| Correspondence to: Kyoko Yamaguchi, Department of Anatomy, Faculty of Medicine, University of the Ryukyus, Uehara 207, Nishihara 903-0215, Japan. E-mail: kyoko777@gmail.com Published online 10 March 2010 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.080718 |
Tooth emergence has been widely used as a marker of growth and development in children (Jelliffe and Jelliffe, 1968, 1973). The trait has long been used in studies of human biology and anthropology where emergence has been examined in many populations for different purposes. As a developmental marker, tooth emergence, particularly deciduous tooth emergence, has been widely employed as a means for aging children. For example, Gillett (1998) used permanent tooth emergence to develop age standards for rural Zambian children. In forensic anthropology, tooth emergence is used for age estimation and individual identification (Iscan, 2005). Because tooth emergence is believed to be relatively buffered against the effects of malnutrition, it is frequently employed as an age marker for assessing somatic growth in subadult skeletal samples (Saunders, 1992; Saunders et al., 1993).
Furthermore, because dental material is the best-preserved evidence of past human populations, the sequence and timing of tooth emergence has been used to interpret the fossil record (e.g. Garn et al., 1957). The timing of tooth emergence is also important in pediatric dentistry and orthodontics for planning treatments (Leroy et al., 2003). For this reason, dental and public health researchers have developed population standards for permanent tooth eruption (Diamanti and Townsend, 2003; Eskeli et al., 1999).
Most previous studies of permanent tooth eruption have been based on cross-sectional observations, in which children are examined once. For example, there have been numerous cross-sectional studies of permanent tooth emergence in Japanese children (Hamano, 1929; Okamoto, 1934; Takakuwa, 1956; Kamijo et al., 1952; Kunimoto, 1954; Matsui, 1961; Inoue, 1961; Japanese Society of Pedodontics, 1988; Nagasaka et al., 2000). Longitudinal studies, in which the dental development of children is repeatedly examined over long periods, are relatively rare. One reason for this is the long time scale (over 12 years) needed to observe the emergence of all permanent teeth. Recently, Aizawa and Yuki (2006) published a cohort study on the eruption of permanent teeth in relation to the physical development. In the study, Japanese school children were examined annually for 6 years from age 6 to 12. This would cover only the first half of the emergence of permanent teeth. Kitamura (1942a, b) and others (Yanagisono, 1960a, b; Ohta, 1966) published longitudinal studies of permanent tooth emergence in Japanese children, although both of these studies pre-date the advent of modern statistical methods to deal with the analytical difficulties such as right-censoring of the observations and agenesis that arise in longitudinal studies (Holman and Jones, 1998). Right-censoring occurs when a subject withdraws from the study prior to the emergence of a tooth, resulting in a missing observation of time to emergence. Agenesis of teeth, also known as congenitally missing teeth, refers to failure of teeth to develop. Standard statistical methods assume all teeth will eventually emerge, so that including agenic teeth leads to an overestimation of the mean time to eruption.
Very few studies have attempted to examine the effect of infant nutritional and health variables on the timing of permanent tooth emergence although the timing of deciduous tooth emergence has been widely studied giving mixed results of the effect of nutritional status on tooth emergence (see Holman and Yamaguchi, 2005). In part, such studies on permanent teeth are rare because they require examination of individuals over a 20-year period. Psoter et al. (2004) reviewed the literature on how protein-energy malnutrition in the first 5 years of life affects permanent tooth emergence and found only two relevant studies. The study of Toverud (1956) found no effect of early childhood malnutrition on the timing of emergence of the permanent dentition. The 6-year study of Alvarez (1995) concluded that emergence of incisors and first molars was accelerated in malnourished children. A 6-year cohort study by Aizawa and Yuki (2006) found correlation between the eruption of permanent teeth and physical development in boys, but not in girls.
Many factors have been found to correlate with the timing of tooth emergence, such as sex, breastfeeding pattern, socioeconomic status (SES), ethnicity, environment, and secular pattern when tooth emergence is used as a developmental marker. For example, Garn et al. (1973) concluded that lower SES was associated with delays in permanent tooth emergence although the magnitude of the effect was not as large as for genetic factors. Kitamura’s data set provides covariate information for health, nutrition, and the sex of each child (Kitamura, 1942a, b). Health assessments were conducted for each child during both infancy and childhood. Additionally, the intensity of breastfeeding was recorded for each child.
