2013 年 36 巻 11 号 p. 1739-1746
2013 年 36 巻 11 号 p. 1739-1746
Fingolimod (FTY720) is known to have a significant therapeutic effect in various autoimmune disease models. Here, we examined FTY720 in a model of rheumatoid arthritis, induced by immunizing DBA/1 mice with a peptide consisting of residues 325 through 339 of glucose-6-phosphate isomerase (GPI325–339). The efficacy was evaluated in terms of macroscopic findings, inflammatory cell infiltration and autoantibody level. Prophylactic administration of FTY720 from the day of immunization significantly suppressed the development of paw swelling, but therapeutic administration of FTY720 from onset of symptoms on day 8–9 was less effective. Interestingly, however, combination treatment with FTY720 plus GPI325–339 for 5 d after onset of symptoms significantly reduced the severity of symptoms in all mice, and no relapse occurred after booster immunization. Taking into account the reported mechanism of action of FTY720, these results indicate that combination treatment with FTY720 plus pathogenic autoantigen might efficiently induce immune tolerance by sequestering circulating autoantigen-specific lymphocytes from blood and peripheral tissues to the secondary lymphoid tissues. Combination treatment with FTY720 plus pathogenic autoantigen may become a breakthrough treatment for remission-induction in patients with autoimmune diseases including rheumatoid arthritis.
Rheumatoid arthritis (RA) is a refractory autoimmune disease, characterized by synovial hyperplasia, mononuclear cell infiltration, cartilage degradation, joint destruction, pannus formation, and so on.1,2) Biological agents such as anti-tumor necrosis factor (TNF)-α monoclonal antibody (mAb) and anti-interleukin (IL)-6 receptor mAb, that target specific molecules, provide an effective means for therapeutic management of RA due to their specificity and powerful functional capabilities.3) However, such agents suffer from various disadvantages, such as induction of neutralizing antibodies and the occurrence of relapse after discontinuation of the drug. Thus, a therapy able to induce complete remission would be highly desirable.
Fingolimod (FTY720) is a synthetic structural analogue of myriocin (ISP-I), a metabolite of Isaria cinclarii.4,5) The structure of ISP-I was subsequently modified in order to reduce its toxicity, to improve its physicochemical properties and to identify the essential structure for immunosuppressive activity, and a candidate compound, FTY720, was obtained.6) FTY720 was discovered by Tetsuro Fujita, of our group, in collaboration with Taito Co. and Yoshitomi Pharmaceutical Industries, Ltd. in Japan. Its efficacy has been well established in preclinical transplantation models (rat heart, liver, skin, and small intestine, dog kidney and monkey kidney).7) Also, FTY720 has been reported to be effective in preventing the development of immunological diseases in various animal models, including models of multiple sclerosis,8) myasthenia gravis,9) atopic dermatitis10,11) and type 1 diabetes mellitus.12,13) The mechanism of action of FTY720 differs from that of established immunosuppressants, such as tacrolimus hydrate and cyclosporine. FTY720 is converted in vivo by sphingosine kinase to FTY720 monophosphate (FTY720-P), which is the active form of the drug. FTY720-P acts as a high-affinity agonist for sphingosine 1-phosphate (S1P) receptors. FTY720 blocks S1P signaling by inducing receptor internalization and intracellular partial degradation.14–16) As a result, FTY720 suppresses immune response by sequestering circulating mature lymphocytes from blood and peripheral tissues to the secondary lymphoid tissues and thymus. Even though the mechanisms of pharmacological action of FTY720 remain to be fully clarified, it is clear that FTY720 has no inhibitory effect on cytokine production, in contrast to established immunosuppressants.17,18)
In this study, we used glucose-6-phosphate isomerase (GPI) peptide-induced arthritis as an animal model of RA, because the model seems to be akin to human RA. GPI-induced arthritis is induced by immunization of DBA/1 mice with recombinant human GPI.19) GPI-induced arthritis is different from collagen-induced arthritis (CIA), a commonly used animal model of human RA, with regard to the priority of T cells and B cells.20,21) In GPI-induced arthritis, administration of CD4 monoclonal antibody after onset rapidly ameliorates the arthritis, despite the absence of changes in anti-GPI antibody titer. Fragment crystallizable γ receptor (FcγR) γ-chain-deficient mice are resistant to GPI-induced arthritis, and adoptive transfer of purified immunoglobulin G (IgG) antibodies alone is not able to induce arthritis in these mice.20,21) The therapeutic efficacy of biological agents (anti-TNF-α mAb, anti-IL-6 mAb and cytotoxic T-lymphocyte antigen 4-immunoglobulin (CTLA 4-Ig)) on GPI-induced arthritis are similar to those in human RA.21)
