Abstract
The antimicrobial agents vancomycin and metronidazole have been used to treat Clostridium difficile infections (CDIs). However, it remains unclear why patients are at risk of treatment failure and recurrence. Therefore, this study retrospectively examined 98 patients with CDIs who were diagnosed based on the detection of toxin-positive C. difficile to determine the risk factors affecting drug treatment responses and the recurrence of CDI. No significant difference was observed in the cure rate or dosage between the vancomycin and metronidazole groups. The 90-d mortality rate and total number of drugs associated with CDIs, including antiinfective agents used within 2 months before the detection of toxin-positive C. difficile, were significantly lower in the treatment success group than in the failure group. The total number of antiinfective agents and gastric acid-suppressive agents used during CDI therapy was also significantly lower in the success group than in the failure group. The period from the completion of CDI therapy to restarting the administration of anticancer agents and steroids was significantly longer in patients without than in patients with recurrence. These results indicate that the total number of drugs associated with CDIs should be minimized to reduce the risk of CDIs, that not only antibiotics but also gastric acid-suppressive agents should be discontinued during CDI therapy to increase therapeutic efficacy, and that the use of anticancer agents and steroids should be delayed as long as possible after patients are cured by CDI therapy to prevent recurrence.
Clostridium difficile is the most common cause of nosocomial diarrhea. Potential risk factors for C. difficile infections (CDI) include host factors, poor infection control practice,1) the use of gastric acid-suppressive agents,2,3) and antibiotic use.2,4–7) Antibiotics may disrupt host defenses provided by indigenous microflora in the colon and, therefore, increase the risk of CDI.6) The antibiotics most commonly associated with CDI are clindamycin, penicillins, cephalosporins, and quinolones.4,6,7) On the other hand, overall antibiotic use rather than a single group of antimicrobial agents has been associated with C. difficile incidence rates.8) Interventions to reduce overall antibiotic use may be more successful in controlling the incidence of CDI than interventions that focus on certain groups of antibiotics.8) Furthermore, immunosuppression has been identified as an independent risk factor for the development of CDI.9–13)
The antimicrobial agents vancomycin and metronidazole have been used to treat CDI. Clinical practice guidelines recommend that the dosages of metronidazole and vancomycin are 500 mg orally 3 times per day and 125 mg orally 4 times per day for 10–14 d, respectively.14) A systematic review revealed that the percentage of patients initially cured with vancomycin and metronidazole ranged from 84% to 94% and from 73% to 94%, respectively.15) Zar et al. suggested that metronidazole and vancomycin were equally effective in the treatment of mild CDI, but that vancomycin was superior for treating patients with severe CDI.16) However, these treatments are unsuccessful in some patients, and recurrence has been reported in other patients that had been treated successfully. The recurrence rate was previously shown to range from 7% to 17% with vancomycin and from 5% to 21% for metronidazole.15) Monaghan et al. reported previously that recurrent disease occurred in 15–35% of CDI patients.17) Nevertheless, it remains unclear why patients are at risk of failure of the drug treatment and recurrence. Therefore, this study retrospectively examined 98 patients with CDI who were diagnosed based on the detection of toxin-positive C. difficile in order to determine the risk factors that affect drug treatment responses and the recurrence of CDI.
PATIENTS AND METHODS
PatientsThis study was approved by the Ethics Review Board of Kagoshima University Hospital (#401). We retrospectively assessed data obtained between January 2007 and March 2013 for 98 adult patients in whom the C. difficile toxin was detected for the first time after ≥72 h of hospitalization at Kagoshima University Hospital. CDI was diagnosed based on the detection of toxin-positive C. difficile. Recurrent CDI was diagnosed based on the detection of toxin-positive C. difficile within one month after successful CDI therapy. Information including drug history, age, sex, body weight, white blood cell counts, body temperature, and C-reactive protein values and so on were extracted from electronic medical records to investigate the effects of the total number of drugs associated with CDI used within two months before toxin-positive C. difficile was detected and during CDI therapy on therapeutic efficacy. Drugs associated with CDI are antibiotics, antifungals, antivirals, gastric acid-suppressive agents, anticancer agents, immunosuppressive agents, and steroids. Furthermore, the number and starting days of drugs associated with CDI used within one month after CDI therapy were investigated in toxin-positive C. difficile patients within one month after CDI therapy. Twenty-four patients who were discharged within one month of completing the therapy were excluded.
Detection of the C. difficile ToxinC. difficile toxin A/B was detected using TOX A/B QUIK CHEK® (Nissui Pharmaceutical, Tokyo, Japan) as the rapid diagnostic test kit.
Assessment of Clinical EffectsThe frequency of diarrhea and stool characteristics were extracted from electronic medical records. Treatment success was defined as a decrease in the number of diarrhea, and an improvement from watery, loose and muddy stool to normal formed stool.
Statistical AnalysisData were analyzed using SPSS software (version 15.0 J; SPSS Japan Inc., Tokyo Japan). Parametric variables were analyzed using the t-test, while nonparametric variables were analyzed by the Mann–Whitney U-test. A p value of <0.05 was considered significant.