In this paper, we reanalyze published data from a 20-year longitudinal study conducted by Kitamura (1942a, b). The data include the emergence age of individual teeth on two cohorts of Japanese children examined prospectively from birth to 20 years of age. The published data included some information on infant and child health and nutrition. The data also included information from dental X-rays of some children, which helped Kitamura to determine that either (1) the tooth was erupting eventually, or (2) the tooth was agenic and would never emerge.
We use parametric logistic-survival analysis to analyze both emergence and agenesis of the permanent dentition in Japanese children. One basic question we address is whether the normal or log-normal distribution better describes permanent tooth emergence. Previous parametric studies have used the normal distribution as a parametric model to describe tooth emergence (e.g. Okamoto, 1934; Matsui, 1961; Inoue, 1961). Ideally, the selection of a parametric distribution would be dictated by theory of the etiologic process leading to tooth emergence. We know of no strong etiologic theory that suggests which distribution better describes tooth emergence, but a weak etiologic model is built from the central limit theorem of statistics. If many environmental insults and alleles at many loci each have a small additive effect on a character, then the resulting distribution will be normal; if effects are multiplicative on a character, the distribution will be log-normal (Wright, 1968). Galton (1879) suggested the use of the log-normal distribution for systems in which a constant percentage increment in growth occurs per unit time rather than an absolute increment. An additional advantage of the log-normal distribution is that it is constrained to positive ages only, whereas the normal distribution has a range that includes negative emergence times.
Kitamura’s observations included a significant number of right-censored teeth because some children withdrew from the study or died prior to emergence of all permanent teeth. Holman and Jones (1998) found a low prevalence of agenesis in deciduous teeth, and little bias by assuming that all teeth will eventually emerge. For the permanent teeth, however, controlling for agenesis is much more important, as many teeth exhibit significant levels of agenesis. We are not aware of a previous study of permanent tooth emergence that simultaneously controls for right-censoring and agenic status.
Our reanalysis of Kitamura’s data on permanent tooth emergence contributes a new analysis of the effect of infant health, infant feeding, and child health on both the timing of tooth emergence and the probability of dental agenesis. More specifically, our objectives are (1) to demonstrate a new statistical model for permanent tooth emergence that simultaneously controls for right-censoring and agenic status, (2) to determine whether the normal or log-normal distribution better describes permanent tooth emergence, and (3) to assess the effect of covariates such as nutrition, breastfeeding behavior, and a child’s sex on the emergence time of permanent teeth.
Kitamura (1917, 1942a, b) collected and published emergence histories of permanent teeth for 49 children born in January 1914 (cohort 1), and 65 children born in January 1924 (cohort 2). The children resided primarily in the Ushigome-Ku, Yotsuya-Ku, Koishikawa-Ku, and Kojimachi-Ku areas in central Tokyo. Each child was visited in regular intervals from birth until the permanent teeth emerged (sometimes excluding the 3rd molar), the child died, or the child was withdrawn from the study. Kitamura (1942a, b) published emergence ages for permanent teeth reported in intervals of about 2 months for 40 children born in 1914, and intervals of 3 months for 60 children born in 1924. Times and the reasons for leaving the study were carefully documented.
Kitamura assessed ‘clinical emergence’, defined as the point at which any part of the tooth pierces the gingiva (Lysell et al., 1962). Although some researchers have defined emergence in other ways (at least 2 mm out of gingival: Iwasawa, 1959; within 1 mm of the gingival surface: Japanese Society of Pedodontics, 1988), clinical emergence is the most commonly used criterion in human biology.
For a subset of teeth that did not emerge, Kitamura took dental X-rays to determine whether the tooth was present or absent. In cases where X-rays were taken, Kitamura clearly labeled whether or not the tooth was agenic.