As for CIA, autoantibodies are important players. Adoptive transfer of polyclonal IgG antibodies purified from the sera of arthritic mice with CIA can induce arthritis even in mouse strains that are not susceptible to actively induced CIA,22) while, most attempts to induce CIA in mice by T-cell transfer have been unsuccessful and CD4-deficient mice develop CIA with unaltered incidence and severity.23–25) Anti-IL-1 and anti-IL-12 mAbs significantly suppressed arthritis in CIA,26–29) however, anti-TNF-α mAb had little effect,26) and anti-CD20 and anti-IL6 receptor mAbs had no effect on established CIA.21,30,31) Anti-CD4 mAb was ineffective in CIA mice after the mice had produced anti-CII antibody.20,32,33) These findings indicated that the pathophysiological mechanisms of CIA might be different from those of human RA. Thus, it is considered that GPI-induced arthritis is a more suitable model for examining the effect of treatment.21) Moreover, Iwanami et al. reported that the major epitope of CD4+ T cells in GPI-induced arthritis was human GPI325–339, and immunization with this peptide induced severe polyarthritis (designated here as GPI325–339-induced arthritis).34)
It has already been demonstrated that intravenously (i.v.) administration of pathogenic autoantigen ameliorates symptoms by inducing tolerance in another animal model of RA.35) Also, administration of antigen induces rapid accumulation of antigen-specific cells in the parracortical regions of all lymph nodes, and most of the cells then rapidly disappeared, leaving behind a population that was hyporesponsive to antigenic stimulation.36) Thus, antigen-specific memory and tolerance can be influenced by antigen administration.36) We considered that immune tolerance might be efficiently induced in the GPI325–339 model if FTY720 induced efficient sequestration of autoantigen-specific lymphocytes in secondary lymphoid tissues. Based on the above considerations, we hypothesized that combination treatment with FTY720 plus pathogenic autoantigen might be an effective approach for inducing complete remission of RA. In the present study, we tested this idea in the GPI325–339-induced mouse model of RA.
DBA/1 mice bred under specific pathogen-free conditions were purchased from SLC Inc., Shizuoka, Japan. The mice were given γ-ray-irradiated food (CRF-1, Oriental Bio Co., Kyoto, Japan) and distilled water for injection (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan). This study was performed according to Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan, June 1, 2006) and the protocol was approved by the institutional animal care committee of Setsunan University. Throughout the experimental procedures, every effort was made to minimize the number of animals used and their suffering.
Agents2-Amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol hydrochloride (FTY720) was kindly provided by Yoshitomi Pharmaceutical Industries, Ltd. (Japan). Peptide 325(IWYINCFGCETHAML)339 of glucose-6-phosphate isomerase (GPI325–339) was purchased from Operon Biotech. K.K. (AL, U.S.A.).
Preparation of GPI325–339-Induced Arthritis Mouse ModelDBA/1 mice (7-week-old males) were immunized by intracutaneous injection of GPI325–339 (10 µg) with Freund’s complete adjuvant containing Mycobacterium tuberculosis H37Ra (Difco) at the base of the tail. Pertussis toxin (200 ng; PT, Calbiochem, CA, U.S.A.) was injected i.v. on days 0 and 2.34) The clinical symptoms of GPI325–339-induced arthritis in each limb were checked daily and graded on a clinical arthritis score of 0–4, defined as follows: 0, no evidence of erythema and swelling; 1, erythema and mild swelling confined to the tarsals or ankle joint; 2, erythema and mild swelling extending from the ankle to the tarsals; 3, erythema and moderate swelling extending from the ankle to metatarsal joint; 4, erythema and severe swelling encompass the ankle, foot and digits, or ankylosis of the limb.1) The clinical scores of the four limbs were totaled for each mouse, yielding a maximum score of 16.
