| * Correspondence to: Yutaka Kunimatsu, Primate Research Institute, Kyoto University, Kanrin, Inuyama 484-8506, Japan. E-mail: kunimats@pri.kyoto-u.ac.jp Published online 24 July 2010 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.100122 |
Among human dental anomalies, dental agenesis has long been studied in various populations (Fujita et al., 1995; Polder et al., 2004). In recent years, it has also become an interesting subject in relation to genetic studies to understand the developmental mechanisms of human dentition (Line, 2003; Matalova et al., 2008).
Catarrhine primates, including our own species Homo sapiens, have two incisors, one canine, two premolars, and three molars on one side of both the upper and lower jaws. In recent human populations, however, congenitally missing third molars are commonly observed (Fujita et al., 1995). Second molars may become absent when the third molars behind them are missing, but such cases are quite rare (Ma, 1949; Sumiya, 1959). Congenital absence of first molars, especially lower ones, is not observed at all (Ma, 1949; Sumiya, 1959; Goya et al., 2008) or is extremely rare (Polder et al., 2004). In textbooks of human dentistry (e.g. Fujita et al., 1995), the general rule in dental agenesis is that teeth become missing sequentially from either the mesial or distal extremity in each of the incisor, canine, premolar, or molar domains (Fujita et al., 1995). In this rule, permanent molars are regarded as the sequel to the deciduous molars (Figure 1). The first molar is the central tooth in the deciduous/permanent molar domain (dp3, dp4, M1, M2, M3), and is considered to be the last molar that becomes missing (Fujita et al., 1995). Dental agenesis in non-human primates has been studied much less than in humans, but the above-mentioned general rule also seems to be applicable to cases of dental agenesis in non-human primates (Lavelle and Moore, 1973; Miles and Grigson, 1990; Jablonski, 1992). But is this rule always true?
 View Details | Figure 1. Incisor, canine, premolar and molar domains in the first and second series of the dentition. Deciduous teeth are more stable than permanent teeth in dental agenesis, and in the molar domain, dental agenesis usually proceeds from the distal extremity (= M3) (Fujita et al., 1995). |
In our research of intraspecific dental variation in Japanese macaques (Macaca fuscata), we found two individuals that showed an unusual pattern of molar agenesis, which apparently contradicts the above-mentioned general rule. In these individuals, the lower first molars were congenitally missing, while the more posterior molars (M2 and M3) did exist. These cases are interesting not only because they showed an extremely rare dental anomaly but also because these two monkeys belonged to the same maternal lineage (mother and son). The repeated occurrence of this extremely rare pattern of molar agenesis in closely related individuals suggests that it is very likely a genetically inherited anomaly shared within this maternal lineage. Here we describe in detail these two cases of the molar agenesis in a single maternal lineage of Japanese macaques. To our knowledge, this is the first report of familial dental agenesis in non-human primates.
The following abbreviations are used in this article: Mff: prefix for captive Macaca fuscata fuscata individuals reared in the Primate Research Institute, Kyoto University (PRI), KUPRI: prefix for skeletal specimens in the PRI osteological collection.
We observed a total of 515 skulls (284 males and 231 females) of Japanese macaques from 16 regions in Japan (Yamamoto, 2007; Yamamoto and Kunimatsu, 2002, 2006a, 2006b). Except for the individuals related to Mff 277, all the specimens were adult, i.e. all permanent teeth were erupted. In Japanese macaques, the permanent dentition is completed at an age of around 7.2–8.3 years (2633–3023 days) (Yamamoto and Kunimatsu, 2006b). The two individuals (Mff 277 and Mff 618) that showed the M1 agenesis belonged to the Takahama troop, one of the captive troops of Japanese macaques in the Primate Research Institute (PRI), Kyoto University. The original stock of this troop was derived from Takahama in Fukui prefecture (central Japan). Their original bloodline has been preserved under captivity (Yamamoto, 2007; Yamamoto and Kunimatsu, 2002, 2006b). The skeletons of Mff 277 and Mff 618 are preserved in the osteological collection of PRI under the accession numbers KUPRI 3990 and KUPRI 4008, respectively. Fourteen individuals related to Mff 277 were identified according to the Database of Non-human Primates in PRI (Matsubayashi et al., 2004), and skeletal specimens were available for eight of them (Figure 2). They belonged to the maternal lineage that originated from a female Japanese macaque (Mff 90) introduced from Takahama into the PRI (Figure 2).
 View Details | Figure 2. Pedigree of the maternal lineage of the Japanese macaques with M1 agenesis. Individual numbers (prefix: Mff), age at death (in years), and sex are indicated. |
Dental measurements were taken according to the methods of Swindler (2002) and Benefit (1993), using digital calipers (Mitsutoyo Co.). Comparative data of dental measurements of M. fuscata were taken from Yamamoto (2007). In order to assess the tooth types (M1 or M2) of Mff 277 and Mff 618, discriminant analysis was performed with the statistical software JMP 6 (SAS Institute, Inc.) for each sex. Mesiodistal length (md) and mesial buccolingual breadth (blm) of M1 and M2 were used for the discriminant analysis. X-ray photographs were taken of the mandibles of Mff 277 and Mff 618 in order to check whether there are unerupted teeth within the mandibular body.