Subjects were selected as young infants without regard to income or father’s occupation. The children came from a wide range of socioeconomic conditions, and from low- to high-income households. In general, good economic conditions prevailed in Japan during the first 5 years of the study period. The economy was growing by about 3% annually, and exports to Europe related to World War I fueled economic growth from 1915 to 1920. After World War I, however, the economy in Japan declined until 1935. The growth rate of GNE (gross national expenditure) of the period from 1920 to 1924 fell to 0.6% from 6.9% in the period 1915–1919. The GNE slowly increased up to 5.5% between 1935 and 1939 (Ohkawa and Shinohara, 1979). Throughout the study period, elementary schooling to age 12 years was nearly universal in Japan (Alexander, 2002).
The effects of covariates on the timing of tooth emergence were assessed by parametric survival analysis. The distribution of tooth emergence was assumed to follow either a two-parameter normal or a log-normal distribution (Holman and Jones, 1998). The two-parameter log-normal probability density function (PDF) is given by
For our analytical methods we worked with the normal survival function, 1 − Φ[(t − a)/b], or the log-normal survival function, 1 − Φ[ln(t − a)/b], where Φ(t) is a standard cumulative normal density, t is age taken from birth, a is a scale parameter, and b is a shape parameter. The mean of the log-normal distribution is aexp(b2/2), the median is exp(a), and the variance of the distribution is a2exp(b2/2)[exp(b2/2) − 1] (Evans et al., 2000).
Covariates
Kitamura (1942a, b) provided several covariates for each child. He assessed each child’s overall health at two points in time. Early health status was assessed during the first year of life for each child, and was categorized as good, medium, or poor. Likewise, later health status was assessed for each child as good, medium, or poor. Unfortunately, Kitamura (1917, 1942a, b) did not provide the specific objective criteria used to assign these rankings, and no yardstick was given against which health could be compared. Furthermore, Kitamura did not specify the age at which each child’s health was assessed. For analytic purposes, early and late health statuses were coded as dummy variables and the good category was taken as the comparison group.
The infant breastfeeding status of each child was assessed as fully breastfed, partially breastfed, or not breastfed. These categories were coded as dummy variables and the fully breastfed category was taken as the comparison group. We included a dummy variable that coded whether the child belonged to cohort 1 (the early cohort), born in 1914, or cohort 2 (the later cohort) born in 1924. Cohort 1 was the reference category. Finally, the sex of a child was coded as 0 for a female and 1 for a male. The sample sizes and characteristics of subjects are given in Table 1.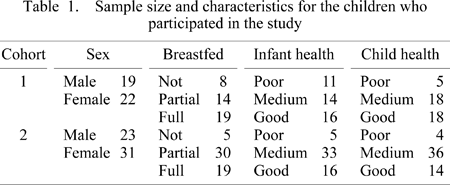
Following the method of Holman and Yamaguchi (2005) for analyzing covariate effects on deciduous tooth emergence, covariates are incorporated into the analyses as effects on the hazard of emergence. The hazard is defined as the instantaneous probability of emergence in an arbitrarily small interval at time t. For the ith child, the model includes an M × 1 vector of covariates, xi = (x1i, x2i, …, xMi)′, and coefficients effects, β = (β1, β2, …, βM); the effects of multiple covariates are combined additively, hence xi′β = x1iβ1 + x2iβ2 + … + xMiβM. (see Holman and Yamaguchi, 2005). Under a log-linear hazard specification, xi′β affects the hazard of emergence as hi(t|a, b, β) = h(t|a, b)exp(xi′β). The corresponding survival distribution is Si(t|a, b, β) = S(t|a, b)exp(xi′β) (Elandt-Johnson and Johnson, 1980).
Agenic fraction
Observations for some teeth were right-censored, meaning the child died, moved away, withdrew from the study, or the study ended prior to emergence of a tooth. Standard survival analytic methods assume all teeth will eventually emerge. However, in some cases, a tooth may be agenic or otherwise permanently fail to emerge. For example, roughly 80% of the 3rd molars did not emerge by the end of Kitamura’s study. Some of these teeth might have emerged had Kitamura carried on the study beyond 20 years. Other 3rd molars probably would not have emerged.
Although there are a number of mechanisms that cause a tooth to fail to emerge, for convenience we will refer to teeth that will never emerge as ‘agenic’ teeth. Statistically, the presence of non-erupting teeth is a violation of underlying assumptions of standard survival-analytic methods. Holman and Jones (1998) statistically modeled an agenic fraction, under the assumption that all right-censored observations might potentially be agenic. For Kitamura’s study of the permanent dentition, however, dental X-rays were taken for some children. Therefore, Kitamura was able to determine that either (1) the tooth was erupting, or (2) the tooth was agenic. Here we modify our methods to accommodate this additional information.
