| * Correspondence to: Tanya M. Smith, Department of Human Evolutionary Biology, Harvard University, 11 Divinity Avenue, Cambridge, MA 02138, USA. E-mail: tsmith@fas.harvard.edu Published online 1 May 2010 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.091006 |
Enamel thickness has figured prominently in studies of human evolution over the past three decades and is recognized as an important morphological difference between thinly enameled African apes and thickly enameled Homo (e.g. Gantt, 1977; Molnar and Gantt, 1977; Martin, 1983, 1985). It has been studied in both extant and extinct primate species to understand taxonomic and phylogenetic relationships (e.g. Schwartz, 2000a; Kono, 2004; Smith et al., 2005, 2006, 2008, 2009a; Olejniczak et al., 2008a, b; Suwa et al., 2009) and tooth function (e.g. Macho and Berner, 1993, 1994; Macho, 1994; Schwartz, 2000b). Information on enamel thickness is also valuable for clinical preventative and therapeutic measures (e.g. Stroud et al., 1998; Jarvis, 1990; Rossouw and Tortorella, 2003; Hall et al., 2007). However, little work has been done to quantify variation in enamel thickness across the dentition (but see Smith et al., 2008) or to assess population-specific differences in enamel thickness and distribution in teeth other than molars.
Enamel thickness studies from physical sections necessitate partial destruction of the crown to view and measure internal structures (e.g. Macho and Berner, 1993, 1994; Macho, 1994; Schwartz, 2000a, b; Grine, 2002, 2005; Smith et al., 2005, 2006, 2008), which often limits sample sizes. Two-dimensional X-ray projection radiography has been used to quantify enamel thickness non-destructively in fossil specimens (e.g. Zilberman et al., 1990; Zilberman and Smith, 1992) and living subjects (e.g. Stroud et al., 1994; Harris et al., 2001). However, measurements from clinical radiographs are typically confined to marginal enamel from the mesial and distal interproximal regions of the crown. Moreover some conventional radiographic techniques are not adequate to accurately measure enamel thickness, as measurements have been shown to be overestimated (or variably exaggerated) (Grine et al., 2001). X-ray projection radiography is often insufficient for dental tissue analysis due to poor absorption contrast and the lack of the third dimension, which largely limits the utility of data (Tafforeau et al., 2006). The third dimension is accessible in images produced by computed tomographic techniques, and the application of high-resolution microtomography provides an accurate and reproducible means of quantifying enamel thickness in two and three dimensions (2D and 3D) (Olejniczak and Grine, 2006; Olejniczak et al., 2007), while facilitating large study samples (e.g. Suwa and Kono, 2005; Olejniczak et al., 2008b).
When compared to studies of human cranial or dental size and shape variation, relatively little is known about population-level variation in dental enamel thickness. Most studies have employed physically sectioned samples of European molars (e.g. Martin, 1983; Macho and Berner, 1993; Schwartz, 2000a, b; Smith et al., 2009b; but see Grine, 2002, 2005; Smith et al., 2006). To date, the sample of 257 human molars employed by Smith et al. (2006) represents the largest available global sample, and did not show differences among populations in average enamel thickness (or dentine area, enamel area, or enamel–dentine junction (EDJ) length) for any maxillary or mandibular molar position, save for the mandibular third molar. In this instance, significantly greater values for average enamel thickness and component variables were found in three groups relative to a fourth group studied; these differences were largely due to thinner enamel in a medieval Danish population, which may correlate with relatively poor health (Boldsen, 2005) when compared with more recent human populations studied. No differences in average enamel thickness were found among southern Africans, North Americans, or northern Europeans, despite variation in tooth size among groups.
Even less is known about 3D molar enamel thickness among human populations. Kono (2004) employed a sample of 41 recent and archaeological Asian molars in her groundbreaking analysis of hominoid 3D enamel thickness and distribution. Feeney (2009) recently employed a 3D sample of 49 known-sex molar teeth from a variety of European, African, Asian, and North American populations. However, to statistically assess population variation in 3D enamel thickness requires larger sample sizes, which are difficult to obtain due to natural wear, lesions, or fractures in clinical samples or museum specimens.
