| Correspondence to: Hitoshi Fukase, Department of Biological Science, Graduate School of Science, The University of Tokyo, Hongo 7-3-1, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: fukase@um.u-tokyo.ac.jp Published online 20 October 2009 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.090513 |
The size and shape of the mandibular corpus have often been interpreted from a biomechanical perspective in association with masticatory forces that are inferred mainly from dietary preference and feeding behavior (e.g. Beecher, 1979; Hylander, 1985, 1988; Bouvier, 1986; Daegling, 1992, 2007; Anton, 1996; Ravosa, 1996, 2000; Daegling and Hylander, 1998; Vinyard and Ravosa, 1998; Taylor, 2006a, b; Daegling and Grine, 2007). However, the results of these studies did not always conform to the expected patterns of covariance between mandibular morphology and masticatory loads, and it has been suggested that biomechanical factors alone cannot explain variation in corpus dimensions and proportions (e.g. Takahashi and Pan, 1994; Daegling and McGraw, 2001, 2007; Taylor, 2002; Williams et al., 2002; Vinyard et al., 2003; Fukase and Suwa, 2008).
In order to evaluate whether population differences in mandibular morphology are accounted for by difference in postnatal masticatory (mechanical) environments, we recently compared two human populations, the modern Japanese and the prehistoric Jomon (Fukase and Suwa, 2008). The latter is considered to have a ‘robust’ mandibular morphology associated with a presumed heavier masticatory load. Results showed that most of the adult Jomon characteristics (e.g. the overall large size of the corpus and ramus, developed angular region, wide bilateral breadth, and thick cortical bone) are seen early in infancy. In addition, the growth rates of these mandibular metric attributes did not differ significantly between the two populations. These observations suggest that the Jomon ‘robust’ mandible is largely manifested by genetically based developmental patterns acting early in ontogeny, and that postnatal masticatory environments had comparatively limited effects on adult mandibular morphology (cf. Cole, 1992) in the two studied populations. It is possible that selection for general skeletal robusticity and/or a developed masticatory apparatus was responsible for (a part of) the Jomon morphogenetic pattern, but that this was not the case in the historical to modern Japanese populational lineages. Alternatively, some or much of the modern Japanese morphology may stem from characteristics inherited from the Yayoi immigrant populations (e.g. Hanihara, 1993; Nakahashi, 1993; Kaifu, 1997).
The previous results also demonstrated that, while the modern Japanese juvenile mandible had a symphyseal height lower than that of the Jomon, adult symphyseal height was conversely higher in the modern Japanese, corresponding to an anteriorly increasing corpus height (Kaifu, 1997; Fukase and Suwa, 2008). Although the mandibular symphysis is under masticatory stress due to its structural function as a bridge between the right and left mandibular corpi (e.g. Hylander, 1985), in the modern Japanese, masticatory loads are considered reduced because of increasing sophistication of cooking practice (Koyama and Goshima, 1985; Saitou, 1987; Katoh et al., 1994; Watanabe and Komoto, 2000). Thus, there are no apparent reasons to expect an increase of biomechanical demand in the symphysis. It is therefore probable that factors other than mechanical requirements are involved in the differences expressed between the modern Japanese and Jomon mandibular symphysis.
The spatial requirements of the anterior teeth, particularly of the large canine of nonhuman primates, have been suggested as one non-mechanical factor that may play a significant role in determining anterior corpus dimensions of the mandible (e.g. Wolpoff, 1975; Wood, 1978; Dean and Beynon, 1991; Plavcan and Daegling, 2006). A simple tooth-jaw model would expect individuals or species with large teeth to have a correspondently large mandible. In fact, the crown and root sizes of the permanent incisor and canine teeth are known to be larger in the modern Japanese than in the Jomon (Brace and Nagai, 1982; Matsumura, 1994; Fukase and Suwa, 2008), although the crown size of the deciduous teeth is conversely larger in the Jomon (Matsumura, 1991; Kitagawa et al., 2002). Therefore it is possible that the higher symphysis of the adult modern Japanese in part results from local spatial requirements for accommodating the larger permanent anterior teeth. However, such a simple correlation model between tooth size and mandibular corpus size has been refuted by many earlier studies of humans (Garn et al., 1963, 1980; Smith et al., 1986, 1989; Kieser and Groeneveld, 1988) and nonhuman primates (Smith, 1983; Taylor, 2000; Plavcan and Daegling, 2006).