Administration Schedules of Test DrugsTo examine the prophylactic effect of FTY720 and GPI325–339, mice with GPI325–339-induced arthritis were divided into four groups. The FTY720 group was given FTY720 in water (1.0 mg/kg, orally, six times a week; this dosing regimen is similar to that reported by Tsunemi et al.37)) from the day of immunization with GPI325–339 to day 22. The GPI325–339 group was given GPI325–339 alone in phosphate buffered saline (PBS) (10 µg/mouse, i.v. once daily) from the day of immunization to day 5. The placebo group was given the vehicle alone on the same schedule as the FTY720 group. The non-immunized group was injected with Freund’s complete adjuvant alone.
To examine the therapeutic effect of FTY720 and GPI325–339 on GPI325–339-induced arthritis, the same procedure as above was employed, except for timing of administration. FTY720 was administrated from the day of onset of symptom (day 8–9) to day 22. In GPI325–339 group, the treatment period was 5 d, from the day of onset of symptom.
To examine whether combination treatment with FTY720 plus GPI325–339 would induce immune tolerance, mice with GPI325–339-induced arthritis were divided into four groups. The FTY720 group was given FTY720 alone (1.0 mg/kg, orally, once daily). The GPI325–339 group was given GPI325–339 alone in PBS (10 µg/mouse, i.v. once daily). The combination group received both FTY720 and GPI325–339 at the above dosages, and the placebo group received vehicle alone. In these groups, the treatment period was 5 d, from the time of onset of symptoms (day 9 after immunization) until day 13.
Histochemical StainingOn day 22 after immunization, the front limb joints were removed, fixed in 10% buffered formalin solution (Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 2 d, and decalcified with K-CX (Falma Co., Ltd., Tokyo, Japan) for 7 d. Tissues were processed, embedded in paraffin and cut into 5 µm sections. The sections were stained with hematoxylin–eosin (H&E) using Mayer’s Hematoxylin Soln. (Wako Pure Chemical Industries, Ltd.) or with goat anti-mouse CD3 mAb (Santa Cruz Biotechnology, Inc., CA, U.S.A.). Synovial hyperplasia and lymphocyte infiltration of carpal-metacarpal joints were histologically graded as follows: 1, normal; 2, mild; 3, moderate; 4, severe (histological score).38) The histological score was evaluated blindly by three investigators and mean values were calculated.
Measurement of Anti-GPI325–339 Total IgG Antibody LevelSerum samples were collected for measurement of anti-GPI325–339 antibody level by quantitative colorimetric ELISA. Polystyrene microtiter wells (EIA flat plate-1, Sanko Junyaku Co., Tokyo, Japan) were coated with GPI325–339 (1.0 µg/mL) in 0.1 M sodium phosphate buffer, pH 7.5, containing 0.1% NaN3, at 4°C overnight. After incubation, antigen solution was removed by aspiration, and the wells were washed 4 times with 0.25 mL of 10 mM sodium phosphate buffer, pH 7.0, containing 0.1 M NaCl. The washed wells were incubated with 0.25 mL of 10 mM sodium phosphate buffer, pH 7.0, containing 0.1 M NaCl, 0.1% bovine serum albumin (BSA, Nacalai Tesque Co., Ltd., Kyoto, Japan) and 0.1% NaN3 at 4°C for 3 h. After incubation, the buffer was removed by aspiration, and the wells were incubated with serum samples that had been previously diluted (5000-fold) with the same buffer (0.15 mL), at 37°C for 3 h and 4°C overnight. After incubation, the diluted serum was removed by aspiration, and the wells were washed as above. The washed wells were incubated with goat (anti-mouse IgG H+L) Fab′-horseradish peroxidase conjugate (Medical and Biological Laboratories Co., Ltd., Nagoya, Japan), which had been previously diluted 7500-fold, with 10 mM sodium phosphate buffer, pH 7.0, containing 0.1 M NaCl and 0.1% BSA (0.15 mL), at 37°C for 3 h. Finally, the conjugate solution was removed by aspiration, the wells were washed as above, and peroxidase activity bound to the wells was measured by colorimetry with o-phenylenediamine as a hydrogen donor. The absorbance at 490 nm was measured with a plate reader (Model 450, Bio-Rad Laboratories, Hercules, CA, U.S.A.).
Measurement of Anti-GPI325–339 IgG Subclass Antibody LevelAnti-GPI325–339 IgG subclass (IgG1, IgG2a, IgG2b, IgG3) levels were detected with Mouse Monoclonal Antibody Isotyping Reagents (Sigma-Aldrich Co., LLC, MO, U.S.A.) according to the manufacturer’s instructions.