Data on enamel thickness in anterior teeth and premolars are more restricted than currently available data on human molars. Schwartz and Dean (2005) and Saunders et al. (2007) reported enamel thickness values from physical sections of mandibular canines, and the latter study also included third premolars. Variation in lateral enamel thickness has also been examined in mandibular premolars from clinical radiographs (Stroud et al., 1994) and in maxillary incisors (Harris and Hicks, 1998). These studies all focused on single populations. Smith et al. (2008) published the first account of human enamel thickness across the entire maxillary and mandibular dental arcade of a mixed-population sample from physical cross-sections, but did not test for population differences due to limited sample sizes within populations. Given the large amount of metric and non-metric dental variation documented among African, European, and Asian populations (Hanihara and Ishida, 2005; Hanihara, 2008), and developmental differences between African and European anterior teeth and premolars (Reid and Dean, 2006; Reid et al., 2008), it is unclear if significant differences exist in internal dental tissue structure (e.g. enamel thickness). The current study is the first to apply microtomographic imaging to a sample of Asian maxillary and mandibular canines and third premolars in order to assess potential variation within and among modern human populations.
The Asian sample consisted of 15 canine and 17 third premolar teeth (n = 32) extracted at dental clinics in Jakarta, Bekasi, and Palembang, Indonesia. The sample derives from western Indonesians and a few individuals of mixed Chinese–Indonesian heritage. A larger sample of teeth was examined for pathology and attrition; only those deemed unworn or lightly worn and caries-free were used for this analysis. When both left and right antimeres were available, a single tooth was employed.
Specimens were scanned using a SkyScan 1172 desktop microtomographic system (Department of Human Evolution, Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany). All scans were performed with an aluminum–copper filter at a voltage of 100 kV and amperage of 100 μA, with an angular increment (step rotation) of 0.12 (i.e. 2400 views per rotation). Images were output at a resolution of 2048 × 2048 pixels per slice and at 16 bits per pixel (subsequently converted to 8 bits during the reconstruction process); the resultant voxels were isometric (equal length, width, and depth) ranging from 12.9 to 17.4 μm3 for different teeth.
Virtual cross-sections of each tooth were prepared using VGStudio MAX 2.0 software (2008, Volume Graphics GmbH) and measured using ImageJ software (v. 1.42, NIH). The canines were virtually sectioned by first orienting the 3D model to view the labial surface of the tooth, and then adjusting this model so that the cusp tip and maximum labial cervical enamel extension were aligned vertically. A grid of lines was then established on the dentine horn tip and the labial cervical enamel extension in the labiolingual longitudinal 2D section. By using an orthogonal (labiolingual) view, the primary dentine horn tip was chosen as the center of rotation, and slight rotation and translation of the tooth yielded a section passing through the dentine horn tip and both the labial and lingual maximum cervical enamel extensions (typically the widest labiolingual bi-cervical diameter (BCD)).
Premolars were sectioned according to the following protocol. Homologous planes of section were located by first orienting the 3D microtomographic models to view the occlusal surface. In the corresponding 2D occlusal view window, the image stack was then scrolled through apically until the base of the crown was located. In this orientation the stack was then adjusted by translation and rotation to acquire a ring of enamel with uniform width near the cervix. After this reorientation, the image stack was scrolled through cuspally to view the buccal and lingual dentine horn tips (Figure 1a). Once these were located, the image stack was adjusted so that both dentine horn tips were aligned horizontally (Figure 1b). The image stack was then centered at the buccal dentine horn tip and then slightly adjusted through mesiodistal rotation to acquire a plane that passes through both dentine horn tips and an average position between the maximal BCD and maximum cervical enamel extension (Figure 1c).
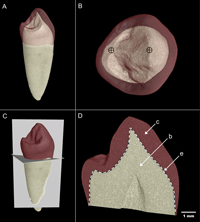 View Details | Figure 1. Orientation protocol for assessment of enamel thickness from virtual microtomographic planes of section. (A) View of a buccolingual orientation of the tooth. (B) Buccal and lingual primary dentine horn tips are aligned horizontally. (C) Using both the 2D (not shown here) and 3D perspectives, the tooth is oriented to obtain a buccolingual virtual section (i.e. longitudinal plane) that includes both dentine horn tips and the mid-plane between the lowest cervical enamel extension points and maximum bi-cervical diameter. A transverse section marks the longest enamel cervical extension along the buccolingual section (i.e. a horizontal plane). (D) Once an ideal plane is located, the area of the enamel (c), dentine (b), and the length of the enamel-dentine junction (e, dotted line) are measured. |
Once virtual sections of canines and premolars were created, a scale bar was inserted and the image was exported into Adobe Photoshop CS3 (v. 10.0) and subsequently saved as a TIFF image prior to measurement with ImageJ. The borders between the tissue interfaces (i.e. between air–enamel and enamel–dentine) were clearly distinguished in all images. The area of the enamel cap (c), area of coronal dentine (b), length of the EDJ (e), and BCD were measured (Figure 1d). Following Martin (1983), average enamel thickness (AET) was calculated as: AET = c/e, yielding a value in millimeters. A subsample of five teeth of mixed tooth types was assessed by two observers (oriented, sectioned, and measured) to determine interobserver error, and the mean present differences in AET was found to be 3.7% (n = 5, range = 0.1–5.7). Several statistical tests were employed to examine differences in c, e, b, AET, and BCD among populations, between maxillary and mandibular analogs, and between canines and premolars using SPSS software (v. 17.0, SPSS Science, Inc.). A comparative sample of 107 canines and third premolars from histological sections of northern European and southern African individuals was employed (preparation detailed in Reid and Dean, 2006; Reid et al., 2008). Sections used for the comparative sample were carefully selected by choosing specimens that were unworn or lightly worn, preserving at least one of two cervices, and sectioned in a similar orientation as the virtual sections (non-obliquely). Differences between populations were examined using the Kruskal–Wallis test with population as the factor. When a significant result was found, Conover’s post-hoc comparisons were made to determine which populations accounted for the significant differences (performed with custom software written by Anthony Olejniczak (personal communication)). The Mann–Whitney U-test was employed to examine differences between both maxillary and mandibular analogs, between canine and premolar tooth positions, and between the sexes of the pooled populations (for n > 3).