By using lateral-view radiographs of the mandible, Smith et al. (1989) evaluated the relationship between postcanine tooth size and corpus height in prehistoric Maori and Australian aborigine populations. These interpopulation comparisons showed that the crown and root sizes of postcanine teeth were larger in the Australian aborigine than in the Maori people. However, the Maori people conversely displayed greater mandibular corpus height and alveolar height superior to the mandibular canal. Smith et al. (1989) therefore concluded that tooth size and mandibular corpus height do not correlate. However, the results of this study may be dependent on the Polynesian specific mandibular form of the Maori, what is called the ‘rocker jaw.’ In ‘rocker jaws,’ projection of the gonial process is lacking and the inferior corpus and posterior ramus profiles form a continuous convex curve (Marshall and Snow, 1956; Snow, 1974; Schendel et al., 1980; Kean and Houghton, 1982, 1990). Such a specialized mandibular form may exhibit a tooth-jaw relationship distinct from other populations. Therefore it remains to be seen whether or not Smith et al.’s (1989) conclusion is applicable to other comparisons.
In another comparative study, Plavcan and Daegling (2006) examined the external dimensions of the tooth crown and mandibular corpus among a large number of anthropoid species. Interspecific comparisons did not exhibit covariance between postcanine corpus and molar breadths. Positive correlations were shown between the canine diameters and anterior corpus depth in many of the intra- and interspecific comparisons. They suggested that this was probbaly an expression of sexual dimorphism within species, and interspecific trends for association of large male canines with a deeper mandibular corpus. Recently, Ward et al. (2007) suggested that correlation between crown/root dimensions and facial proportions differed among species, and that canine size cannot universally explain facial dimorphism in anthropoids.
Thus, to what extent and under what circumstances spatial requirements of the dentition determine or significantly influence adult corpus dimension is largely unknown. Most previous studies have focused on the relationships between the sizes of the adult tooth crown and mandible, and therefore the influence of the developing teeth on mandibular growth remains unclear. It would be ideal to examine the spatial requirements of the totality of the developing dental tissue, and its interaction with the developing mandible. In the earlier growth periods, this would include not only the crown and what is formed of the root, but also the entire tissue that forms the developing tooth bud. This would be expressed osteologically by the bony crypts of the teeth. In later periods, dental tissue mostly comprise the crown and root, so that both need to be looked at, since the sizes of the two do not necessarily correlate (Garn et al., 1978; Smith et al., 1989; Spencer, 2003; Paulus et al., 2007). In the present study, by use of microcomputer tomography (CT) scanning, we examined the size and placement patterns of the developing teeth and crypts in the skeletal growth series of the prehistoric Jomon and modern Japanese. We then evaluated the possible interaction between crypt/tooth size and anterior mandibular size and proportions. In particular, our aim was to examine whether space requirements of the developing dental tissue could explain the higher anterior corpus of the adult modern Japanese mandible compared to that of the Jomon.
The prehistoric Jomon people were Neolithic hunter-gatherer-fishers, who lived in the ancient Japanese archipelago from c. 13000 to 2350 BP. The population history of the Japanese has not been entirely clarified, but the bulk of the evidence suggests admixture between the Jomon people and the immigrant population lineages of the Yayoi period (c. 2350–1650 BP) thought to have come from the Asian continent. A dominant genetic influence of the latter is generally supposed (Hanihara, 1987, 1991, 1993; Dodo and Ishida, 1990; Nakahashi, 1993; Omoto and Saitou, 1997), but the timing, extent, and nature of admixture appears to be regionally and temporally complex and remains the subject of active study (Ossenberg et al., 2006; Matsumura, 2007; Manabe et al., 2008; Nakahashi and Iizuka, 2008; Hanihara and Ishida, 2009; Ishida et al., 2009; Kawakubo et al., 2009).