RESULTS
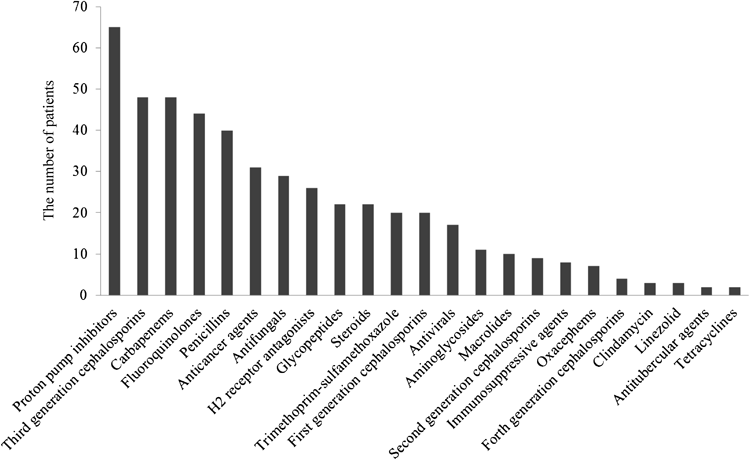
Patient characteristics are shown in Table 1. Fifty-six men and forty-two women, with a mean age of 64.0±16.5 years (mean±S.D.) and body weight of 53.2±10.5 kg, were evaluated in this study. Figure 1 shows the number of patients for each group of drugs associated with CDI used within two months before toxin-positive C. difficile was detected. Patients could be counted more than once in Fig. 1 if they received drugs from multiple groups. The groups most frequently implicated in the development of CDI were proton pump inhibitors, third generation cephalosporins, carbapenems, and fluoroquinolones. Proton pump inhibitors alone, H2 receptor antagonists alone, and these drugs concomitantly were given to 51, 12 and 14 patients, respectively. That is, a total of 77 patients (78.6% of 98 patients) were administered any gastric acid-suppressive agents. Third generation cephalosporins, carbapenems, and fluoroquinolones were given to 16, 11 and 5 patients, respectively. Coadministration of third generation cephalosporins and carbapenems was found in 7 patients, third generation cephalosporins and fluoroquinolones in 9 patients, and carbapenems and fluoroquinolones in 14 patients, respectively. All these 3 antibacterial groups were concomitantly given to 16 patients. That is, a total of 78 patients (79.6% of 98 patients) were administered third generation cephalosporins, carbapenems, and/or fluoroquinolones.
Table 1. Characteristics of the 98 Patients Included in the Study
| Characteristics | Number or mean±S.D. (range) |
|---|
| Sex | |
| Male : female | 56 : 42 |
| Age (years) | 64.0±16.5 (18–90) |
| Body weight (kg) | 53.2±10.5 (36.0–88.4) |
| Underlying disease | |
| Malignant tumor | 94 |
| Cardiovascular disease | 11 |
| Liver disease | 9 |
| Immune disease | 9 |
| Respiratory disease | 4 |
| Infectious disease | 4 |
| Neurological disease | 3 |
| Others | 4 |
Tables 2 and 3 show the therapeutic efficacy of oral vancomycin and metronidazole in CDI patients. Duration of vancomycin therapy in the success and failure groups were 10.1±3.1 and 12.2±4.6 d, respectively. Duration of metronidazole therapy in the success and failure groups were 9.9±2.4 and 17.1±10.1 d, respectively. The percentages of patients cured with vancomycin and metronidazole were 78.1% and 85.2%, respectively. No significant difference was observed in the cure rate or dosage between the two drugs. Four out of seven patients (57.1%) who were not administered both agents improved. The characteristics of patients with and without therapeutic efficacy were compared in Tables 4 and 5. No significant differences were observed in the white blood cell count or C-reactive protein value between these patients (Table 4). Furthermore, although data are not shown in Table 4, no significant difference was observed in the underlying disease, blood urea nitrogen, serum creatinine, total bilirubin, serum aspartate aminotransferase and serum alanine aminotransferase. No severe and complicated patients with hypotension, shock, ileus, magacolon and white blood cell count >50000 were included in this study. The 90-d mortality, but not 30-d mortality, was significantly lower in the success group than in the failure group (p<0.05, Fisher’s exact test; Table 4). The total number of drugs associated with CDI and anti-infective agents (antibiotics, antifungals, and antivirals) used within two months before toxin-positive C. difficile was detected was significantly lower in the success group than in the failure group (Table 5). Furthermore, the total number of drugs associated with CDI, anti-infective agents, antibiotics, and gastric acid-suppressive agents used during CDI therapy were significantly lower in the success group than in the failure group (Table 5). Especially on during CDI therapy, many risk factors (90-d mortality, and the total number of CDI-associated drugs, anti-infective agents, antibiotics, and gastric acid-suppressive agents) were found, and thus multivariate logistic regression analysis was additionally performed. The analysis showed that the therapeutic efficacy was significantly reduced by three of the five factors: 90-d mortality (odds ratio=5.58), and the total number of anti-infective agents (odds ratio=1.95) and gastric acid-suppressive agents (odds ratio=2.96). The other two factors were not significant due to confounding, because the total number of CDI-associated drugs was correlated with the total number of anti-infective agents, and the class of antibiotics was fully included in the wider class of anti-infective (antibiotics, antifungals, and antivirals).