Likelihood
Kitamura’s observations of tooth emergence can be classified as interval-censored or right-censored (see Holman and Jones, 1998, for a more detailed discussion of censoring in tooth emergence data). Briefly, interval-censored observations are those for which the child’s age is known at some examination time prior to emergence and an examination time following emergence. The two dates define the interval within which emergence is known to have taken place. Right-censored observations are those for which the child has not emerged the tooth of interest at that child’s last examination in the study. We use the maximum likelihood to estimate the parameters of a logistic log-normal survival model that includes parameters to quantify the effects of covariates on the distribution of emergence, an agenic fraction, and effects of covariates on the agenic fraction.
The likelihood for a given individual tooth emergence depends how it was observed. For observations in which the tooth was observed to have emerged over the half-opened interval (tu, te], the individual likelihood is L = (1 − p)[S(tu|a, b, β) − S(te|a, b, β)]. For right-censored observations, the likelihood depends on whether or not the observation was ascertained to be agenic based on X-rays, not agenic based on the X-rays, or whether this additional information is unknown because X-rays were never taken. If the tooth was still known to be erupting at the last observation, the likelihood is L = (1 − p)S(tu|a, b, β). For observations in which the tooth is known to be agenic, the likelihood is simply L = p. Finally, for right-censored cases where the status of the tooth is not known because no dental X-ray was taken, the likelihood is L = (1 − p)S(tu|a, b, β) + p.
The likelihood for all of these possibilities combined, and for all children for a given tooth, is:
where N is the number of children observed for a given tooth, te is infinity for right-censored cases, ci is an indicator variable for the ith child that is 1 if the tooth is known with certainty to be agenic and 0 otherwise, and ki is an indicator variable for the ith child that is 1 if the tooth is known with certainty to present (either erupting or already emerged) and 0 otherwise.
The agenic proportion is modeled as a logistic regression, where pi = 1/[1 + exp(p′ + xi′βp)]. The vector of parameters βp quantifies the effects of covariates, xi, on the probability of agenesis.
Maximum-likelihood estimates of the parameters are values of a, b, β, p′ and βp that maximized equation (2), and were found using mle version 2.1 (Holman, 2003). The mle program and the code to do the analysis are available from one of the authors (D.J.H.). Standard errors of mean emergence times (SEMs) were computed numerically using the delta method (Elandt-Johnson and Johnson, 1980). Effective sample size (i.e. the number of observations that provides the equivalent statistical information about the distribution of tooth emergence) was computed for each tooth from the standard deviation (SD) and SEM as Neff = (SD/SEM)2 (Holman and Jones, 1998).
The Akaike Information Criterion (AIC) was used to select between the normal and log-normal models and for selecting the most parsimonious set of covariates (Akaike, 1973, 1992; Burnham and Anderson, 1998). This criterion is computed as twice the negative log-likelihood added to twice the number of parameters in the model. The model that minimizes AIC is taken to be the most parsimonious model, i.e. the model with the best trade-off between goodness-of-fit and the minimum number of parameters.
The analyses only included the left dentition. Pooling of the right and left dentition is statistically improper because of the high correlation between the sides (Holman and Jones, 1991). In this paper, a superscript and subscript is used to indicate maxillary (upper) and mandibular (lower), respectively.
Basic two-parameter models (with a and b parameters) and three-parameter model (a, b, and p) were initially fit to each tooth using a normal and a log-normal distribution. As assessed by AIC, the log-normal distribution always fit better than the normal distribution, suggesting that permanent tooth emergence exhibits a distribution with something of a long right tail. This result is consistent with findings for deciduous tooth emergence (Holman and Jones, 1998).
Parameter estimates for the most parsimonious models for each of the 16 permanent teeth are given in Table 2. The cumulative distributions of emergence times are given in Figure 1. The means, SDs, and SEMs are given in Figure 2. The proportion of agenic teeth, computed from the parameter estimates (Table 2) are shown in Figure 3.
