2014 年 37 巻 11 号 p. 1803-1810
2014 年 37 巻 11 号 p. 1803-1810
Osteoarthritis (OA) is a worldwide disease in aged people, causing not only physical suffering to the patients themselves, but also a great burden on their families and on society. Here we used a mouse OA model induced by destabilization of the medial meniscus (DMM), and studied the therapeutic effect of recombinant human midkine (rhMK) on this OA model. Our results indicated that the DMM surgery induced mechanical allodynia and locomotor activity obstacles, together with cartilage injury in the C57BL/6 mice. The rhMK treatment mitigated the OA related mechanical allodynia, improved locomotor activity capacity, and prevented degradation of the cartilage. Considering the safety issue of rhMK used as a biologic, we also inspected the main organs in the rhMK treated mice throughout the process and found no pathological change. These results suggest that rhMK could be used as a biologic to treat OA or OA related pain.
Osteoarthritis (OA) was earlier thought to be a disease with joint articular cartilage loss reflected by radiographic joint space narrowing, but recently most researches demonstrate OA as a whole joint disease with both destruction of cartilage and inflammatory components (e.g., synovitis and bone marrow lesions).1) Pain is the main clinical symptom of OA, which is also the foremost reason cause the disability. Pain in OA, like other pain, is a complex integration of sensory, affective and cognitive processes,2) however, being different from the normal physiological pain which plays important role for the body defense of tissue injury,2,3) the pain in OA is a pathophysiological state (neuropathic pain)3,4) involving the sensitization of the peripheral and central nervous system induced by the destruction of the homeostasis and low grade chronic inflammation inside the joint.2,3,5) Until now, the therapeutic drug for pain of OA is mainly focused on non-steroidal anti-inflammatory drugs (NSAIDs), steroids and opioid analgesics, which only attenuated the pain of partial OA patients, even with severe side effects.2) Developing drugs focus on the etiological factors, the degraded articular cartilage and pathologically altered nervous system, should be the fundamental therapy for pain of OA or OA itself.2)
Midkine (MK) is a heparin-binding growth factor, whose another family member is pleiotrophin (PTN). MK and PTN are known for having multi-biological functions, involving nerves6) and skeletal7) system development, injured tissue repair (e.g., ischemic myocardial8–10) and brain11,12) injury, bone fracture13)) and regeneration (e.g., liver,14) skeletal muscle15) and hematopoietic stem cells16)), immune regulation,17) inflammation18,19) and tumorigenesis.20–22) MK and PTN not only play an important role in nervous system formation at the embryo stage6) but also maintain its functions at the adult stage by protecting the injured neuron from apoptosis, preventing degradation of the peripheral and central nerves and promoting the regeneration and new synapses formation of the neuron.23) MK also have the potency for cartilage regeneration: in vitro promoting the chondrocyte proliferation and cartilage related extracellular matrix expression24,25) and in vivo increasing the thickness of joint cartilage.24)
Here we studied the therapeutic effect of the recombinant human midkine (rhMK) on a mouse OA model induced by destabilization of the medial meniscus (DMM).26) The DMM OA model was recognized as mimicking the clinical OA well primely for its long pathological process and pain features.26,27) We found that intraperitoneal injection of rhMK significantly attenuated the mechanical allodynia, improved the capacity of activity and prevented the degradation of the cartilage without harmful effects on the main organs in the C57BL/6 mice after DMM surgery.
Eight weeks old male C57BL/6 mice were bought from Slrc Laboratory Animal Co. (Shanghai, China), and fed for another 2 weeks under the condition of 21°C (±2°C), 12 h cycles of light and dark (7:00 a.m. to 7:00 p.m.), and food and water ad libitum in the animal center of school of pharmacy, Shanghai Jiao Tong University (Shanghai, China). All animal experiments have been approved by the Animal Ethical Committee of Shanghai Jiao Tong University. 120 mice were randomly assigned into four groups: I. Performing the DMM surgery, and then after 18 weeks duration the mice were treated with rhMK (DMM+rhMK); II. Performing the DMM surgery, and then after 18 weeks duration treated with phosphate buffered saline (PBS) (DMM+PBS); III. Sham surgery without any treatment (Sham) and IV. Naive group without any treatment (Naive).
