| * Correspondence to: Hajime Ishida, Department of Human Biology and Anatomy, Faculty of Medicine, University of the Ryukyus, Uehara 207, Nishihara, Okinawa 903-0215, Japan. E-mail: ishidaha@med.u-ryukyu.ac.jp Published online 23 December 2010 in J-STAGE (www.jstage.jst.go.jp) DOI: 10.1537/ase.100928 |
Human nonmetric cranial variations have been described worldwide for a long time, alongside other macroscopic variations in human anatomy (e.g. Poirier, 1896). In Japan, Akabori (1933) reported many nonmetric variations using about 200 recent Japanese crania. Hauser and De Stefano (1989) reviewed the gross anatomy, function, development, and genetics of nonmetric cranial variations on the basis of huge previous anatomical studies.
Physical anthropologists have long studied cranial metric data in order to reconstruct population affinities (e.g. Koganei, 1893; Woo and Morant, 1934; Howells, 1973). On the other hand, the application of nonmetric cranial variations to population studies only became popular in the late 20th century (Laughlin and Jörgensen, 1956; Berry and Berry, 1967; and many others).
Yamaguchi (1967), Dodo (1974, 1986a), Ossenberg (1986), and Mouri (1986) have undertaken refined studies of nonmetric cranial traits, which proved effective for reconstructing the Pan-Pacific population history. Yukio Dodo and his collaborators have intensively studied nonmetric cranial traits from the viewpoint of their stability within populations and diversity among populations (Dodo, 1974, 1986a, b, 1987; Dodo and Ishida, 1987, 1990, 1992; Dodo et al., 1992; Ishida and Dodo, 1993, 1997; Dodo et al., 1998; Dodo and Kawakubo, 2002). On the basis of these series of studies, the nonmetric cranial variations of populations worldwide have been investigated (Ishida, 1993, 1995; Hanihara et al., 1998; Ishida and Kondo, 1999; Hanihara and Ishida, 2001a, b, c, d, e; Komesu et al., 2008; Dodo and Sawada, 2010; Nakashima et al., 2010). Furthermore, the efficacy of nonmetric cranial analysis was confirmed with reference to classic genetic and linguistic research on worldwide human populations (Hanihara et al., 2003).
Population studies have been performed on the assumption that the appearance of nonmetric cranial variations is fairly well controlled by genetic factors (Dodo and Ishida, 1990). In fact, the affinity of Ainu and Okinawa populations suggested by Hanihara (1991) was supported by both genetic studies (e.g. Omoto and Saitou, 1997) and by nonmetric study (Fukumine et al., 2006). However, only a limited number of studies have reported the heritability estimates of nonmetric cranial traits based on human family studies, or human or other primate skeletons (Saunders and Popovich, 1978; Cheverud and Buikstra, 1981a, b; Sjøvold, 1984; Mouri, 1997; Velemínský and Dobisíková, 2005).
Recently, the genetics of normal human morphological characteristics has radically evolved through the use of genome-wide association studies and strategies based on population genomics (e.g. Weedon et al., 2008; Fujimoto et al., 2008; Kimura et al., 2009). For example, variation in the EDAR gene determines the presence of shovel-shaped incisors (Kimura et al., 2009), which is one of the major characteristics in East Asian and American peoples (Hanihara, 1966; Haneji et al., 2007). Although these visible characteristics are easily measured using living samples, there have been few genetic studies on human anatomical variants.
The newest and most advanced computer tomography (CT) and magnetic resonance imaging (MRI) methods can evaluate anatomical variations of living human subjects, and this has already enabled analysis of the anatomy of the bronchial arteries and their relationships to adjacent nerves and veins (e.g. Oshiro et al., 2009; Morita et al., 2010). Here, we preliminarily applied these up-to-date methods to examine the presence or absence of nonmetric cranial variations in order to obtain basic data for genetic analysis.
Two mainland Japanese males, subject 1 (a 53-year-old male) and subject 2 (a 35-year-old male), volunteered for CT imaging. They had no previous history of head or neck surgery. Written informed consent was obtained from both individuals following the regulations of the University of the Ryukyus.
Whole-head CT scans were obtained for the two males with a 320-detector row MDCT scanner (Aquilion One, Toshiba, Tokyo, Japan) using a scanning protocol (120 kV, auto-mA, helical pitch 41.0, small focus, slice thickness; 0.5 × 64 mm) at the University Hospital of the Ryukyus on 22 July 2010.