 View Details | Figure 1. Distributions of tooth emergence in the permanent dentition of Japanese children. The vertical bars indicate standard deviation of cumulative proportion of tooth emergence. The distribution for each tooth asymptotically approaches the fraction of teeth that are expected to ever emerge (1 − the agenic proportion). |
 View Details | Figure 2. The mean, standard deviation, and standard error of the mean emergence time for the permanent dentition in Japanese children. |
 View Details | Figure 3. The estimated fraction of agenesis in the permanent dentition (mean ± one standard error) of Japanese children. The teeth are ordered from earliest to latest mean emergence time. |
As seen in Table 2, for all teeth except I2, PM1, I2, and M3 the effect of cohort (βcohort) was significantly different from 0, with the effect of cohort 1 delaying the emergence relative to cohort 2. Males exhibited accelerated emergence in PM1, PM2, and M3.
Children who were not breastfed (βbfnot) showed delayed emergence in I1, PM2, and I2, relative to the fully breastfed reference group. Children who were partially breastfed (βbfpartial), however, showed mixed results, exhibiting delayed emergence in M1, PM1, M1 but accelerated emergence in PM1, M2, I2, M2. Overall, breastfeeding, while significant for some individual teeth, did not show consistent effects on time to emergence over all teeth.
The parameters to quantify the effect of infant health status (βih_medium, βih_poor) were significantly different from zero in seven teeth, with the effect of medium or poor health status delaying the emergence in five teeth (I1, M1, M2, M1, M3) but accelerating emergence in I2 and M3, relative to the reference group with good health. Children of medium child health status (βch_medium) showed significantly delayed emergence of PM2 and M3. Also, poor child health status (βch_poor) delayed the emergence of I2, I2, PM2, and PM1.
Parameter p quantifying agenesis was significant for all teeth except I1, I2, M1, M2, M1, and M2 (Table 2). Covariates showed few effects on the fraction of agenic teeth (see βpsex and βpcohort in Table 2). Only sex and cohort significantly affected agenesis in the full logistic-survival model, and each affected only one tooth (sex on PM2 and cohort on PM2). That is, males compared with females had a significantly increased estimated fraction of agenesis in PM2 (12.7% compared to 2.1%). Of the known agenic teeth for PM2, five cases were found in males and one in females. Cohort 1 showed a higher estimated probability of agenesis compared to cohort 2 in PM2 (21.4% compared to 3.6%). Of the known agenic teeth in PM2, seven were found in cohort 1 and two were found in cohort 2.
Our results contradict some of Kitamura’s analysis (1942b) on the effects of nutritional status and sex on the emergence of the permanent dentition. Kitamura (1942b) concluded that there was a tendency for the teeth of females to emerge earlier than those of males. Our reanalysis, however, found that, when agenesis is controlled for and its influence is eliminated, males had an earlier mean emergence time for PM1, PM2, and M3 and no significant differences for the rest of the dentition (see βsex in Table 2). The median age of emergence for PM1 was 3.5 months earlier in males; for PM2 males were earlier by 5.2 months, and for M3 males were earlier by 17.4 months. Other studies in Japanese children have found that the permanent dentition of females emerges earlier than that of males, and usual sex differences in eruption timing are from 0 to 16.8 months, with the averages ranging from 5.22 to 5.65 months (Okamoto, 1934; Takakuwa, 1956; Japanese Society of Pedodontics, 1988).
There are several possible explanations for our results. First, we are working with a small sample size where we were unable to detect a significant sex difference for most teeth. Second, we properly included right-censored observations and explicitly controlled for agenesis, so that any sex differences in agenesis would not affect the distribution of emergence. Finally, there may have been unmeasured difference in health or nutrition that had sex-specific effects on the timing of tooth emergence. For instance, males’ good health could have been better than that of females, which would accelerate tooth emergence.
Perhaps the greatest weakness of the Kitamura data is the unknown nature of the health assessments. The definitions of “infant health status” and “child health status” are never fully specified. In his 1917 and 1942 papers, Kitamura uses the term “health status” and “nutritional status” interchangeably. Furthermore, in Kitamura’s study, there is no mention of whether an infant’s or child’s health status reflects health at a specific age, or an average over time.