Average cross-sectional dentine area (b), enamel cap area (c), EDJ length (e), AET, and BCD are given for three human populations in Table 1. No significant differences were found among human populations for the AET index (Figure 2) or BCD at any tooth position (Table 2). Differences were found for upper third premolar dentine area, enamel cap area, and EDJ length. Post-hoc comparisons revealed that both the Asian and European samples showed greater dentine area and EDJ length than the African population, and that the Asian sample had greater enamel area than both the European and African populations (Table 3). Population differences were also found for lower canine dentine area and EDJ length. The post-hoc comparisons showed that the European samples had greater dentine area and EDJ length than the Asian sample.
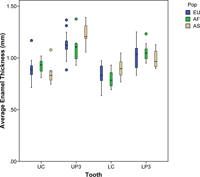 View Details | Figure 2. Average enamel thickness in Asian (AS), African (AF), and European (EU) maxillary and mandibular canines and third premolars. Box plots depicting the range of average enamel thickness in maxillary (U) and mandibular (L) canines (C) and third premolars (P3). Significant differences were not found among populations (see text and Table 2). The outliers are indicated as closed circles. |
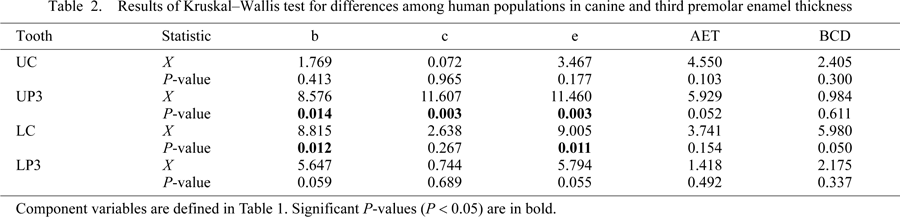

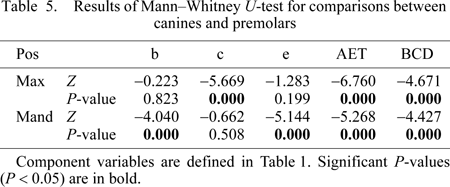
Given that AET did not significantly differ among populations, the samples were subsequently combined to test for differences in AET between dental arcades, between tooth positions, and between sexes. Highly significant differences were found for comparisons of maxillary and mandibular third premolars (Table 4). Maxillary premolars have greater dentine area, enamel cap area, EDJ length and BCD than mandibular premolars, resulting in significantly greater AET. No significant differences were found for comparisons of maxillary and mandibular canines. (AET was slightly greater in maxillary canines, although this difference was not significant.) When canines and premolars were compared within the dental arcade, maxillary premolars showed significantly greater enamel cap area (and BCD) than maxillary canines, leading to significantly greater AET (Table 5). In contrast, mandibular canines showed significantly greater dentine area, EDJ length, and BCD than mandibular premolars. This resulted in significantly lower AET in mandibular canines relative to mandibular premolars. Finally, no significant sex differences in AET were found, although males tended to have greater dentine area, enamel area, EDJ length, and BCD (Table 6). These differences in dentine area and EDJ length were significant for maxillary canines and premolars (Table 7).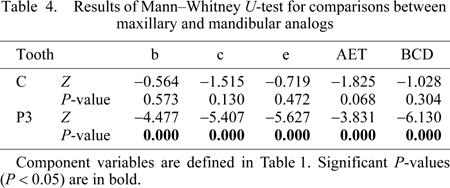


Non-metric dental characteristics and external metric variation are traditionally used to describe recent human geographic populations and samples of fossil hominins (e.g. Wolpoff, 1971; Kieser, 1990; Wood, 1991; Scott and Turner, 2000; Hanihara and Ishida, 2005; Hanihara, 2008). While most odontometric studies focus on crown size differences, few researchers have attempted to quantify tissue differences to account for population variation in disperse geographic groups. Schwartz (2000a) pointed out that the majority of published data on molar enamel thickness in modern humans are derived from European populations, which may not be representative of the global species mean (possibly underestimating variation). More recently, Grine (2002, 2005) conducted an investigation of crown tissue components in regionally disparate modern human populations and found values similar to those reported for European samples, with low levels of variation within and among populations. Likewise, Smith et al. (2006) did not find consistent differences in molar enamel thickness among larger samples of disparate human populations. The results of this study also suggest that canine and premolar AET is similar across populations, rendering mixed-population samples suitable for comparison with hominin taxa.