The mandibles used in the present study are housed in the University Museum, the University of Tokyo. The Jomon materials that we used span a chronological age of approximately 5000 to 2350 BP. The ‘modern’ Japanese materials come from the anatomy department collections made from approximately 1890 to 1920. For the comparison of adult specimens, a total of 26 Jomon and 32 modern Japanese male mandibles with at least one M3 erupted were chosen. Sex of the Jomon adult specimens was evaluated from pelvic morphology, and from appendicular morphology in the few cases that lacked sufficiently preserved associated pelvic remains. The age at death of the modern Japanese adult specimens ranged from 20 to 40 years (mean = 28.0, SD = 5.9), according to archival records. The age at death of the Jomon adult samples was evaluated by dental wear (e.g. Lovejoy, 1985) and pubic symphyseal form (Brooks and Suchey, 1990), and controlled so that excessively aged individuals were not included. For the growth series comparison, a total of 39 Jomon and 33 modern Japanese specimens ranging in dental age from nine months to 15 years were selected for study (Table 1). Dental age was determined referring to Ubelaker’s (1989) standard. In particular, attention was focused on the formation stages of permanent incisors and canine until the time of canine eruption (10 years of dental age). For individuals with intermediate conditions in Ubelaker’s (1989) dental age criteria, the mean value of the corresponding dental ages was used. The sex of all Jomon specimens was considered unknown, and the recent Japanese materials were of mixed sex. Individuals without tooth loss, tooth extraction, apparent skeletal deformation, or pathological traits were selected.
A series of cross-sectional images of the whole mandible were obtained for each specimen by use of the microfocal X-ray CT system (TXS225-ACTIS, Tesco) of the University Museum, the University of Tokyo. The CT imagery was taken at 130 kV, 0.24 mA. The slice thickness and interval was determined depending on the size of each specimen, and ranged from 180 to 450 μm. Cross-sectional images were reconstructed in a 512 × 512 matrix with the pixel size equivalent to the slice thickness. The sagittal plane was defined as the plane perpendicular to both the mandibular basal plane and a line passing through the posteriormost points of the condyles. The horizontal plane was then set to be perpendicular to the sagittal plane and parallel to the alveolar line passing through two points on the alveolar crests of the right corpus (dc/dm1 or C/P3 and dm2/M1 or M1/M2). CT image processing and analysis were carried out by use of the software Analyze 7.0 (Mayo Clinic).
In the frontally projected whole-volume CT images (equivalent to a conventional X-ray projection, but without perspective errors), corpus heights were measured at three positions passing through the lowest points of incisor and canine crypts or sockets. The actual coordinates of the measured points were determined by scrolling through the serial CT imagery. Corpus height was defined as the vertical distance from the mandibular basal margin to a line connecting the tops of the interdental alveolar crests. The position of the mandibular canal is known to move little during mandibular development (Bjork, 1963, 1969; Bjork and Skieller, 1983), and has been often used as a reference to define alveolar and basal portions of the mandibular postcanine corpus (Darling and Levers, 1975; Whittaker et al., 1985; Whittaker et al., 1990; Ulm et al., 1993; Varrela et al., 1995; Xie et al., 1996; Boughner and Dean, 2004). In the present study, the level of the upper border of the mandibular canal was determined in the coronal plane passing the top of the alveolar crest of dm2/M1 or P4/M1. We used this level to subdivide corpus height into alveolar and basal heights, i.e. three variables, anterior corpus height, anterior alveolar height, and anterior basal height, were examined (Figure 1a). The means of heights taken at I1, I2 and C positions were analyzed.