DMM SurgeryThe DMM surgery was carried out according to the method described by Glasson et al.26) Briefly, the mouse was anesthetized by intraperitoneally injection of pentobarbital (50 mg/kg body weight), and the ventral portion of the right knee was unhair using depilatory paste and sterilized with antiseptic solution. The surgical process was performed under a dissecting microscope. Using surgical blade (11#) to incise the skin along the ventral midline of the right knee, then the joint cavity was opened through the standard medial parapetellar surgical approach. Stretching the suture line which transpassed the patellar ligament and medial collateral ligament to get a good view of the cavity and using ophthalmic surgery blade to incise the medial meniscotibial ligament. After that, a forceps was used to touch the anterior half of the medial meniscus to confirm its clear displacement form the tibial plateau. After surgery, the joint cavity and the skin was closed with absorbable and nonabsorbable fine sutures respectively. The sham surgery process was the same as the DMM surgery without the incising of the medial meniscotibial ligament.
Mechanical Allodynia MeasurementMechanical allodynia was tested by measuring the 50% paw withdrawal thresholds (PWT) to calibrated Von Frey filaments (Stoelting Co., Illinois, IL, U.S.A.) using the up-down method.28) The test was executed during the day time (7:00 a.m.–7:00 p.m.). Placing the mice into a plastic cage with a framed metal mesh bottom and allowing the mice to accommodate the circumstance for 30 min, 8 serial Von Frey hairs (0.02 g, 0.04 g, 0.07 g, 0.16 g, 0.4 g, 0.6 g, 1.0 g, 1.4 g) were chosen to do the test. The 0.16g hair as the initial trial was applied perpendicular to the mid-plantar surface of the hind paw for 6–8 s with a force that can bend the hair. A positive response was defined as a fast withdrawal of the hind paw upon stimulation. Repeating stimulation needs at least 5 min interval allowing the previous stimulus effect vanish. Whenever a positive response to a stimulus occurred, the next hair of descending weight was applied, and whenever a negative response occurred, the nest hair of ascending weight was applied. Test was consisted of five more stimuli after the first occurrence of a change in response, and the pattern of response was converted to a 50% PWT using the method described by Dixon.28)
Locomotor Activity AssessmentThe spontaneous locomotor activity test was executed during the night time (7:00 p.m.–7:00 a.m.) in the locomotor apparatus (Taimeng, Chengdou, China). The mice were placed into the individual chamber with infrared array sensors for 12 h in a dark and quiet circumstance. The movement number and standing number were recorded automatically.
rhMK Protein Production and TreatmentA DNA sequence (GenBank NM_001012334) encoding the mature human MK (hMK) protein was expressed in E. coli BL21 (DE3) using pET30a (+) vector (Novagen, Beijing, China) as described previously.29) Briefly, isopropylthio-b-D-galactoside (IPTG) was used to induce the expression of rhMK at the final concentration of 1 mM. The midkine was efficiently expressed as the form of inclusion bodies. After expression, bacteria were harvested and sonicated. The inclusion bodies were pelleted and dissolved in 6 mol/L guanidine HCl. The solubilized proteins were refolded by dropwise dilution into a defined protein folding buffer. After centrifuging (18000 rcf) at 4°C for 30 min, the refolded protein supernatant was purified using a S-Sepharose column (Amersham, Shanghai, China), and the Column fractions were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot. The purity of the purified protein was 98% as assessed by reverse phase high-performance liquid chromatography, and proved to be biologically active in the murine NIH3T3 cell line proliferation assay. The purified protein was passed through a sterile 0.22 µm filter and stored at −80°C. The endotoxin level of the final formulated rhMK protein solution was 0.03 EU/mg protein.
For rhMK treatment of the OA mice induced by DMM surgery, 300 μg/kg body weight of rhMK in 100 μL PBS was peritoneally injected once a day for 2 weeks.24)
Histological Staining and Scoring of PathologyMice were sacrificed by cervical dislocation following isolation of the whole knee joint. After 24 h fixation in the 4% paraformaldehyde prepared in PBS at 4°C, the sample was washed for 2 h with flowing water and then decalcified in 10% ethylenediaminetetraacetic acid (EDTA) (pH 7.4) solution with agitation at 4°C for 2 weeks with changing the decalcification solution every 3 d. Using syringe needle to check the sample with easily penetrating of the bone portion means perfect decalcification. After washing with milliQ water, the sample was processed by the conventional paraffin section procedure. The section was performed along the dorsoventral direction of the joint with thickness of 6 µm. The slide was stained with 1% toluidine blue for 10 min and then counterstained with hematoxylin and eosin (H–E staining). For each knee, 5 slides of the middle portion was chosen to do the scoring of pathology on the optical microscope (Nikon, Japan). Modified mankin score30) which focused on the structure, cellularity, matrix staining and tidemark integrity of the knee was used to do the evaluation of the pathology (Supplement, Table I).