Statistical AnalysisThe statistical significance of differences was evaluated by using the Mann–Whitney U-test. p<0.05 was considered significant.
In order to examine the prophylactic effect of FTY720, mice with GPI325–339-induced arthritis were treated from the day of immunization to day 22. The clinical arthritis scores during this period are shown in Fig. 1A. In the placebo group, GPI325–339-induced arthritis-associated symptoms were observed at day 7, and became severe within the following week (Figs. 1A, B). In contrast, the administration of FTY720 from the day of immunization significantly suppressed the onset of GPI325–339-induced arthritis to a level similar to that of the non-immunized mice injected with adjuvant alone (Figs. 1A, B). To examine synovial hyperplasia and inflammatory cell infiltration, paraffin-embedded front carpal–metacarpal joints were stained with H&E or anti-mouse CD3 mAb. Synovial hyperplasia and infiltration of CD3+ cell (T cell)-based inflammatory cells were observed in the placebo group, but were significantly suppressed in the FTY720 group (Fig. 2).

Mice with GPI325–339-induced arthritis were treated with FTY720 or GPI325–339 from the day of immunization. (A) Clinical symptoms of arthritis were evaluated from the day of immunization to day 22, ●: the placebo group (n=4), ■: the FTY720 group (n=4, FTY720 1.0 mg/kg, orally, 6 times a week, from the day of immunization to day 22), ▲: the GPI325–339 group (n=7, GPI325–339 10 µg/mouse, i.v. once daily, from the day of immunization to day 5) and ◇: the non-immunized group (n=3). The significance of differences in clinical scores was examined (* (the FTY720 group vs. the placebo group), ♭ (the GPI325–339 group vs. the placebo group) denotes p<0.05). (B) Photographs showing typical paws of non-immunized mice and GPI325–339-induced arthritis mice with or without FTY720 or GPI325–339 treatment, taken on day 22 after immunization.

Mice with GPI325–339-induced arthritis were treated with FTY720 or GPI325–339 from the day of immunization as described in Fig. 1. Then, carpal–metacarpal joint tissues were excised and stained with hematoxylin–eosin (H&E) and anti-CD3 mouse mAb. (A–D): H&E. (E–L): CD3. (A, E, I): placebo mice. (B, F, J): FTY720 treated mice. (C, G, K): GPI325–339 treated mice. (D, H, L): non-immunized mice. A representative image from one mouse in each group is shown. The arrow indicates synovial hyperplasia and inflammatory cell infiltration. (M) Histological score is indicated as the mean+S.D. for all mice in each group (* denotes p<0.05).
In order to examine the therapeutic effect of FTY720, mice with GPI325–339-induced arthritis were treated from the day of onset of symptoms (day 8–9) to day 22. FTY720 reduced the severity of GPI325–339-induced arthritis-associated symptoms (Fig. 3A). The maximum score in the FTY720 group (10.9±5.3, S.D.) was significantly lower than that of the placebo group (15.1±1.0, S.D.). In addition, synovial hyperplasia and lymphocyte infiltration were significantly suppressed as compared with the placebo group (Fig. 3B).

Mice with GPI325–339-induced arthritis were treated with FTY720 or GPI325–339 from the day of onset of symptom (day 8–9). (A) Clinical symptoms of arthritis were evaluated from the day of immunization to day 22, ●: the placebo group (n=8), ■: the FTY720 group (n=10, FTY720 1.0 mg/kg, orally, 6 times a week, from the day of onset of symptom to day 22), ▲: the GPI325–339 group (n=11, GPI325–339 10 µg/mouse, i.v. once daily, from the day of onset of symptom to day 5) and ◇: the non-immunized group (n=3). The significance of differences in clinical scores was examined (* (the FTY720 group vs. the placebo group), ♭ (the GPI325–339 group vs. the placebo group) denotes p<0.05). (B) Histological score is indicated as the mean+S.D. of all mice in each group (* denotes p<0.05).