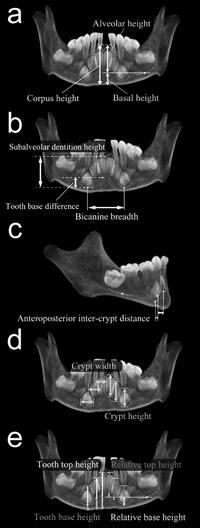 View Details | Figure 1. Mandibular metrics in frontal (a, b, d, e) and lateral (c) CT image projection. (a) Corpus height, alveolar height, and basal height. Corpus height was subdivided into alveolar and basal heights at the level of the mandibular canal (at dm2/M1 or P4/M1). The broken line indicates the trajectory of the upper border of the mandibular canal. (b) Bicanine breadth, tooth base difference, and subalveolar dentition height. (c) Anteroposterior intercrypt distance, defined as the anteroposterior distance between the lowest points of tooth crypts. (d) Crypt width and height. (e) Tooth top height and tooth base height, defined as the distances from the mandibular basal margin to the highest and lowest points of each tooth crypt or alveolus, respectively. Relative top height and relative base height were similarly determined in reference to the level of the mandibular canal. |
Several measurements were defined to examine the placement pattern of the incisor and canine crypts (see Figure 1b and c). Bicanine breadth was taken as the transverse distance between the lowest points of the canine crypts. Tooth base difference was determined as the vertical distance between the lowest points of central incisor and canine crypts. Subalveolar dentition height was defined as the vertical height from the lowest point of the canine crypt to the highest point of the central incisor crypt, or after incisor eruption, to the midlevel between the tops of the alveolar crests of I1/I1 and I1/I2. In the case of the presence of a thin ascending canal in the top of the central incisor crypt, the height was measured up to the midpoint of the rim of the hole. Anteroposterior intercrypt distance was measured between the lowest points of central and lateral incisor crypts and of lateral incisor and canine crypts. With the specimens of 15 years of dental age and adults, these measurements were likewise taken by using the lowest points of the dental sockets, instead of the dental crypts. Furthermore, maximum transverse widths and vertical heights of the dental crypts (Figure 1d) were measured for the unerupted tooth crypts (using the serial CT imagery). The sums of crypt width and crypt height were also examined. Crowding index was derived as the ratio of the sum of crypt widths to half of bicanine breadth.
In order to investigate crypt size increase and tooth eruption patterns, the variables ‘tooth top height’ and ‘tooth base height’ were defined as the distances from the mandibular basal margin to the highest and lowest points of each tooth crypt or socket, respectively (Figure 1e). Relative top height and relative base height were similarly determined in reference to the level of the mandibular canal.
In order to further evaluate the relationship between tooth size and corpus height, root lengths of the permanent teeth were taken from the serial CT imagery of the adult mandible. Root length of the lower I1 to P4 was determined as the distance from the tooth root apex to the lowest point of the labial cervical line. Molar root length was defined as the distance from the mesial tooth root apex to the intersection point between the mid-buccal face of the mesiobuccal cusp and the buccal cervical line.
We used least-squares (LS) regressions of each variable on dental age in comparing Jomon and modern Japanese growth patterns. Alternatively, reduced major axis (RMA) regression is often stated to be the preferred method, when the independent variable includes a substantial (measurement) error component (e.g. Sokal and Rohlf, 1995). However, Smith (2009) provides an instructive and useful summary of the actual complexities underlying such routine characterizations, and emphatically recommends that the appropriate regression model depends on the biological relationship being represented by the two variables. In our previous (Fukase and Suwa, 2008) and present studies, our aim is to investigate how morphological attributes change through growth, so that LS regression with age as the independent variable is appropriate. Similar analyses have been performed by other workers (e.g. Pinhasi et al., 2005; Mays et al., 2008).