Statistical AnalysisTwo-way repeated measures ANOVA analysis followed by least significant difference (LSD) post-hoc test was used for the repeated measures data (mechanical allodynia and locomotor activity) and Mann–Whitney test was used for the no-parametric mankin score analysis using SPASS 18.0 software (p<0.05 was set as the level of statistical significance).
The whole experimental process was schemed as Fig. 1, 10 weeks old C57BL/6 mice were used for the DMM surgery, and after 18 weeks duration, the mice were treated with rhMK (300 µg/kg) once a day for 2 weeks. Set the time when the rhMK treatment was done as time 0 weeks (0 w), choose the time points −6, −4, −2, 0, 2, 4, 6, 8, 10 w to test the mechanical allodynia and locomotor activity behavior, and at certain times points (−5, 3, 6, 10 w), certain number of mice was sacrificed for detection of the pathology of the knee and other organs.
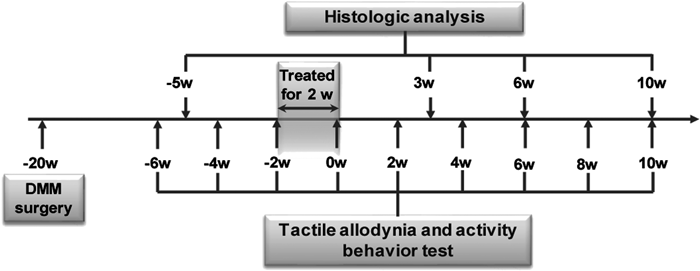
Ten weeks old C57BL/6 mouse was used to build the OA model through surgical destabilization of the medial meniscus (DMM). Eighteen weeks after the model building, the mouse was treated with rhMK (300ug /kg of body weight) for 2 weeks through intraperitoneal injection. Set the time when the rhMK treatment was done as time 0 weeks (0 w), mechanical allodynia, activity behavior and histology of the joint were detected at different time points.
The DMM surgery induced OA in C57BL/6 mice was reflected by the disorders developed after the surgery (Fig. 2). The mechanical allodynia reflected by 50% PWT (Figs. 2a–c) and locomotor activity reflected by movement No. (Fig. 2d) and standing No. (Fig. 2e) were repeatedly measured at −6, −4, −2 weeks (before rhMK treatment, corresponding to 14, 16 and 18 weeks after DMM surgery). For the DMM model group, 50% PWT of the right paw (ipsilateral side with DMM surgery) was significantly lower than the left paw (contralateral side without DMM surgery) (Fig. 2c). In addition, for the ipsilateral paw, 50% PWT of the DMM model group was significantly lower than that in the Sham and Naive group while there was no significant difference between the Sham and Naive group (Fig. 2a), however, similar result was found for the contralateral paw (Fig. 2b) even without DMM surgery. These results indicated that the DMM surgery induced mechanical allodynia in the mice, and this allodynia occurred not only on the ipsilateral paw, but also on the contralateral side. The locomotor behavior assessment showed that the movement No. (Fig. 2d) and standing No. (Fig. 2e) of the DMM model group in 12 h duration was significantly lower than that in the Sham and Naive group, which means the DMM surgery induced decrease of the locomotor activity on the operated mice. Corresponding to the behavior outcomes, the pathological analysis (Fig. 2f) of the ipsilateral knee 15 weeks after DMM surgery showed roughness on the surface of the cartilage with loss of chondrocyte and irregular arrangement. The tidemark was not integrity and some of the chondrocyte performed hypertrophic and vacuolated conversion.

Fourteen, sixteen and eighteen weeks (corresponding to −6, −4 and −2 weeks, before rhMK treatment) after the DMM surgery, the allodynia (50% paw withdrawal threshold (PWT)) (a, b and c), activity behavior (standing No. and movement No. in 12h) (d and e) was measured repeatedly. The allodynia of the right paw (ipsilateral) (a) indicated that the 50% PWT of DMM model group was significantly lower than that in the Sham and Naive group, while there was no difference between the Sham and Naive group. Similar result happened on the left paw (contralateral) (b), and the 50% PWT of the ipsilateral side was significantly lower than that in the contralateral side in the DMM group (c). The standing activity of the DMM model group was significantly lower than that in the Sham and Naive group, while there was no difference between the Sham and Naive group (d). Similarly, the movement activity (e) of the DMM model group was significantly lower than that in the Sham and Naive group, while there was no difference between the Sham and Naive group. The histological analysis of the joint cartilage after 15 weeks DMM surgery (f) indicated that there was trauma on right surgical joint cartilage surface, the chondrocyte was disorganized, missing and hypertrophic, the tidemark was partially lose. For allodynia measurement N=8; for activity measurement N=6; two way repeated measures ANOVA analysis; * p≤0.05, ** p≤0.01.