Next, to examine the effects of FTY720 on GPI325–339-specific antibody production, serum samples were collected from mice in all groups on days −1, 8, 15 and 22 after immunization, and the anti-GPI325–339 antibody level was measured by quantitative colorimetric enzyme-linked immunosorbent assay (ELISA). In the FTY720 group, the production of anti-GPI325–339 total IgG, IgG2a, IgG2b and IgG3 was significantly reduced as compared with the placebo group (FTY720 group vs placebo group on day 22: total IgG, 1.28±0.72 vs. 2.65±0.93; IgG2a, 0.69±0.29 vs. 1.35±0.27; IgG2b, 1.35±0.58 vs. 2.05±0.65; IgG3, 0.23±0.18 vs. 0.49±0.22), while, no difference was observed in IgG1 level between FTY720 group (1.41±0.69) and placebo group (1.40±0.60) (Fig. 4). These results suggested that FTY720 might suppress the severity of symptoms by blocking synthesis of the complement-fixing IgG subclasses IgG2a, IgG2b and IgG3. However, FTY720 showed only a partial effect and was less effective than previously reported agents (anti-TNF-α mAb, anti-IL-6 mAb and CTLA 4-Ig).21)
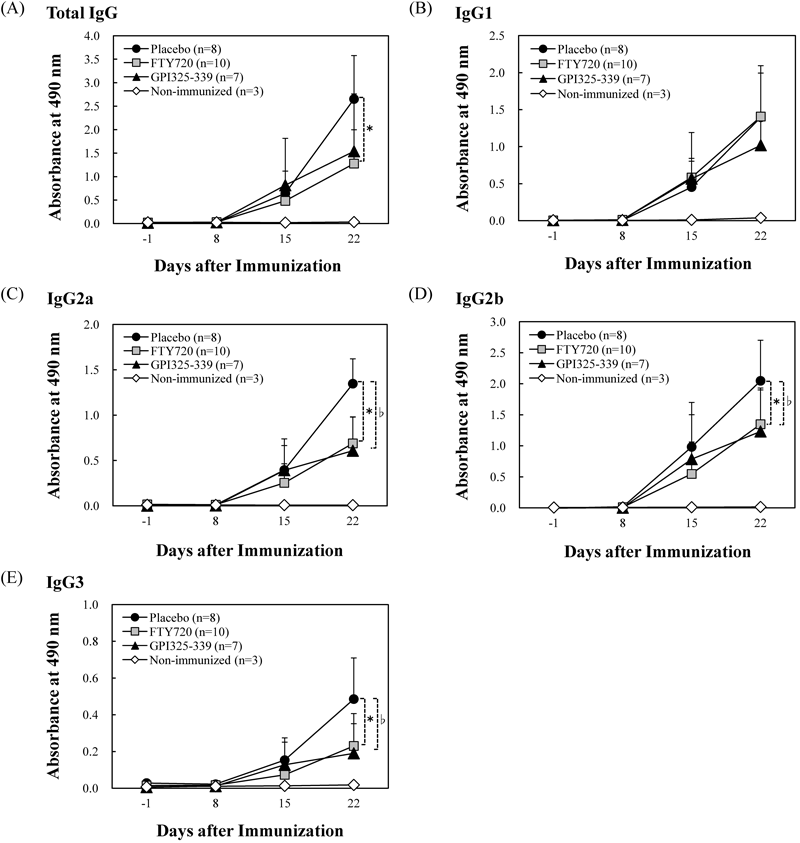
Mice with GPI325–339-induced arthritis were treated with FTY720 or GPI325–339 from the day of onset of symptoms (day 8–9). On days −1, 8, 15 and 22 after immunization, peripheral blood samples were collected, and the levels of anti-GPI325–339 IgG antibody subclasses in serum were measured by means of enzyme-linked immunosorbent assay (* denotes p<0.05), ●: the placebo group (n=8), ■: the FTY720 group (n=10, FTY720 1.0 mg/kg, orally, 6 times a week, from the day of onset of symptom to day 22), ▲: the GPI325–339 group (n=7, GPI325–339 10 µg/mouse, i.v. once daily, from the day of onset of symptom to day 5) and ◇: the non-immunized group (n=3). The significance of differences in antibody level was examined (* (the FTY720 group vs. the placebo group), ♭ (the GPI325–339 group vs. the placebo group) denotes p<0.05).