The majority of odontometric variation is found within groups rather than between groups (e.g. Scott and Turner, 2000), which appears to be true for AET as well. Hanihara and Ishida (2005) reported that less than 20% of the overall variation in dental metrics (external length and breadth dimensions) derives from between-group differences. In addition, variation in geographic patterning in tooth size is generally consistent with non-metric trait data (e.g. Scott and Turner, 2000; Hanihara, 2008). Europeans demonstrate the lower limit of contemporary global metric variation, and Asians (including southeast Asians) together with sub-Saharan African groups form an average metric group (Hanihara and Ishida, 2005). One surprising finding in this study is the lack of differences in enamel cap area between European and African samples. Despite their smaller average size (Hanihara and Ishida, 2005), northern European canines and premolars form over longer periods than southern Africans on average. Reid and Dean (2006) reported mean population differences of 185 and 372 days for upper and lower canines, respectively. Reid et al. (2008) found a similar pattern of short crown formation times in southern African third premolars, which took on average 309–315 days longer to form in northern Europeans (depending on the cusp analyzed). Cuspal enamel thickness was found to be fairly similar in both populations (less than 0.3 mm on average: Smith et al., in prep); differences in total formation times were due to shorter imbricational formation times in southern Africans. These differences do not appear to have impacted the total amount of enamel formed (in the 2D plane examined here), nor the relationship between enamel cap area and EDJ length (AET).
The difference found in AET and related components between maxillary and mandibular premolars may be due to differences in the size of primary cusps; human lower third premolars have small lingual cusps that do not greatly contribute to coronal area. Maxillary third premolars typically have two large primary cusps, creating a broader and taller profile, which appears to include proportionately more enamel area (leading to greater AET). Maxillary and mandibular canines are more similar in cross-sectional shape, although maxillary canines are typically larger than mandibular canines (and have non-significantly thicker enamel). Smith et al. (2006) found that maxillary molars had significantly greater AET than mandibular molars, which they suggested was due to differences in their buccal–lingual configurations. When canines were compared to premolars, premolars were found to have significantly greater AET in both maxillary and mandibular rows. However, canine–premolar comparisons were found to show different patterns in the components of enamel thickness for maxillary and mandibular comparisons. Thus the trend in increasing AET throughout maxillary and mandibular dental arcades reported by Smith et al. (2008) is supported, although it appears to be due to different relative tissue patterns in each arcade. Additional 3D study is needed to examine the changing distribution of dental tissues across the human dentition. These data could be coupled with information on crown formation times in order to elucidate volumetric rates of enamel development (e.g. Smith and Tafforeau, 2008), which are likely to vary among human populations.
Studies of metric differences in crown size in modern humans demonstrate that males and females show overlapping dimensions, although males show slightly greater mean dimensions than females (2–7%), with canines showing the greatest dimorphism and the premolars showing the least (Hillson, 1996; Scott and Turner, 2000). In the current study, AET was not found to differ significantly between males and females for canines or premolars. However, significant differences in the components of enamel thickness were found in the maxillary canine and first premolar, with males having significantly greater values for dentine area and EDJ length. In contrast, Schwartz and Dean (2005) found that female mandibular canines have significantly greater AET than males, due to greater enamel cap area and small dentine area in females (also see Saunders et al., 2007). AET in four male canines in our study (0.84) is greater than this value (0.61) in Schwartz and Dean (2005). It is possible that expanded global samples of mandibular canines will show significant sex differences. Overall, the addition of a relatively large sample of Asian teeth contributes to a better understanding of variation in recent humans. The present findings suggest that human populations can be combined for enamel thickness comparisons with fossil hominins.
Michelle Haenel and Pam Walton are thanked for assistance with clinical samples. Two anonymous reviewers provided helpful comments on the manuscript. Anthony Olejniczak is acknowledged for use of unpublished statistical software. This study was funded by the Max Planck Society and Harvard University.