Two-tailed analysis of covariance (ANCOVA) was conducted to test for population differences in developmental patterns (slope and y-intercept derived from least-squares regressions) (cf. Vinyard and Ravosa, 1998; Ravosa, 2000; Taylor, 2002). Because many of the variables examined in this study, such as those that relate to the inferior extent of tooth crypt or root position, are directly affected by status of tooth eruption, we conducted the above metric comparisons separately for each of three stages of anterior teeth eruption (see below). This was done in order to examine the effect of dental tissue expansion separately from eruption itself. Considering that the eruption of the incisor and canine teeth takes place in approximately seven and ten years, respectively (The Japanese Society of Pedodontics, 1988; Liversidge et al., 1998), analyses were carried out for the following three stages: before incisor eruption (from zero to six years of dental age), during tooth eruption (from seven to nine years of dental age), and after canine eruption (from ten to fifteen years of dental age). When regression slopes significantly differed between the two populations, tests for y-intercept were not conducted. In the cases that the regressions against dental age were not significant, a two-tailed Mann–Whitney U-test was performed to test for differences between the two populations. With the measurements in the mandibles of 15 years of dental age and adults, a two-tailed Mann–Whitney U-test was conducted for interpopulation comparisons. For all analyses, either the value of either side or the mean of right and left sides was used because of small sample sizes. The statistical analyses were carried out using SPSS 10 software (SPSS Inc.). Statistical significance of P < 0.05 is referred to in describing the results.
The anterior view projections of the developing mandibles and teeth are displayed as a growth series representation in Figure 2. In both populations, the incisor crowns are completed before the canine crown, which forms near the mandibular basal margin and moves upwards with root formation. The order and relative timing of tooth formation did not appear to differ between the two populations.
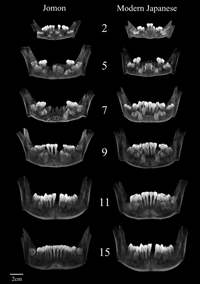 View Details | Figure 2. Growth series of teeth and mandibles. The numbers of each row are the dental age (years). The broken arched line in the third row passes through the lowest points of the canine and central incisor crypts. Note that the Jomon mandible shows a wider and lower arch than that of the modern Japanese. |
