a CACCC box (−140 to −144), and a TATA box (−27 to −30) (De Vooght et al., 2007). The TATA box composes the core promoter, where the transcription initiation complex (RNA–polymerase II and the general transcription factors) docks. The CCAAT and the CACCC boxes comprise the proximal promoter where regulatory transcription factors bind, and therefore influence the transcription rate of the RNA polymerase II (Fang et al., 2004). The γ-globin TATA and CACCC elements are the major regulators of embryonic β-globin silencing, and the CACCC element has stage-specific effects in γ- to β-globin gene switching (Sargent and Lloyd, 2001). The CACCC box is largely responsible for the down-regulation of the γ-gene in adult erythropoiesis (Li et al., 2004).
Several polymorphisms in the promoters of the HBG1 and HBG2 genes have been hypothesized to be potential modulators of HbF synthesis. Diminished expression of Aγ globin has been observed in subjects with a 4-basepair (bp) deletion of AGCA from nucleotide positions −222 to −225 in the promoter of the HBG1 gene (Gilman et al., 1988; Manca et al., 1991). This gene deletion affects the Gγ:Aγ ratio of both newborns and adults. The 4-bp deletion is a common polymorphism in Americans of European and African ancestry (Coleman et al., 1991). By contrast, an elevated expression of the Aγ globin has been associated with a G→A substitution at position −117 adjacent to the distal CAAT box of the Aγ gene promoter, possibly by inhibiting binding of a repressor (Mantovani et al., 1989; Pistidda et al., 1995). This polymorphism has been found in Mediterranean populations. Elevated HbF levels, due to increased Gγ globin expression, have been associated with a C→T substitution at position −158 (Gilman and Huisman, 1985) and an A→G substitution at position −309 of the HBG2 promoter in adults (Zertal-Zidani et al., 2002).
Keeping these findings in mind, it seems quite interesting to investigate in evolutionary terms whether polymorphisms, being associated with altered fetal γ-globin expression, differ in their frequencies between native highland and lowland populations. However, no such study has been undertaken until now. Therefore, the objective of the present pilot study was to investigate whether a native Andean highland population differs from a native lowland population in promoter polymorphisms of the HbF genes HBG1 and HBG2, which are associated with altered HbF production. The question is addressed whether allele differences might be explained by genetic adaptation as a consequence of natural selection during evolution.
Subjects were representatives of native highland and lowland populations. The highlanders comprised 50 indigenous adults of Aymara origin. They have resided in the Chilean Andes for several generations at altitudes above 3500 m. The lowlanders included 50 adults of European ancestry, living in northern Germany. All subjects were unrelated and healthy. The study design was approved by the ethics committee at the Medical Faculty of the University of Chile, Santiago de Chile. The volunteers gave their written informed consent prior to data collection.
Genomic DNA was isolated from peripheral EDTA venous blood using the Lahiri and Nurenberger (1991) techniquie or from buccal mouth mucosa probes using the following protocol (modified after Ausubel et al., 1997). The volunteers rinsed out their mouth with 50 ml tap water which was transferred into a 50 ml Falcon tube. The cells were spun at 1800 g for 5 min at room temperature. The pellet was resuspended in 400 μl 50 mM Tris pH 8/10 mM EDTA/2% SDS solution. It was incubated at 65°C for 5 min. Then 250 μl 4.5 M NaCl was added and spun for 4 min at 10000 g at room temperature. The supernatant was transferred into a new tube and precipitated with an equal volume of isopropanol. It was spun at 10000 g at room temperature for 10 min. The pellet was washed with 70% ethanol and resuspended in 100 μl HPLC water. It was stored for two days at 4°C and finally at −20°C.
From the extracted DNA, the promoters of the genes HBG1 and HBG2 (NCBI accession no. M91036) were amplified by polymerase chain reaction (PCR). Forward (F) and reverse (R) promoter primers were as described in Duan et al. (2001). Primers for the HBG1 promoter were F-5′-tctattactgcgctgaaactgtg and R-5′-gtctggactaggagcttattgat, respectively (PCR product size: 684 bp). For the HBG2 promoter, primers were F-5′-aactgttgctttataggatttttca and 5′-gtctggactaggagcttattgat (PCR product size: 666 bp). The same reverse primers were used for HBG1 and HBG2. The PCR reaction volume (50 μl) contained 2 μl DNA (300 ng/μl), 0.5 μl dNTP (25 mM each base), 1 μl of each primer (10 μM), 5 μl 10× PCR buffer, 0.4 μl (5 U/μl) Taq polymerase (Roche Diagnostics, Mannheim, Germany), and 40.1 μl HPLC water. DNA was amplified in a gradient thermocycler (Whatman Biometra, Göttingen, Germany) under the following conditions: pre-treatment temperature of 94°C for 5 min, 37 cycles of 94°C for 1 min, 56°C for 1 min, 72°C for 1 min, and a post-treatment temperature of 72°C for 10 min. The amplified PCR products were controlled on a 1% agarose gel, stained with ethidium bromide, visualized under ultraviolet transillumination, and photographed.