Mff 277 is the first specimen in which we detected M1 agenesis. It was not possible to determine whether her mother (Mff 90) had the same dental anomaly or not, as her skeleton had not been preserved. Of the three siblings of Mff 277, the skeleton is available only for one individual (Mff 339), which showed normal dentition (Figure 1). Among the eight offspring of Mff 277, whose skeletons were available except for one individual (Figure 2), only Mff 618 had the anomalous dentition with M1 agenesis as in Mff 277. Detailed descriptions of the two anomalous individuals (Mff 277 and Mff 618) are given below (for the dental measurements of these two individuals, see Table 1). The X-ray photographs of the mandibles of Mff 277 and Mff 618 showed that neither of them has any hidden molars within the mandibular body.
In this individual, the left lower dentition shows abnormal morphology, while the right lower dentition is normal for Japanese macaques (Figure 3). In humans, it is often difficult to precisely identify the molar types by shape and size. However, identification of each molar type in Japanese macaques is much easier and more reliable. In fact, within the same sex, the first, second, and third lower molars can be identified nearly perfectly even based on size alone.
 View Details | Figure 3. Skull of Mff 277 (KUPRI 3990): (a) inferior view of the cranium; (b) occlusal view of the mandible; (c) right side of the skull; (d) left side of the skull. Scale = 2 cm. |
The ‘first’ molar on the left lower dentition is considerably larger than the right M1. It is similar to the M2 on the right side in size and shape (Figure 3, Figure 4, Figure 5). Although there is some overlap in size between M1 and M2 of Japanese macaques (Figure 5), in most cases, they can be properly classified in the discriminant analysis (Table 2). According to the discriminant analysis, the left ‘first’ molar of this specimen is classified as a M2 with a very high probability (0.999) (Table 3). The ‘second’ molar on the left side exhibits a typical M3 morphology. The crown is mesiodistally elongated and tapers distally with a prominent hypoconulid. In Japanese macaques, as in other cercopithecids, M1 and M2 are tetracuspid with no hypoconulid. It is only M3 that develops the hypoconulid. The left ‘third’ molar is a simple, peg-like tooth, resembling the M4s that sometimes occur in primates as a dental anomaly (Miles and Grigson, 1990). This M4-like tooth occludes with the left M3 (Figure 3d).
 View Details | Figure 4. Enlarged occlusal view of the mandible of Mff 277 (KUPRI 3990). Scale = 2 cm. |
 View Details | Figure 5. Right and left lower molar measurements of Mff 277 (KUPRI 3990) plotted with comparative data of normal female Japanese macaques. MD, mesiodistal length (mm); BLm, mesial buccolingual breadth (mm). |


There is no diastema between the last lower premolar and the left ‘first’ molar (corresponding to M2 in normal dentition). This pattern on the left dentition appears as if the formation of the left M1 had been omitted in the process of dental development and the permanent molar row morphogenesis had been started from M2, instead of M1, and continued posteriorly to produce even an extra M4 at the distal extremity.
This individual is one of the two sons of Mff 277. The upper permanent dentition is fully erupted and is normal. In the lower dentition, however, there are only two molars on each side. In size and shape, these molars are identical to M2 and M3 in the normal dentition of other Japanese macaques (Figure 6, Figure 7, Figure 8). In humans, it may be difficult to determine with certainty whether the missing teeth are actually first molars or not. On the other hand, in Japanese macaques, the first molars are much smaller than the second molars, and there is little overlap in size between them within the same sex (Figure 8). According to the discriminant analysis, both of the right and left ‘first’ lower molars of Mff 618 are classified as M2 with fairly high probabilities (0.856 for the right and 0.860 for the left side: Table 3). Apparently, both of the right and left M1s were never formed in this individual, and the permanent molar row morphogenesis was started from M2, followed by M3, having no diastema between the last premolars and M2s, as in Mff 277. In contrast to the case of his mother (Mff 277), there is no indication of an M4-like ‘third’ molar on either side. Instead, there is a large retromolar space between the M3 and the anterior margin of the ascending ramus of the mandible. Consequently, the upper third molars do not occlude with any lower tooth (Figure 6c and 6d).