The slopes and y-intercepts of the least-squares regression of each measurement against dental age are listed in Table 2. ANCOVA shows that the regression slopes differ significantly between the two groups only in some of the comparisons and that this was confined to the period before incisor eruption. Significant differences in y-intercepts were observed in some of the comparisons throughout the examined growth periods (outlined below).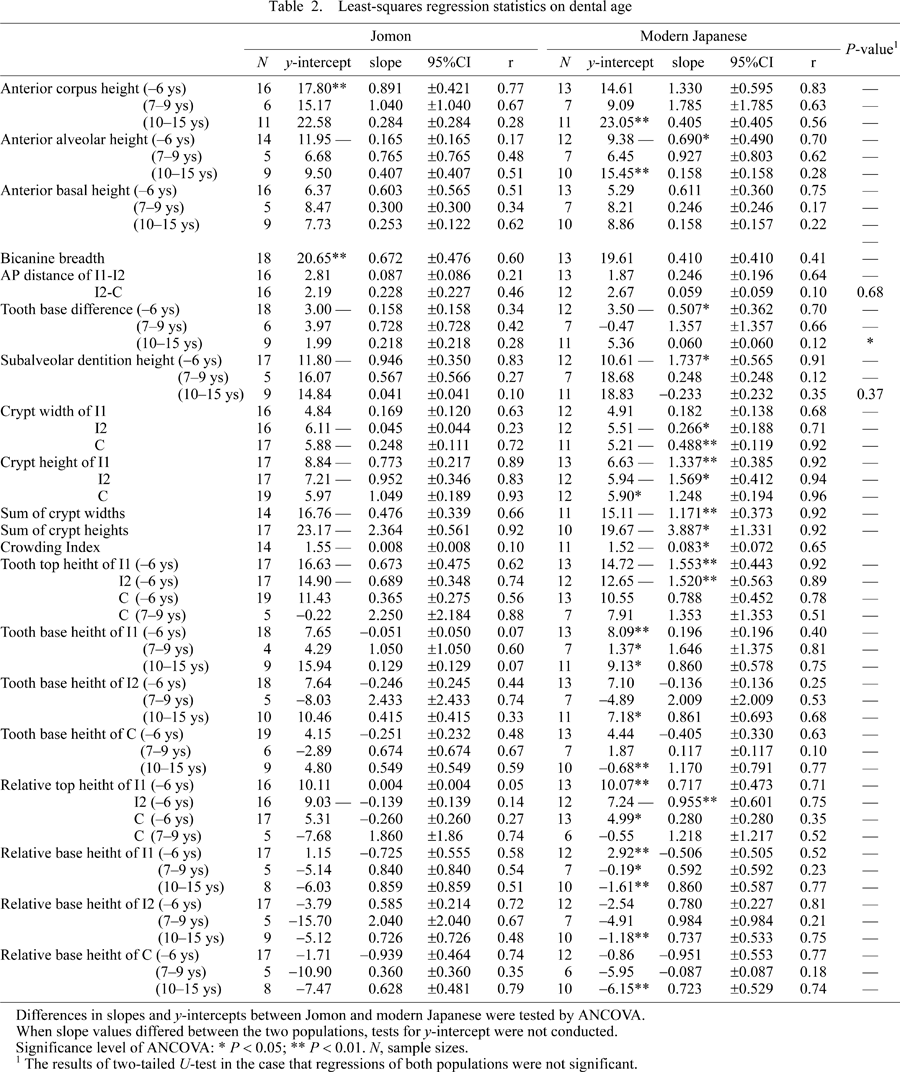
The growth pattern of anterior corpus height is shown in Figure 3. The regressions show that anterior corpus height and anterior alveolar height were initially larger in the Jomon than in the modern Japanese, but afterwards the modern Japanese overtook the Jomon. The regression slope of anterior alveolar height before incisor eruption was significantly greater in the modern Japanese. In anterior basal height, population differences in both slope and y-intercepts were not significant in all three age categories, showing that the basal mandible is invariable between the two populations.
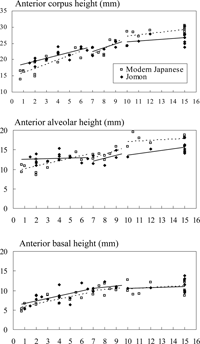 View Details | Figure 3. Growth related changes in the measurements of corpus height. The horizontal axis represents dental age (years). Solid and broken lines indicate the least-squares regressions of the Jomon and modern Japanese, respectively. |
The placement patterns of the incisor and canine crypts are shown in Figure 4. ANCOVA shows that the Jomon bicanine breadth was significantly greater than that of the modern Japanese during early growth. The anteroposterior intercrypt distance did not differ between the two populations in both I1-I2 (P = 0.86) and I2-C (P = 0.68). In both tooth base difference and subalveolar dentition height before incisor eruption, the regression slopes were found to be significantly greater in the modern Japanese than in the Jomon. Thereafter, the tooth base difference of the modern Japanese remained larger than that of the Jomon throughout growth and in the adults (Table 3). The growth rate of the individual crypts (width and height) tended to be greater in the modern Japanese than in the Jomon, resulting in a significantly greater growth rate of the sums of the crypt widths and heights in the modern Japanese. The crowding index increased during growth in the modern Japanese, while it hardly changed in the Jomon.