The PCR products were purified by enzymatic digestion with shrimp alkaline phosphatase (SAP) and exonuclease I (ExoI) in order to remove dNTPs and primers. The 10 μl reaction mixture contained 0.075 μl ExoI (10 U/μl, Amersham Pharmacia), 0.3 μl SAP (1 U/μl, Roche Diagnostics, Mannheim, Germany), 1.625 μl dH2O, and 8.0 μl PCR product. After incubation at 37°C for 15 min, the enzymes were inactivated at 72°C for 15 min. The purified PCR products relating to the HGB1 and HBG2 promoters were subsequently sequenced. Sequencing primers were the same as those used for amplifying by PCR. The sequencing reaction (10 μl) contained 1.5 μl 5× sequencing buffer, 1.0 μl forward or reverse primer (3.2 μM), 2.0 μl purified PCR product, 0.7 μl Big Dye® Terminator Cycle Sequencing Kit Version 1.1 (Applied Biosystems, Darmstadt, Germany), and 4.8 μl dH2O. Sequencing conditions were pre-treatment of 96°C for 1 min, 25 cycles of denaturation at 96°C for 10 s, annealing at 50°C for 5 s, and extension at 60°C for 4 min. The sequencing products were purified by Sephadex G50 Superfine (Amersham Biosciences, Freiburg, Germany) in 96-well multiscreen-HV filter plates (Millipore, Schwalbach, Germany). 2 μl of purified sequencing product were sequenced in both directions on an ABI PRISM 3730-xI Genetic Analyzer using POP7 polymer.
The statistical analyses have been performed with SPSS version 12 for Windows PC (SPSS, Inc., Chicago). Allele and genotype frequencies were calculated by direct counting. The Hardy–Weinberg equilibrium was tested for each group with the chi-squared goodness-of-fit test. Absolute and relative allele frequencies, allele carrier frequencies, and genotype frequencies were calculated separately for highland and lowland populations. Allele carriers were defined as those subjects who carried the allele either in homozygous or heterozygous state. Highland and lowland populations were compared for independence in alleles, allele carriers, and genotype frequencies using chi-square tests or Fisher’s exact test, where appropriate. A P value of <0.05 (two-tailed) was defined as statistically significant.
The fixation index (FST) was estimated in order to determine levels of genetic divergences between populations, using Wright’s formula, FST = (HT − HS)/HT, where HT represents expected heterozygosity per locus of the total population and HS represents expected heterozygosity of a subpopulation (Wright, 1951). Blast analysis was performed aligning genomic HBG1/HBG2 sequences of Homo sapiens against chimpanzee Pan troglodytes (NCBI: NW_001222274) in order to search for ancestral alleles.
Sequencing of the promoter regions of the HbF genes HBG1 and HBG2 established seven polymorphic sites in the highland residents (Table 1). These were six single nucleotide polymorphisms (SNPs) and one deletion. For two of these polymorphisms, significant differences in allele frequencies between highlanders and lowlanders were observed: a 4-bp deletion at positions −222 to −225 (AGCA) in the HBG1 promoter and a −158 C→T polymorphism in the HBG2 promoter (Table 1). The corresponding genotype distributions were found to be in Hardy–Weinberg equilibrium, both for the HBG1 −222 to −225 AGCA→del polymorphism (highlanders, χ2 = 0.62, P = 0.432; lowlanders, χ2 = 0.01, P = 0.926) and the HBG2 −158 C→T polymorphism (highlanders, χ2 = 0.38, P = 0.539; lowlanders, χ2 = 1.26, P = 0.262). We did not observe, neither in the highland nor in the lowland population, the −117 G→A substitution in the HBG1 promoter (Ottolenghi et al., 1988) which is associated with increased HbF production. Alignment of genomic HBG1/HBG2 sequences of H. sapiens against chimpanzee P. troglodytes (NCBI: NW_001222274) showed that the major allele of each polymorphism, given in Table 1, was present in P. troglodytes.