 View Details | Figure 6. Skull of Mff 618 (KUPRI 4008): (a) inferior view of the cranium; (b) occlusal view of the mandible; (c) right side of the skull; (d) left side of the skull. Scale = 2 cm. |
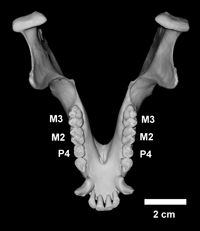 View Details | Figure 7. Enlarged occlusal view of the mandible of Mff 618 (KUPRI 4008). Scale = 2 cm. |
 View Details | Figure 8. Right and left lower molar measurements of Mff 618 (KUPRI 4008) plotted with comparative data of normal male Japanese macaques. MD, mesiodistal length (mm); BLm, mesial buccolingual breadth (mm). |
By examining 515 skulls of Japanese macaques, we discovered two individuals with a rare pattern of dental anomaly, i.e. M1 agenesis with the more posterior molars (M2 and M3) fully erupted, within a single maternal lineage in the Takahama troop of PRI. These cases are interesting from the viewpoints of patterns in dental agenesis and dental development, because the congenitally missing teeth are the anteriormost molars, while the dentition preserves the more posterior molars completely, and in one case (Mff 277), an M4 is even formed. This greatly differs from the ordinary pattern of dental agenesis in both humans and other mammals. In general, when molars are congenitally missing, such anomalies occur from the distal extremity of the molar row. In modern humans, congenitally missing or unerupted third molars are commonly observed (Fujita et al., 1995). In his study on modern Japanese, Sumiya (1959) found no case of congenitally missing upper and lower first molars. Goya et al. (2008) also reported that the upper and lower first molars were never absent in their sample of 2072 Japanese individuals. According to the compiled data in Polder et al. (2004), congenitally missing first molars have been reported previously, but the first molars are more stable than the second and third molars. Most notably, the lower first molar is the most stable molar in the upper and lower dentitions. The prevalence of M1 agenesis in humans is extremely rare (only about 0.01%) (Polder et al., 2004).
In non-human primates, the reduction of the molar tooth row usually occurs from the posteriormost molars, both phylogenetically and pathologically. In Miles and Grigson (1990), there are a number of examples of congenitally missing teeth in primates. However, there is no case of congenitally missing first molars. When molars are congenitally missing, nearly all the cases are upper or lower third molars. Lavelle and Moore (1973) reported prevalence of tooth agenesis in extant primates. Their data indicated that molar agenesis is quite rare in non-human primates. For example, among 978 individuals of cercopithecoids, they found only nine individuals with molar agenesis (0.9%). In particular, in 350 specimens of macaques (M. mulatta, M. sinica, and M. irus (= M. fascicularis)), they detected no case of molar agenesis. Although Lavelle and Moore (1973) did not indicate which molars were missing, they stated that the anomalies in the molar teeth were almost all in the third molar region. Hence, congenitally missing first molars must be an extremely rare phenomenon in non-human primates, if it occurs at all.
It is highly probable that the M1 agenesis we observed in Japanese macaques is a genetically inherited dental anomaly, because this rare anomaly repeatedly occurred within a single maternal lineage while no other case was detected among more than 500 specimens of Japanese macaques that we examined. In humans, the relationships between familial dental agenesis and mutations in genes such as MSX1 or PAX9 have become a hot topic and have been extensively studied in recent years (Vastardis et al., 1996; Stockton et al., 2000; van den Boogaard et al., 2000; Jumlongras et al., 2001; Nieminen et al., 2001; Frazier-Bowers et al., 2002; Line, 2003; Matalova et al., 2008). However, these genes seem to be related to severe congenital loss of teeth (hypodontia or oligodontia), and the conditions of dental agenesis in the patients with mutations in these genes are not identical to those in Mff 277 and Mff 618. There are other genes that may regulate the dental development in humans (Matalova et al., 2008) and similar genetic studies have started in non-human primates recently (Perry et al., 2006), but it is still beyond the scope of this paper to locate the precise genetic cause behind the dental anomaly observed in the two Japanese macaques. However, these cases may hint a possibility of future genetic studies on dental development in non-human primates.
The findings of the present study give a new perspective to studies in human dentistry. According to the conventional view, the congenital loss of molars proceeds from the third molars to the anterior direction, i.e. M3 > M2 > M1 in order (Fujita et al., 1995). The present two cases of Japanese macaques clearly contradict this convention. Previously, if a patient had only two molars erupted on one side of the upper or lower jaw with no diastema between the fourth premolar and the anterior molar, such a case would be conventionally interpreted that the erupted teeth are the first and second molars with the third molar congenitally missing. Taking the results of the present study into account, it is possible that even in modern humans, M1 agenesis with M2/M3 being preserved may occur, but such cases might have been overlooked in the past, because of the ambiguity in determining the molar types (M1, M2, or M3) in human dentition. The new perspective given by the present study in Japanese macaques suggests that we should be more cautious in interpreting individual cases of molar agenesis in studies of human dentistry.
We are grateful to the present and past staff of the Primate Research Institute and the Laboratory of Physical Anthropology of Kyoto University, Tochigi Prefectural Museum, Hakusan Nature Conservation Center (Ishikawa prefecture), Kanagawa Prefectural Museum of Natural History, and Dr Kosei Izawa and the Research Group of Monkeys in Miyagi Prefecture for allowing us to examine the specimens under their care. We thank Mr Seiji Hayakawa for his assistance in taking the X-ray photographs. The present study was financially supported by Grants-in-Aid for Scientific Research (#15570193, #18370098, #16310156) and by the Global COE for Biodiversity and Evolution (Program A06) of Kyoto University.