 View Details | Figure 4. Growth related changes in the measurements of dental placement and dimensions. The horizontal axis represents dental age (years). Solid and broken lines indicate the least-squares regressions of the Jomon and modern Japanese, respectively. |
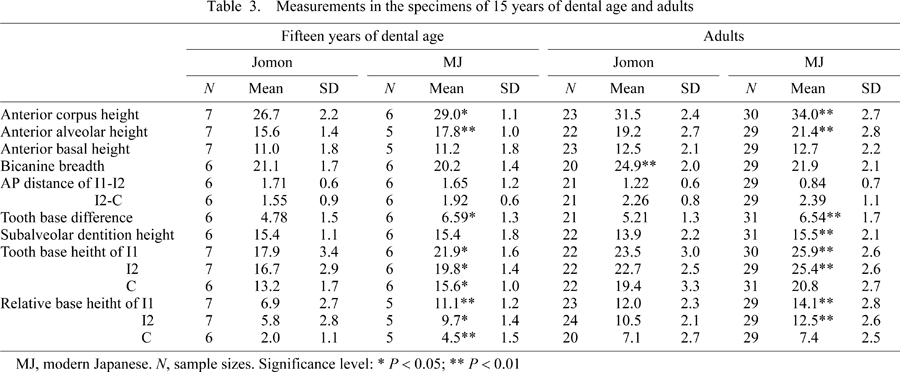
Figure 5 shows crypt expansion and tooth eruption patterns. The tooth top heights increased until tooth eruption in both populations, while the relative top heights of the Jomon did not increase. Slopes of both the tooth top height and relative top height were significantly greater in the modern Japanese than in the Jomon in the I1 and I2. The tooth base heights and relative base heights generally decreased and increased before and after tooth eruption, respectively. Those of the I1 were found to be higher in the modern Japanese than in the Jomon throughout the age categories examined. Regarding the canine, tooth base height and relative base height did not differ significantly before incisor eruption (P = 0.54 and P = 0.14, respectively) and during tooth eruption (P = 0.56 and P = 0.09, respectively). However, after canine eruption, the canine position of the modern Japanese was significantly higher than that of the Jomon, suggesting a greater eruption distance of the modern Japanese canine.
 View Details | Figure 5. Patterns and amounts of tooth crypt expansion and eruption. The horizontal axis represents dental age (years). Solid and broken lines indicate the least-squares regressions of the Jomon and modern Japanese, respectively. Tooth top height is the projected distance from mandibular base to top of tooth crypt, and tooth base height is from mandibular base to bottom of tooth crypt. After eruption, only the tooth base height (base of mandible to alveolus) is shown. |
Table 3 shows the descriptive statistics in the mandibles of 15 years of dental age and adults. The results showed that, in both age groups, the anterior corpus height and anterior alveolar height were greater in the modern Japanese than in the Jomon. The anterior basal height values did not significantly differ between the two (P = 0.64 and P = 0.543 in 15 years of dental age and adults, respectively). The results of the dental placement patterns were also similar to the results of the growth series comparisons.
Comparisons of tooth root lengths are listed in Table 4 and exhibited as line plots in Figure 6. The Jomon teeth had significantly shorter roots than those of the modern Japanese in the anterior teeth and premolars, but not the molars. In particular, the third molar root was significantly longer in the Jomon.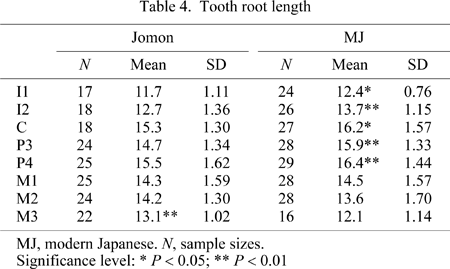
 View Details | Figure 6. Distribution pattern of tooth root length. |
The comparisons of the placement of the tooth crypts showed that, although the anteroposterior distances between the adjacent teeth did not differ significantly between the two populations, the juvenile Jomon mandible had a larger bicanine breadth than the modern Japanese mandible and the vertical positions of the tooth crypts differed between the two populations. In particular, the tooth base difference of the modern Japanese increased faster and became greater than that of the Jomon by the time of incisor eruption. This resulted in the arch-like line connecting the lowest points of the incisor and canine crypts to be relatively wide and low in the Jomon mandible, and narrow and high in the modern Japanese mandible (Figure 2). What can account for this differential dental placement of the two populations?