The HBG1 promoter 4-bp (AGCA) deletion polymorphism was observed both in highlanders and lowlanders (Table 1). The frequency of the AGCA allele was significantly increased in highlanders compared with lowlanders (90% vs. 76%), whereas the frequency of the 4-bp deletion allele was decreased (10% vs. 24%, χ2 = 6.95, P = 0.014). Carriers of the HBG1 4-bp deletion allele, i.e. subjects carrying the allele in either homozygous or heterozygous state, occurred less frequently among highlanders than among lowlanders (20% vs. 42%, χ2 = 5.66, P = 0.030). The AGCA/AGCA genotype was increased in highlanders and the del/del genotype completely absent, compared with lowlanders (P = 0.030, Figure 1).
 View Details | Figure 1. Genotype distributions in highlanders and lowlanders with respect to (A) HBG1 promoter −222 to −225 (AGCA→deletion) polymorphism and (B) HBG2 −158 C→T polymorphism. The AGCA/AGCA genotype is increased in the highlanders, whereas the del/del genotype is completely absent, as compared with lowlanders (χ2 = 7.04, P = 0.030). The C/C genotype is increased in highlanders and the T/T genotype is absent, as compared with lowlanders (χ2 = 21.34, P = 0.00002). |
The HBG2 promoter −158 C→T polymorphism was observed in both highlanders and lowlanders (Table 1). However, the frequency of the C allele was strongly increased in highlanders vs. lowlanders (92% vs. 66%), while the frequency of the T allele was decreased in highlanders vs. lowlanders (8% vs. 34%, χ2 = 20.37, P = 0.0000085). Highlanders carried the HBG2 −158 T allele less frequently than lowlanders (16% vs. 60%; χ2 = 20.54, P = 0.00001). The C/C genotype was increased and the T/T genotype absent in the highlanders, compared with lowlanders (P = 0.00002, Figure 1).
None of the highlanders with the AGCA 4-bp deletion carried the HBG2 T allele, in contrast to 43% of lowlanders (χ2 = 6.04, P = 0.030, Figure 2). Only 20% of highlanders with the AGCA allele carried the T allele compared with 72% of lowlanders (χ2 = 18.96, P = 0.00002).
 View Details | Figure 2. Relationship between HBG1 promoter AGCA→del and HBG2 promoter −158 C→T polymorphisms in highlanders and lowlanders. None of the highlanders with the AGCA 4-bp deletion carried the HBG2 T allele, in contrast to 43% of lowlanders (χ2 = 6.04, P = 0.030). Only 20% of highlanders with AGCA carried the T allele, compared with 72% of lowlanders (χ2 = 18.96, P = 0.00002). Abbreviations: AGCA+, AGCA is present; AGCA−, AGCA is deleted. |
Next, the level of genetic divergence between highlanders and lowlanders was estimated using the fixation index (FST). With respect to the AGCA/del polypmorphism, interregional genetic variation between highlanders and lowlanders was small (FST = 0.035). For the −158 C > T polymorphism, pairwise FST values indicate somewhat higher subpopulation divergence in allele frequencies for highlanders and lowlanders (FST = 0.181), leading to a reduction in heterozygosity on average for the two subpopulations. For the −158 C > T polymorphism, further data were available from Macedonians. Comparing Macedonians with lowlanders (Germans), there was almost no genetic divergence (FST = 0.002). Here, the expected heterozygosity in the subpopulations and in the total population was nearly equal, expected in the ideal case where the populations are panmictic. The subpopulations divergence between Macedonians and highlanders was only slightly higher (FST = 0.083).
This pilot study investigated for the first time sequence variations in HbF genes in a highland population. The highlanders differed significantly from lowlanders in the allele frequencies of two common promoter polymorphisms, HBG1 AGCA→del and HBG2 −158 C→T. Both polymorphisms, first, are associated with altered HbF γ levels and, second, are influencing the fetal to adult globin switch after birth.