With these caveats, the effect of poorer child’s health was always negative or non-significant on the hazard of emergence. That is, children of medium or poor health showed delayed emergence relative to children of good health. The pattern for infant health, which was assessed during the first year of life for each child, is less clear. Children of medium infant health, relative to those of good infant health, had no significant differences in emergence except for earlier emergence in I2 and delayed emergence in M2. Children of poor infant health were delayed for I1, M1, M2, M1, and M3, but showed accelerated emergence for M3. These teeth, except M3 and M3, start forming by 1 year after birth according to a well-known diagram of tooth formation and eruption by Schour and Massler (1941); therefore, poor infant health could delay formation of these teeth. For deciduous teeth in these same children (Holman and Yamaguchi, 2005), those with poor and medium infant health had later emergence times than infants with good health. Apparently, the effects of medium and poor infant health on emergence of the permanent dentition is somewhat ameliorated with time, although the effect of poor infant health remained for some of the permanent teeth. The significant effect of poor infant health on M3 and M3 might reflect some unmeasured variables that are related to infant health since these teeth are likely to be affected by the health status of later growth stages.
Seven of the 16 teeth yielded a significant relationship to breastfeeding. Even so, the overall pattern was inconsistent. Four teeth (I1, M2, I2, and M2) emerged faster for children who were partially or not breastfed relative to children who were fully breastfed. Three teeth (M1, PM1, and M1) showed delayed emergence for children who were partially breastfed relative to those who were fully breastfed. Among these three teeth, PM1 begins to form during the late second year through the third year when the effect of breastfeeding seems to be small, whereas M1 and M1 initiate earlier, when children are still breastfed. This inconsistent pattern further supports the idea that infant nutrition and health have a smaller effect on permanent tooth emergence than does childhood health.
We have presented a method for finding unbiased estimates of the distribution of emergence and agenesis from mixed observations. In this Japanese sample we found that ten teeth exhibited significant agenesis (see p in Table 2). Consistent with Endo et al. (2006) the most common agenic tooth after M3 and M3 was PM2 with just over 10% agenesis (Figure 3). Although males had a significantly increased fraction of agenesis in PM2, the other teeth with agenesis showed no significant sex differences in the fraction of agenesis. This is mostly consistent with a study in Japanese orthodontic patients that found no sex differences in agenesis (Endo et al., 2006).
A major advantage of our approach is that we managed to estimate unbiased agenic proportion even for the third molars. Previous studies either exclude the third molar from analysis, or give the fraction of agenic teeth at a fixed age. For example, one cross-sectional study of 46698 children (Japanese Society of Pedodontics, 1988) estimated that M3 and M3 failed to emerge in 77.7% and 70.6% of 19-year-olds, respectively. These percentages are similar to Kitamura’s (1942b) observations that the third molar failed to emerge about 80% of the time for 20-year-olds. However, the agenic fraction we estimated from Kitamura’s data suggests that, in the long run, M3 would never emerge in 46.0% of the children, and M3 would never emerge in 39.5% in both males and females. Our statistical method gives a different estimate of agenic fraction that accounts for the likelihood that some fraction of the unemerged teeth will emerge at an age later than the last observed age.
The order of tooth emergence that we found was slightly different from that reported by Kitamura. We observed that the mean order of emergence was M1, M1, I1, I1, I2, I2, PM1, C1, PM1, PM2, C1, PM2, M2, M2, M3, M3. When we examine the median order of emergence (which is less susceptible to outliers than is the mean order), C1 and PM1 were reversed, as were M3 and M3. Kitamura’s sequence followed our mean sequence except that he found that C1 emerged before PM2. The mean emergence time of PM2 would have appeared later than C1 without controlling for agenic proportion, since the agenic proportion for PM2 was estimated to be about 7% and C1 was only 2%. Our analysis incorporating agenesis, however, provided a different order that is not biased by agenesis or right-censoring.