After confirming that the DMM induced allodynia and activity obstacle along with cartilage pathological alteration in the mice, we treated the DMM induced OA mice with rhMK (300 µg/kg, intraperitoneally) for 2 weeks (Fig. 1) and the therapeutic effect of the rhMK on the activity and mechanical allodynia was tested repeatedly. The rhMK treatment (DMM model+rhMK) increased the PWT of the ipsilateral side to as high as the Naive and Sham group and continuously kept this level to 10 weeks after treatment; the 50% PWT of the rhMK treatment group was significantly higher than that in the PBS treatment group (Fig. 3a). Similar effect happened on the contralateral side paw (Fig. 3b). The standing No. in 12 h increased after rhMK treatment and slightly decreased until 8 weeks after treatment (Fig. 3c). There was significant difference between the rhMK treatment group (DMM model+rhMK) and PBS treatment group (DMM model+PBS). Correspondingly, the movement No. in 12 h increased after rhMK treatment and slightly decreased 8 weeks after treatment, and the 12 h movement No. in the rhMK treatment group (DMM model+rhMK) was significantly higher than that in the PBS treatment group (DMM model+PBS) (Fig. 3d). Over all, these results meant that the rhMK treatment attenuated the allodynia and recovered the activity behavior on the DMM OA mice.
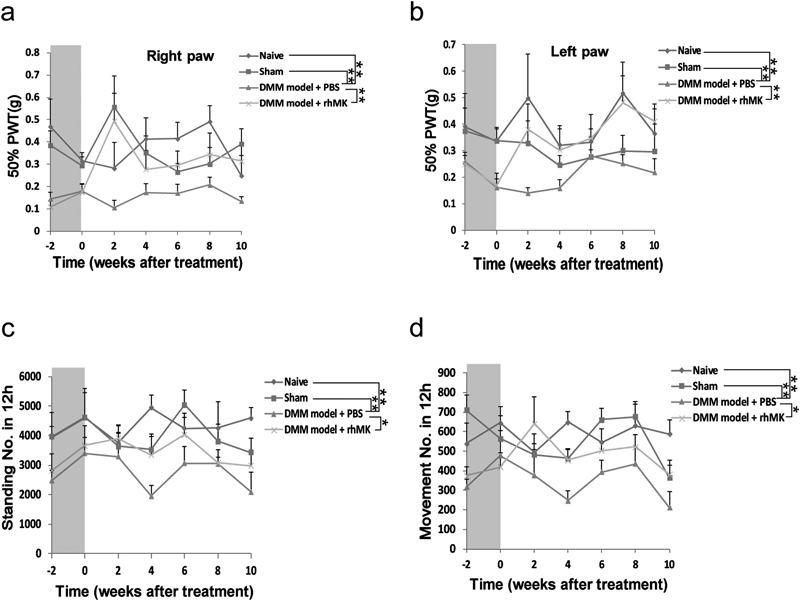
The mice with DMM was treated with rhMK for 2 weeks (gray column), the allodynia (a, b) and activity (c, d) was detected at the time point (−2, 0, 2, 4, 6, 8, 10 weeks), repeatedly. (a) After rhMK treatment (DMM model+rhMK), the 50% PWT of the right paw increased to as high as that in the Naive and Sham group and continuously kept this level to 10 weeks after treatment; the 50% PWT of the rhMK treatment group was significantly higher than that in the PBS treatment group (DMM model+PBS). Similarly, the 50% PWT of the left paw (b) increased up to as high as that in the Sham and Naive group after rhMK treatment (DMM model+rhMK). Comparing with the PBS treatment group, the 50% PWT of the rhMK treatment group (DMM model+rhMK) was significantly higher than that in the PBS treatment group (DMM model+PBS). (c) The standing capacity increased after rhMK treatment (DMM model+rhMK), and slightly decreased until 8 weeks after treatment. There was significant difference between the rhMK treatment group (DMM model+rhMK) and PBS treatment group (DMM model+PBS). (d) Correspondingly, the movement capacity increased after rhMK treatment and slightly decreased 8 weeks after treatment. The 12 h movement No. in the rhMK treatment group (DMM model+rhMK) was significantly higher than that in the PBS treatment group (DMM model+PBS). For allodynia measurement N=8; for activity measurement N=6; two way repeated measures ANOVA analysis; * p≤0.05, ** p≤0.01.