The allele frequency of the 4-bp deletion (AGCA) in the HBG1 promoter was found in highlanders to be as half as high (10%) as that in lowlanders (24%). Other authors also reported higher frequencies of the 4-bp deletion in non-highland populations (Coleman et al., 1994, Table 2). Interestingly, a low frequency of the 4-bp deletion allele was also observed in persons with sickle cell anemia (Coleman et al., 1994). Both sickle cell anemia as well as high-altitude hypoxia result in restricted oxygen supply, and both groups show a lowered frequency of the 4-bp deletion. Here, the 4-bp deletion seems to be disadvantageous, because it correlates with a reduced HBG1 expression resulting in a diminished Aγ HbF level in newborns and adults (Gilman et al., 1988; Coleman et al., 1994). This can be explained by the observation that the 4-bp AGCA deletion occurs within the promoter sequence motif GCAGCA, which possibly interacts via a regulatory transcription factor with an enhancer being located 5′ to the promoter region (Harvey et al., 1992; Coleman et al., 1994; Lu and Steinberg, 1996). One or more of the deleted AGCA nucleotides seem to contribute to the binding of the trans-acting factor (Beldjord et al., 1992). Therefore, the 4-bp deletion is associated with a reduced expression of HBG1 (Harvey et al., 1992), and newborns with this deletion showed a decreased level of total HbF (Manca et al., 1991; Beldjord et al., 1992). The 4-bp deletion not only affects the expression of the Aγ globin gene HBG1, but also that of the Gγ globin gene HBG2 (Coleman et al., 1994). Taken together, this explains why the 4-bp deletion allele occurs less frequently and never in a homozygous state at high altitude. It resulted in a lowered γ-globin level which possibly affects the oxygen supply of the fetus. The 4-bp deletion is also associated with a delay in the fetal to adult globin switch (Beldjord et al., 1992) which might worsen the oxygen supply of newborns after birth at high altitude.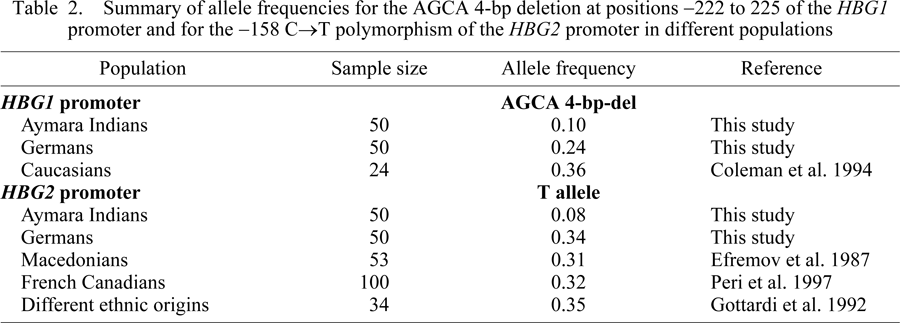
We found that the C allele at position −158 of the HBG2 gene promoter was significantly increased in the highland population (92%) compared with the lowland population (66%). The CC genotype was twice as prevalent in highlanders (84%) compared to lowlanders (40%). By contrast, the T allele was present in only 16% of highlanders, compared with 60% of lowlanders. The highlanders carried the T allele always as heterozygotes, but never as homozygotes. The frequency of the T allele in lowlanders agrees well with frequencies reported worldwide for other non-highland populations (Table 2). Since the T allele is underrepresented in highlanders, it seems not to be advantageous at high altitude. The −158 C→T polymorphism is a modulating factor for the γ-globin gene expression (Tasiopoulou et al., 2007), and the T allele is associated with an increased expression of Gγ chains, resulting in an increased HbF level in adults (Sampietro et al., 1992; Leonova et al., 1996; Zertal-Zidani et al., 2002; Tasiopoulou et al., 2007). The T allele did not significantly affect the expression of γ-genes in healthy people, but increases the HbF production under conditions of erythropoietic stress (Thein, 2005).
Like the HBG1 4-bp deletion, the −158 region of HBG2 T allele is involved in the mechanisms of globin gene expression switching after birth. A 240-kDa activator protein, which is a member of the C/EBP family, binds at position γ−158/−161 (Gilman, 1995). The presence of the −158 C→T substitution resulted in a delayed switchover from fetal to adult hemoglobin (Peri et al., 1997) and a significantly delayed decline of HbF production in infants (Grosso et al., 2008). Therefore, the resulting delay in production of adult hemoglobin might affect adequate oxygen supply in infants after birth under hypoxic conditions at high altitude.