It has already been demonstrated that i.v. administration of pathogenic autoantigen ameliorates symptoms by inducing tolerance in another animal model of RA.35) Thus, we first examined whether treatment with the pathogenic autoantigen GPI325–339 would suppress the development of GPI325–339-induced arthritis-associated symptoms by inducing immune tolerance. Prophylactic administration of GPI325–339 significantly suppressed the severity of symptoms (Figs. 1A, B). However, therapeutic administration of GPI325–339 showed only a partial effect (Figs. 3A, B). These results indicated that the tolerance is easily induced in naive animals, but primed animals are more resistant to the suppressive effect of pathogenic autoantigen administration.
Combination Treatment with FTY720 Plus GPI325–339 Ameliorates Symptoms of GPI325–339-Induced ArthritisWe previously reported that combination treatment with FTY720 plus pathogenic autoantigen could efficiently induce complete remission in experimental autoimmune encephalomyelitis (EAE), which is an animal model for multiple sclerosis.8) Thus, we considered that combination treatment with FTY720 plus GPI325–339 might ameliorate the severity of the symptoms of GPI325–339-induced arthritis. Indeed, the combination treatment for 5 d from the day of onset markedly reduced the severity of symptoms in all animals (n=8) (Fig. 5A). The combination treatment was more effective than administration of FTY720 alone or GPI325–339 alone (Fig. 5A). Synovial hyperplasia and inflammatory cell infiltration were also markedly suppressed in the combination group (Fig. 5B). Next, we examined whether or not aggravation of symptoms appeared after discontinuation of treatment. No clinical deterioration was observed in any of the animals (n=4) up to 4 weeks after discontinuation of treatment (data not shown). It has been reported that a booster immunization of recombinant GPI after resolution of arthritis induced a relapse in recombinant GPI-induced arthritis.39) Mice attained remission with the combination treatment were injected as a booster with GPI325–339 (10 µg) with Freund’s incomplete adjuvant at 12 d after discontinuation of treatment (booster immunization), and clinical symptoms were evaluated. No clinical deterioration was observed until 49 d after the booster immunization in all animals (n=3) in the combination group (data not shown).

Mice with GPI325–339-induced arthritis were treated from the day of onset of symptom (day 9 after immunization) to day 13 with GPI325–339 alone, FTY720 alone or the combination of GPI325–339 with FTY720. (A) Clinical symptoms of arthritis were evaluated from the day of immunization to day 22, ●: the placebo group (n=11), ■: the FTY720 group (n=8, FTY720 1.0 mg/kg, orally, once daily), ▲: the GPI325–339 group (n=11, GPI325–339 10 µg/mouse, i.v. once daily) and ◆: the combination group (n=8, FTY720 1.0 mg/kg, orally, once daily and GPI325–339 10 µg/mouse, i.v. once daily). The significance of differences in clinical scores was examined (* (the placebo group vs. the combination group), ♭ (the FTY720 group vs. the combination group) and # (the GPI325–339 group vs. the combination group) denotes p<0.05). (B) Mice with GPI325–339-induced arthritis were treated, as in (A). Histological score is indicated as the mean+S.D. of all mice in each group at day 22 (* denotes p<0.05).
The novel immunomodulator fingolimod (FTY720) exerts its immunosuppressive effect via a different mechanism from those of established immunosuppressants, such as tacrolimus hydrate, and cyclosporine. FTY720 suppresses immune response by sequestering circulating mature lymphocytes from blood and peripheral tissues to the secondary lymphoid tissues and thymus. At therapeutic doses, FTY720 does not affect T or B cell responses in vitro or in vivo.7,10)
RA is characterized by persistent synovitis, systemic inflammation, and autoantibodies.2) A key feature of the inflammatory response is overexpression and overproduction of TNF, arising from interactions between T and B cells.2,40) In addition, a pathological study showed that most of the lymphocytes infiltrating into the synovium in RA are CD4+ (helper) T cells.41) Thus, we hypothesized that FTY720 might be effective for treatment of rheumatoid arthritis by inducing immune suppression via sequestration of circulating mature lymphocytes from blood and joints. Our present results confirmed that the onset of GPI325–339-induced arthritis was significantly inhibited by prophylactic administration of FTY720. Therapeutic administration of FTY720 also suppressed the progression of symptoms of GPI325–339-induced arthritis, as well as synovial hyperplasia and lymphocyte infiltration.