Pathological evaluation of the ipsilateral knee was quantified using mankin score at different time points (3, 6 and 10 weeks) after rhMK treatment (Fig. 4). For Sham and Naive group, there were no obvious pathological changes happened during all the time; for the modeling groups, the joint injury was become severe along with the time going, while there was difference happened between the PBS and rhMK treatment. At the 3 weeks time point, the joint cartilage injury (cartilage surface fibrosis, crack and fragment, chondrocyte lose and disintegrity of the tidemarker) appeared in the rhMK treatment and PBS treatment group (Fig. 4a); while the chondrocyte cellularity and cartilage matrix staining of the rhMK treatment group was better than the PBS treatment group (Fig. 4a); cellularity mankin score of the rhMK treatment group was significantly lower than that in the PBS treatment group (Fig. 4b). Six weeks after treatment, the cartilage injury of rhMK and PBS treatment group was deteriorated (Fig. 4a), while comparing with the PBS treatment group, the mankin score of the rhMK treatment group was significant lower; and this showed at the better performance of the chondrocyte cellularity and matrix staining in the rhMK treatment group (Fig. 4b). At the 10 week time point, joint cartilage was severely degenerated without cartilage matrix staining in the PBS treatment group (Fig. 4a); while for the rhMK treatment group, the cellularity mankin score of the rhMK treatment group was significant lower than that in the PBS treatment group (Fig. 4b). These results indicated that the rhMK treatment mitigated the pathological deterioration of the joint cartilage induced by DMM, which is especially performed at the cellularity and matrix staining of the joint cartilage.

Eighteen weeks after the DMM surgery, the mice were treated with rhMK for 2 weeks, then the mice were sacrificed at the time points (3, 6 and 10 weeks after the rhMK treatment, see the experiment process Fig. 1) and the right joint (with DMM surgery) was isolated. The paraffin section was stained with toluidine blue and counterstained with H–E (a), the histopathological analysis was quantified using the Mankin score (b). At the 3 week time point, the joint cartilage trauma, (cartilage surface fibrosis, crack and fragment, chondrocyte lose and disintegrity of the tidemarker) appeared in the rhMK treatment and PBS treatment group; while the chondrocyte cellularity and cartilage matrix staining of the rhMK treatment group was better than the PBS treatment group; cellularity mankin score of the rhMK treatment group was significantly lower than that in the PBS treatment group. Six weeks after treatment, the cartilage of rhMK and PBS was severely damaged, while comparing with the PBS treatment group, the mankin score of the rhMK treatment group was significantly lower; and this showed at the better performance of the chondrocyte cellularity and matrix staining in the rhMK treatment group. At the 10 week time point, the joint cartilage of the rhMK treatment and PBS treatment group was extremely damaged; for rhMK treatment group, the joint cavity was chondrified, and the neonatal chondrocytes and cartilage matrix was disorganized; for the PBS treatment group, joint cartilage was severely degenerated without cartilage matrix staining; the cellularity mankin score of the rhMK treatment group was significantly lower than that in the PBS treatment group. Non-parametric test: Mann–Whitney test; for Naive and Sham group N=5, for PBS and rhMK group N=10; * p≤0.05, ** p≤0.01.
Another outcome which needs to be noted is that there was no difference in the structure and tidemark of the cartilage between the PBS and rhMK treatment group (Fig. 4b), and the joint cavity of the rhMK treatment group was chondrified (Fig. 4a). One explanation for this might be that rhMK promotes the regeneration of the cartilage,24,25) while the etiology, destabilized medial meniscus always exist during the process and make the neonatal chondrocytes and cartilage matrix disorganized.