In the present high-altitude population, a 4-bp deletion allele in the HBG1 promoter as well as the −158 T allele in the HBG2 promoter were significantly decreased, compared with lowlanders. The deletion is associated with a decreased expression of the Aγ (HBG1) and negatively influences the expression of Gγ (HBG2). Both the deletion as well as the T allele are associated with a delayed fetal to adult globin switch. This means that after birth, HbF persists longer until adult hemoglobin is produced. While HbF is advantageous during pregnancy because of its higher oxygen affinity than maternal hemoglobin, it seems to be disadvantageous after birth. At that time, the infant will no longer receive its oxygen from its mother, but from the ambient air through its own breathing. HbF will be replaced by HbA which will be produced from birth on. HbF amounts 60–80% of total hemoglobin at birth and drops to about 1% after two years through adult life (Karlsson and Nienhnis, 1985). Since both the HBG1 deletion as well as the HBG2 T allele are associated with a delayed switch of HbF to HbA, this might affect the oxygen supply of the infant at high altitude.
The fixation indices FST indicate that, in general, subpopulation divergence in allele frequencies for highlanders and lowlanders was small. Highlanders and lowlanders are more divergent with respect to the −158 C > T polymorphism than with respect to the AGCA/del polymorphism.
The human HBG1 and HBG2 genes resulted from a tandem duplication of an approximately 5.5 kb DNA fragment which occurred about 47 million years ago (Shen et al., 1981). The AGCA 4-bp deletion and the −158 T allele are not present in P. troglodytes, suggesting that they are not the ancestral alleles. Also, both alleles were less frequent in highlanders than in lowlanders. This indicates that the ancestral alleles tended to be fixed in the highlanders, suggesting that the locus might be under purifying selection. Thus, stabilizing selection might act to conserve a complex physiological system. These characteristics might result from a common descent and are not hypoxia adaptations per se. By contrast, the HBG1 −473 T allele as well as the HBG2 −166 A, −309 G and −369 G alleles were only present in highlanders, but not in lowlanders. They did not occur in P. troglodytes, suggesting that they are not the ancestral alleles. It might be possible that these alleles could favor via directional selection a hypoxia tolerance phenotype in the highlanders. However, there is still no proof that these alleles arose under directional selection for hypoxia tolerance. Additional phenotypic studies are needed to analyze this question.
In addition to natural selection forces, there are alternative possibilities for explaining the different allele frequencies observed between highlanders and lowlanders. Random genetic drift and gene flow also contribute to genetic divergence between populations. Genetic drift may occur in small populations and may be due to a founder effect or a bottleneck event. Mitochondrial DNA studies of South American natives indicate that no significant bottleneck occurred during the colonization of the continent (Monsalve et al., 1994). However, there might be a bottleneck in historic times after the arrival of the European conquerors, leading to a large decline in the Andean populations (Cook, 1981). A bottleneck often causes a reduction in heterozygosity. However, in the present study, there was no noticeable reduction in heterozygosity in the highlanders. Another factor is gene flow, which may act to change allele frequencies. In this context, the influx of European genes might not have altered the gene pool of the highland Aymara significantly, because average Caucasian admixture in contemporary Aymara is only 8% (Salzano and Callegari-Jacques, 1988).
With respect to evolutionary aspects at high altitude, this study showed that highlanders and lowlanders are dimorphic in two common promoter polymorphisms, showing two different alleles. Thus, group differences were not observed in the presence or absence of certain alleles, but in the frequencies of existing alleles. This should be expected because the time that has passed since the settlement of the Andes, 12000 years, is too short for genetic mutations to generate completely new advantageous genetic variants (Rupert and Hochachka, 2001). Further work is needed to confirm the present findings with replication in independent samples. Also, Amerindian lowland populations as well as Asian populations should be analyzed, which was not possible in the present study. HbF levels should be determined and related to the observed polymorphisms. The present results provide a basis for future genetic analysis of fetal globin genes and mechanisms associated with adaptation to high altitude.
We are very grateful to the volunteers for taking part in this study. We thank Dr E. Llop (Laboratory of Population Genetics and Human Evolution, Faculty of Medicine, University of Chile), Mrs J. Schmitz (Department of Human Biology, Zoological Institute, Kiel University, Germany) as well as Mrs A. Dietsch, M. Friskovec, and L. Bossen (Institute for Clinical Molecular Biology, University Hospital of Schleswig-Holstein, Germany) for their technical assistance.
|