CT image processing and visualization were carried out using the software Analyze 10.0 (Mayo Clinic, USA). After the three-dimensional reconstruction of CT images, the last author (H.I.) checked for the presence/absence of 23 nonmetric cranial traits defined by Dodo (1974, 1986a). These 23 characteristics are listed in Table 1.
Figure 1 and Figure 2 show the three-dimensional reconstruction of respective cranial sites of the 23 nonmetric cranial variations, mainly using the CT data of subject 2 because cranial sutures tended to be obliterated in subject 1. Almost all traits were absent, unfortunately (Table 1). Neither metopism (characteristic no. 1) nor supraorbital nerve groove (characteristic no. 2) was seen on the external surface of the squamous part of the frontal bone (Figure 1a). The supraorbital foramen (characteristic no. 3) on the supraorbital margin was present on the right side, while the supraorbital notch was present on the left side of both subjects (Figure 1b and c), which shows the unilateral presence of this trait.
 View Details | Figure 1. Three-dimensional CT simulation images of cranial anatomy in the two Japanese male subjects. (a) Neurocranium viewed from right anterior–superior side. (b) Orbitae viewed from frontal side of subject 2. The supraorbital foramen on the right and supraorbital notch on the left of the supraorbital margin are present. (c) Orbitae viewed from frontal side of subject 1. The supraorbital foramen on the right and supraorbital notch on the left of the supraorbital margin are present. (d) Neurocrania viewed from left posterior–superior side. The upper cranium had an Inca bone (incomplete median type, Hanihara and Ishida, 2001a). (e) Neurocranium viewed from right posterior–lateral side. The lambdoid, occipitomastoid, and squamous sutures are seen. No wormian bones are present within them. (f) Basicanium viewed from inferior side. The right condylar canal is present just posterior to the occipital condyle. (g) Basicanium viewed from inferior side. No precondylar tubercles are seen in the front of the foramen magnum. The paracondylar process is not present lateral to the occipital condyle. (h) Intracranial view enables clear visualization of the hypoglossal canal and related structure. (i) Basicranium from inferior side. The inferior aspect of the tympanic part of the temporal bone and the spine of the sphenoid bone are observed. The pterygospinous foramen is not present. |
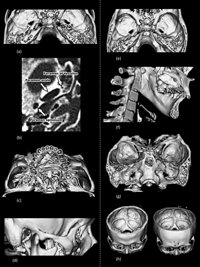 View Details | Figure 2. Three-dimensional CT simulation and axial CT images of cranial anatomy in the two Japanese male subjects. (a) Middle cranial fossa viewed from superior side. The foramina of ovale and spinosum are observed. There is no ovale–spinosum confluence or foramen of Vesalius. (b) Axial CT image at the level of the foramina ovale and spinosum of subject 1 shows the foramen of Vesalius just anterior-medial to the foramen ovale on the left side. (c) The hard palate viewed from the inferior side. Structures of the hard palate cannot be seen owing to noise from dental metal. (d) The zygomatic arch viewed from the lateral side. The temporozygomatic suture is barely visible. The transverse zygomatic suture may not be present. (e) The body of the sphenoid bone viewed from above. The anterior and posterior clinoid processes are observed. There is no clinoid bridging. (f) Inner aspect of the ramus of mandible. The mandibular foramen, lingula, and mylohyoid groove are observed. (g) Posterior cranial fossa viewed from the posterior side. The bilateral jugular foramina with no bridging are seen. (h) Posterior cranial fossa viewed from the antero-superior side. Both grooves for superior sagittal sinus turn to the right. |
From the posterior aspect, the interparietal, or incomplete median Inca bone (Type V, Hanihara and Ishida, 2001a) (characteristic no. 5) was present in subject 1 (Figure 1d, upper). Other wormian bones, including the ossicle at the lambda (characteristic no. 4), biasterionic suture vestige (characteristic no. 6), asterionic bone (characteristic no. 7), occipitomastoid bone (characteristic no. 8), and parietal notch bone (characteristic no. 9), were not found (Figure 1e).
Around the foramen magnum, the left condylar canal (characteristic no. 10) was found just posterior to the occipital condyle, but was not found in the right side (Figure 1f). There were no precondylar tubercles (characteristic no. 11) and paracondylar processes (characteristic no. 12) (Figure 1g). Although we could see the hypoglossal canal (characteristic no. 13) from the inner aspect of the foramen magnum, the bridging was not found in subject 2 (Figure 1h). In the inferior view of the tympanic part of the temporal bone, it was difficult to judge the presence of the tympanic dehiscence (Foramen of Huschke) (characteristic no. 14), as shown in Figure 1i. The spine of sphenoid bone was clearly recognized and the pterygospinous foramen (characteristic no. 16) was not present in this case.