It has been reported that FcγR γ-chain-deficient mice were protected from GPI-induced arthritis, whereas FcγRIIB-deficient mice developed exaggerated arthritis.19) Also, Tanaka-Watanabe et al. reported that complement activation by immune complex was observed in joints of mice with GPI-induced arthritis, and they suggested that local immune complex (GPI-anti-GPI antibodies) activation in the joints also plays an important role in GPI-induced arthritis.42) Here, we demonstrated that administration of FTY720 suppressed the production of the complement-fixing IgG subclasses (IgG2a, IgG2b and IgG3) in GPI325–339-induced arthritis and also confirmed that the complement-fixing IgG subclasses titer was correlated with clinical score (data not shown). Thus, FTY720 might suppress the severity of symptoms by preventing the synthesis of the complement-fixing IgG subclasses, although further study will be needed to establish in detail the mechanism(s) through which changes in autoantibody titer are associated with suppression of the severity of symptoms. However, FTY720 showed only a partial effect, and was less effective than other biological agents. In recipients of FTY720, reductions in circulating T lymphocytes are predominantly seen in the lymphoid homing receptor-expressing naïve T cells and central memory T cells. In contrast, effector memory T cells typically lack expression of CCR7, and thus do not recirculate regularly through lymph nodes.43) Also, examination of blood CD4+ T cells from recipients of FTY720 indicates that the effector memory T cells that are still present in the circulation remain functionally responsive.43) This may be the reason why immune suppression was easily induced in non-immune mice, but primed animals were resistant to the suppressive effect.
It has already been demonstrated that i.v. administration of pathogenic autoantigen ameliorates the severity of RA in another animal model by inducing tolerance.35) As described in the Results, we demonstrated that prophylactic administration of GPI325–339 significantly suppressed the severity of symptoms. However, therapeutic administration was less effective. This result indicated that tolerance was easily induced in naïve mice, but primed mice were relatively resistant to the suppressive effect of pathogenic autoantigen.
As described in the Introduction, Kearney reported that administration of pathogenic autoantigen induces immune tolerance in the parracortical regions of all lymph nodes.36) Thus, we considered that immune tolerance might be efficiently induced if sequestration of autoantigen-specific lymphocytes to secondary lymphoid tissues was induced by FTY720. In the present study, we investigated the effect of combination treatment with FTY720 plus GPI325–339. Administration of GPI325–339 alone or FTY720 alone was relatively ineffective, but combination treatment with FTY720 plus GPI325–339 from the onset of symptoms strongly suppressed the severity of arthritis. Also, no clinical deterioration was observed on booster immunized-mice in combination group. To understand the mechanism(s) of this combination treatment, the anti-GPI325–339 antibody level was measured. However, the antibody titer was not correlated with the clinical score (data not shown). Thus, the mechanism(s) underlying the combination treatment was not related to only suppression of the antibody production. We considered that the combination treatment might influence not only B-cell function but also T-cell function.
We previously reported that relapse of EAE, which is an animal model for multiple sclerosis, occurred within one week after discontinuation of FTY720, with an increase in the number of lymphocytes infiltrating the spinal cord and demyelination.8) The timing of relapse was around the same time that lymphocyte recirculation commenced after the discontinuation. Interestingly, the relapse following discontinuation of treatment was completely suppressed by the combination treatment with FTY720 plus pathogenic autoantigen.8) The combination treatment might have induced anergy of autoantigen-specific T cells responsible for the relapse on EAE. In this study, the similar results were obtained by using GPI325–339-induced arthritis model, and revealed generality of the combination treatment. Thus, we consider that the combination treatment might efficiently induce immune tolerance by sequestering circulating autoantigen-specific lymphocytes in secondary lymphoid tissues and inducing cell tolerance. Further studies will be needed to establish in detail the mechanism(s) through which immune tolerance is induced by the combination treatment with FTY720 plus autoantigen. Possible mechanisms include induction of immunological unresponsiveness, modulation of regulatory T cells and induction of apoptosis of autoantigen-specific T cells.
In conclusion, our results suggest that combination treatment with FTY720 plus pathogenic autoantigen may become a breakthrough remission-induction therapy for autoimmune diseases including RA. Further investigation seems warranted.
This work was supported in part by a Grant-in-Aid for Young Scientists (B) (24790483 and 24791093) from the Japan Society for the Promotion of Science and by Watanabe Memorial Foundation for The Advancement of New Technology.