Safety Evaluation of the rhMK TreatmentOne of the bioactivity of the rhMK is oncogenesis, and a lot of researches indicate that rhMK expression has strong correlation with the invasion and malignancy.22,31) After the rhMK treatment, we inspected the main organs (liver, spleen, kidney, heart, lung, small intestine) of the sacrificed mice at different time points using the conventional paraffin section with H–E and PCNA staining (Supplement data). The results showed that no pathological changes, abnormal cell proliferation and hyperplasia were found on these organs over the 30 weeks experimental duration. The body weight growth curve also showed there was no difference between each group (rhMK treatment, PBS treatment, Sham and Naive). These results meant that rhMK at the dose of 300 μg/kg body weight with intraperitoneally injection once a day for 2 weeks couldn’t cause any pathological or carcinomatous changes on the main organs.
OA is a common disease in elderly people giving great burden to the patients, families and society.32,33) The main clinical syndromes of OA include radiographic changes (joint space width increasing with cartilage layer thinning), pain and physical disability.32,34) Although some researches show that there is no obvious relations between the radiographic change and the pain or physical disability (e.g., 50% patients with radiographic change don’t have pain, while 50% patients with pain don’t have radiographic change32)), but for the patients with both pain and radiographic change features, the degree of the pain and disability does have correlation with the severity of the joint cartilage injury.2) More researches2–4,35) indicate that OA occurrence and development is a long-term process with that the long-lasting knee injury or un-rational mechanical force induced dysfunction of the joint, which leading to the turn-over of the cartilage and bone and even the pathologic remodeling of vascular and peripheral nerve system of the joint, and this will further backward deteriorating the function of the joint over again. Once these pathological changes surpass a certain threshold, the OA is irreversible without any intervention.
Numerous OA animal models have been reported,36–38) but most models have short-term pathological process and just focus on the joint cartilage lesion without noticing the clinical pain and activity obstacle. Here we used the mouse DMM OA model which more closely resembles not only the more slowly-progressing, but also the pain and physical disability syndromes of the human OA.26) The mice DMM OA model was first developed by Morris group,39) and it’s been popularly used for studying the OA associated chronic pain40,41) and pathological mechanism42–47) and identifying OA related genes in genetically modified mice.48–54) In the DMM OA model on C57BL/6 mice, the DMM induced mechanical allodynia and activity obstacle coincidently with cartilage injury was found after 15 weeks of DMM surgery. Similar results also demonstrated by other groups,26,39,55) but in their research, the slight pathological change on cartilage was found 9 weeks after the DMM surgery, different from 15 weeks in our DMM model. This variance might be because of the different mouse strain used for the modeling.
As a multifunctional bioactive factor, midkine has been reported not only stimulating the proliferation and attenuating the differentiation of monolayer cultured chondrocyte in vitro,24,25) but also increasing the thickness of the hyaline cartilage in vivo.24) In the DMM OA model, rhMK treatment also benefited the repair of the cartilage injury especially for the cellularity and cartilage staining but not for the structure and tidemark integrity, which might be because the etiology, DMM always exist in this DMM OA model, making the neonatal cartilage continuously in a turn-over state without ordered organization. What is exiting to us is that rhMK treatment attenuated the allodynia and therefore increased the physical activity behavior of the OA mice. This might attribute to the rhMK treatment prompting the recovery of cartilage whose loss will cause invasion of the vascular and nerve giving rise to pain.56,57) Another possible reason is that the rhMK promoted the retrieval of pathologic changed nervous system that was involved in the OA chronic pain.2,3,5) Because beyond the bioactivity on cartilage, the neurotrophic and neurite outgrowth activities of MK have also been widely reported22,23). In addition, the study on Midkine knockout mice showed that the midkine (−/−) mice has a regenerative delay of the axonal damage after freezing injury of the sciatic nerve.58) Furthermore, another report shows that up regulation of the levels of the highly homologous cytokine PTN in the injured dorsal root ganglia (DRG) of rats with Chronic Constriction Injury (CCI) of the sciatic nerve correlates with faster recovery of neuropathic pain.59)
Currently, the biologic agents used for the earlier phase of the OA was throught to be a strategy for OA therapy through either blocking the catabolism or promoting the anabolism of the cartilage.60) Our study indicated that rhMK application on the DMM OA mice mitigated the OA related pain, improved the activity capacity and prevented the degradation of the cartilage. Considering the safety issue of the rhMK used as a biologics, we also inspected the main organs in the rhMK treated mice during the whole process and didn’t found any pathological changes. These results provide the potency that rhMK could be used as a biologics to cure OA related pain or OA.
We gratefully acknowledge the assistance of Mark Hartman for language revision. This work was supported by National Natural Science Foundation of China (81001388/H3004) and Medicine-Engineering Collaboration Foundation of Shanghai Jiao Tong University (YG2010MS87).