Figure 2a shows the upper view of the foramen ovale and foramen spinosum from the inner side. Because the foramen ovale and foramen spinosum are normally separated by a bony structure, the ovale–spinosum confluence (characteristic no. 15) was not seen. The foramen of Vesalius (characteristic no. 17) was also not recognized in subject 2 (Figure 2a). However, a simple horizontal slice at the level of the foramina ovale and spinosum in subject 1 showed that the foramen of Vesalius was present just anterior to the foramen ovale on the left side (Figure 2b).
Figure 2c represents the inferior aspect of the hard palate and alveolar process. Both subjects had a previous history of dental treatment, and a large amount of noise from metal on the crown disturbed the three-dimensional reconstruction of the hard palate. Therefore, we could not see the medial palatine groove or canal (characteristic no. 18).
The temporozygomatic suture can be faintly seen in Figure 2d. Although we judged that the transverse zygomatic suture vestige (characteristic no. 19) was not present in this case, it may be difficult to identify it. Figure 2e shows the internal cranial fossa from above. The anterior and posterior clinoid processes of the body of sphenoid bone were seen but the middle clinoid process was not, indicating that there was no clinoid bridging (characteristic no. 20) in this case.
The inner aspect of the ramus of mandible is seen in Figure 2f. The mandibular foramen, lingula and mylohyoid groove were completely observed. Unfortunately, the mylohyoid bridging (characteristic no. 21) was not present. A three-dimensional image of the posterior cranial fossa of the superior–posterior aspect was reconstructed (Figure 2g). The jugular foramina, which were continuous from the groove for the sigmoid sinus, were seen, but there was no bridging (characteristic no. 22).
Finally, Figure 2h shows the inner surface of the occipital bone, in which several sinus grooves were present. The groove for the superior sagittal sinus (characteristic no. 23) turned to the right in both subjects.
Bone surface anatomy for 19 nonmetric cranial variations was clearly observed among the 23 variations using this three-dimensional reconstruction from CT images.
The suture variations were not easily detected in the older subject because of suture closure on the external surface. The temporozygomatic suture was not easily observed even in the younger case to score the transverse zygomatic suture. We should improve the X-ray radiation conditions because a clinical study was able to reveal the feature of the temporozygomatic suture (Boeddinghaus and Whyte, 2008).
The two small foramina of the tympanic dehiscence and the foramen of Vesalius could not be detected on the basis of our three-dimensional images. However, we found the foramen of Vesalius on the simple thin-sliced horizontal CT image, as shown in clinical cases (Borges, 2008a). Both three-dimensional and thin-sliced CT images must be combined to find small variations in the foramen.
The hard palate was not seen in these cases because of metal from dental treatments. We need to select other subjects for observation of the hard palate and palatine grooves.
In summary, we would in the future like to examine younger and healthy humans to observe sutures and tiny variations using more refined radiation conditions.
Clinical medicine has investigated not only pathological conditions but also normal variation using CT scanning (Boeddinghaus and Whyte, 2008; Borges, 2008a, b; Goh et al., 2008; Lemmerling et al., 2008; Maroldi et al., 2008). However, because nonmetric cranial variations were often not noticed, this study is quite new.
Nerves and vessels pass through some foramina, including the jugular foramen and hypoglossal canal. The glossopharyngeal, vagus, and accessory nerves pass through the jugular foramen, and the hypoglossal nerve passes into the hypoglossal canal. CT clearly displays bony anatomy while MRI can directly visualize nerves (Alves, 2010; Borges and Casselman, 2010; Kang and Hin, 2010). In order to confirm the existence of the bridging of those foramina, it is better to combine CT and MRI methods simultaneously. In fact, the two subjects underwent a preliminary MRI investigation: the nerves were clearly observed, and no bony bridging was detected (data not shown).
In this study, we definitively showed that cranial anatomy can be used to score nonmetric cranial variations based on CT data from living humans. Thus, these data can be directly compared with huge amounts of genetic data of the same individuals; to date, only osteological analyses have been used to infer heritability (Sanchez-Lara et al., 2007). We will gather much anatomical and genetic data from the same individuals under the approval of our ethical committee to elucidate the morphological background of the human body.
We are deeply grateful to the radiological technologists at the University Hospital of the Ryukyus for radiographic imaging. This study was supported in part by a Grant-in-Aid for Scientific Research (No. 22687023) from the Japan Society for the Promotion of Science.