The balance between general mandibular growth and resulting space available for the developing tooth crypts must be a major determining factor of the positioning of the tooth crypts. Such an interaction is particularly expected in the anterior mandibular corpus, considering the considerably crowded condition of the forming anterior teeth. Our previous study showed the Jomon mandible to have a larger bigonial breadth than that of the modern Japanese throughout development (Fukase and Suwa, 2008). This is consistent with the wider bicanine breadth of the Jomon observed in the present study throughout growth, and indicates a broader space available for tooth development. In terms of tooth crypt size, however, the incisor and canine crypts were found to expand at a greater rate in the modern Japanese, which is expected from their larger crown and root sizes seen in the adults (Brace and Nagai, 1982; Matsumura, 1994; Fukase and Suwa, 2008; this study). Thus, the discrepancy between mandibular space and tooth crypt size can be regarded to be more severe in the modern Japanese during growth. In fact, the crowding index (the ratio of the sum of crypt widths to half bicanine breadth) kept increasing throughout growth in the modern Japanese but not in the Jomon. We therefore suggest that in human mandibles, tooth crypt size does not influence anterior corpus breadth, but does impact its height by means of superoinferior displacement of the developing crypts faced with a lack of sufficient space. This is clearly suggested from subalveolar dentition height, which was found to become increasingly greater in the modern Japanese by the time of incisor eruption.
Longitudinal orthodontic studies on human facial growth have suggested that the increase of mandibular symphyseal height is realized largely by development of alveolar bone rather than basal bone of the anterior corpus, although there is considerable individual variation (Bjork, 1963, 1969; Bjork and Skieller, 1983; Sarnat, 1986; Buschang et al., 1992). In the present study, we therefore examined the growth pattern of the anterior corpus height in reference to the mandiblular canal position, which is considered to be relatively stable during development (Bjork, 1963, 1969; Bjork and Skieller, 1983). The results we obtained were quite instructive in that the greater height increase of the modern Japanese anterior corpus relative to that of the Jomon during incisor formation and eruption was confirmed to stem from differential growth of the alveolar portion of the anterior mandibular corpus.
Another pattern of interest we see from the analysis of corpus height relative to the mandibular canal regards patterning of height growth below the level of the canal. Our results show that, with size increase of the crypts, mandibular height increases not only in the alveolar bone above the level of the canal (discussed above for the modern Japanese incisor), but basally below the canal as well. This was found to occur slightly in the Jomon incisors and strongly in the canines of both the modern Japanese and Jomon. Examination of the anterior view volume projection renderings (Figure 2) suggests that spatial constraints position the canine crypts lower in the anterior corpus than the incisors from early in growth. Thereafter, throughout growth, the canine crypts enlarge, for the most part, basally relative to the level of the mandibular canal in both the Jomon and modern Japanese mandibles. However, our results show that a populational difference does not exist in the amount of basal growth at the canine position.
Subsequently, the modern Japanese appears to exhibit a greater distance of canine eruption, which is accompanied by formation of their longer tooth roots. Our results of root length comparisons show that both the incisors and canine (and premolars as well) have a greater length by about a millimeter in the modern Japanese than the Jomon. It is possible that the greater eruption distance (a reflection of greater corpus height) of the modern Japanese canine is in part caused by the longer root. However, it should be noted that canine eruption takes place at 9.5–10.5 years of age (The Japanese Society of Pedodontics, 1988; Liversidge et al., 1998). This overlaps the period of growth when upper facial height of the modern Japanese is also known to have increased faster than that of the Jomon (Okazaki, 2006). Enlargement of symphyseal height during this period is probably for the most part related to growth of the entire face, regardless of tooth size and eruption. From the present study, it is not apparent whether or not root length was an added factor that actually does influence anterior corpus height during eruption.
From the foregoing considerations, we propose the hypothesis that tooth size and spacing conditions have substantial effects on the growth of the anterior mandibular corpus until the time of tooth eruption. Thereafter, root length and eruption distance may also affect mandibular height dimensions, although this is an issue that needs further investigation. Our results indicate that dental size is in part responsible for the higher mandibular symphysis of the adult modern Japanese compared to the Jomon.
We are grateful to the members of the Physical Anthropology laboratory of the University Museum, the University of Tokyo, and the Department of Anthropology, the National Science Museum, Tokyo, for encouragement and help throughout the various aspects of this research. Ms N. Awaji assisted in the CT scanning operation. This work was supported in part by Global COE Program (Integrative Life Science Based on the Study of Biosignaling Mechanisms), MEXT, Japan.