Our finding offers a clue to understanding recent change in the order of permanent tooth eruption in Japanese. A cross-sectional study of 46698 Japanese children by the Japan Society of Pedodontics (1988) reported that the mean time of eruption for I1 was earlier than that for M1 which for a long time had been the first permanent tooth to emerge for Japanese. The underlying mechanism alternating the emergence time of I1 and M1 remains unclear. Our reanalysis showed that the emergence of I1 was accelerated for children who were partially breastfed or not breastfed relative to fully breastfed, although it was not influenced by infant health or child health. The emergence of M1, however, was delayed for children who were partially breastfed or not breastfed as well as for children with poor or medium infant health or child health. Considering these results, we can expect a child who was partially or not breastfed and/or with poor or medium infant and child health to emerge I1 before M1. This hypothesis, however, contradicts the current situation in Japan where infant and child health status would be better relative to a few decades ago.
The most consistent effect of the covariates was that cohort 1 showed delayed emergence in 12 teeth (I1, C1, PM2, M1, M2, M3, I1, C1, PM1, PM2, M1, and M2). The permanent teeth of the first cohort (born in 1914) started emerging around 1920, given that the mean age for the eruption of M1 was about 6.8 years. The Japanese economy began to decline beginning around 1920 until around 1935. Although the later cohort (born in 1924) also experienced this period, the economy was better during the time most of their dentition was emerging from 1934 until 1939. Economic differences may explain, in part, why it took longer for most of the dentition of cohort 1 to emerge compared to cohort 2. Along with the economic change, the nutritional status of the Japanese people also changed. Available statistics show that average calorie intake per person per day was 2113.9 kcal between 1911 and 1915, increased up to 2308.1 kcal between 1921 and 1925, declined to 2180.8 between 1931 and 1935, and stayed about the same until 1939 (Yamazaki, 1973). Considering that calorie intake was the highest between 1921 and 1925 when cohort 2 was in their infancy, nutritional status during infancy and early childhood might largely affect the eruption timing of permanent teeth.
We expect that economic differences in tooth emergence would act through health. Therefore it is surprising that the cohort effect was strong even while simultaneously controlling for infant and child health to eliminate the influence of health status. Given Kitamura’s (apparently) crude measurements of health, however, there may still have been unmeasured variability in infant and child health that was captured through the cohort variable. Alternatively, Kitamura’s rankings of health may have been calibrated to be relative rankings within each cohort, rather than measured as an absolute standard.
The cohort effect we found might reflect the secular trend in height in Japan, which was a 1 cm increase per decade for students and conscripts born before 1920 (Kouchi, 1996). Kitamura did not record the height or weight of his subjects, but we could follow the average height of Japanese students who were born in 1914 (the same year as cohort 1) and 1924 (the same year as cohort 2) from age 6 to 17 in the ‘Statistical Report on School Health and Hygiene,’ a report published by the Ministry of Education, Culture, Sports, Science and Technology of Japan (2009). The mean height of the students who were born in 1924 was 1.4 cm higher on average between age 6 and 17 (ranging from 0.5 cm to 2.1 cm) relative to those who were born in 1914, except for age 16 and 17 in males because of the influence of World War II. There is some evidence to suggest that body height and tooth eruption are negatively correlated, which means that taller children tend to have advanced tooth eruption relative to shorter children (Garn et al., 1965; Yano, 1971). This may explain why permanent tooth eruption could be earlier for cohort 2 (born in 1924) relative to cohort 1 (born in 1914).
We conclude from this analysis that the log-normal distribution better describes the timing of permanent tooth emergence. Our reanalysis of Kitamura’s data found that males had an earlier mean emergence time for three permanent teeth, controlling for right-censoring and agenesis simultaneously. This contradicts the conclusion of Kitamura’s and other studies that the permanent dentition of females emerges earlier than that of males (e.g. Japanese Society of Pedodontics, 1988). Emergence may be delayed for children of poor and medium health compared to those of good health although infant health and breastfeeding behavior appeared to have a smaller effect. Cohort showed the most consistent effect among the covariates: the later cohort (born in 1924) showed accelerated emergence in 12 teeth relative to the first cohort (born in 1914) although there may have been unmeasured variability in child health that appeared as cohort effect. A definitive test of nutritional effect on the timing of permanent tooth emergence will require data with more specific assessment of nutritional and health status.
We thank Yasuo Ihara and Akiko Nosaka for their help with Kitamura’s original Japanese publications, and Steven Goodreau for helpful comments on the manuscript.
|