Table 2. Therapeutic Efficacy of Vancomycin in Patients with CDI
| Daily dose (g) | Success (n=50) | Failure (n=14) | Cure rate (%) |
|---|
| 0.5 | 29 | 5 | 85.3 |
| 1.0 | 3 | 1 | 75.0 |
| 1.5 | 2 | 0 | 100.0 |
| 2.0 | 16 | 8 | 66.7 |
CDI: Clostridium difficile infection.
Table 3. Therapeutic Efficacy of Metronidazole in Patients with CDI
| Daily dose (g) | Success (n=23) | Failure (n=4) | Cure rate (%) |
|---|
| 0.5 | 5 | 1 | 83.3 |
| 0.75 | 1 | 0 | 100.0 |
| 1.0 | 7 | 0 | 100.0 |
| 1.5 | 10 | 3 | 76.9 |
CDI: Clostridium difficile infection.
Table 4. Identification of Factors Affecting Therapeutic Efficacy
| Factors | Success(n=77) | Failure(n=21) | p Value |
|---|
| Sex (male) | 44 (57.1%) | 12 (57.1%) | 1.00 |
| Age (mean±S.D.) | 65.6±14.7 | 58.1±20.8 | 0.15 |
| Serum albumin concentration (g/dL) | 2.8±1.2 | 2.7±1.3 | 0.45 |
| WBC count (103 cells/mm3) | 8.2±6.4 | 9.0±7.6 | 0.62 |
| WBC count (<103 cells/mm3) | 4 (5.2%) | 3 (14.3%) | 0.15 |
| CRP value (mg/dL) | 4.8±5.5 | 6.5±5.9 | 0.56 |
| Use of gastric acid-suppressive agents within 2 months before the detection of toxin-positive CD | 58 (75.3%) | 19 (90.5%) | 0.11 |
| Use of antibiotics, antifungals, and antivirals within 2 months before the detection of toxin-positive CD | 73 (94.8%) | 20 (95.2%) | 0.71 |
| 30-d mortality | 1 (1.3%) | 2 (9.5%) | 0.12 |
| 90-d mortality | 3 (3.9%) | 5 (23.8%) | 0.01 |
WBC: white blood cell, CRP: C-reactive protein, CD: Clostridium difficile.
Table 5. Effect of the Class and Number of Drugs Associated with CDI on Therapeutic Efficacy
| Factors | Number of drugs per patient |
|---|
| Used within 2 months before the detection of toxin-positive CD | Used during CDI therapy | Used within 1 month after CDI therapy |
|---|
| Success(n=77) | Failure(n=21) | p Value | Success(n=77) | Failure(n=21) | p Value | Patients with recurrence(n=14) | Patients without recurrence(n=39) | p Value |
|---|
| Drugs associated with CDI | 6.0±4.5 | 8.4±3.9 | <0.05 | 1.6±1.7 | 3.1±1.8 | <0.01 | 3.2±2.9 | 2.5±3.0 | 0.45 |
| Anti-infective agents | 3.7±2.9 | 5.4±3.0 | <0.05 | 0.9±1.3 | 1.9±1.7 | <0.01 | 1.5±1.6 | 1.4±1.6 | 0.82 |
| Antibiotics | 3.1±2.2 | 4.0±2.1 | 0.10 | 0.5±0.8 | 1.2±1.1 | <0.01 | 1.3±1.2 | 1.0±1.2 | 0.42 |
| Gastric acid-suppressive agents | 1.2±1.0 | 1.3±0.6 | 0.62 | 0.5±0.5 | 0.8±0.5 | <0.05 | 0.6±0.6 | 0.4±0.5 | 0.23 |
| Anticancer agents | 0.7±1.3 | 1.0±1.6 | 0.31 | 0.03±0.16 | 0.05±0.21 | 0.61 | 0.5±1.0 | 0.5±1.2 | 0.97 |
| Immunosuppressive agents | 0.1±0.3 | 0.1±0.3 | 0.42 | 0.06±0.29 | 0.10±0.29 | 0.68 | 0.07±0.26 | 0.03±0.16 | 0.55 |
| Steroids | 0.3±0.9 | 0.5±0.8 | 0.38 | 0.05±0.22 | 0.24±0.43 | 0.07 | 0.4±0.8 | 0.2±0.5 | 0.37 |
CDI: Clostridium difficile infection.
The characteristics of patients with and without recurrence were compared in Tables 5–7. No significant differences were observed in age or serum albumin concentrations between the 2 groups (Table 6). The total number of drugs associated with CDI used within one month after CDI therapy was not significantly different between both groups (Table 5). The starting days of drugs associated with CDI, anticancer agents, and steroids after CDI therapy were significantly longer in the patients without recurrence than in the patients with recurrence (Table 7).
Table 6. Identification of Factors Affecting Recurrence
| Factors | Patients with recurrence(n=14) | Patients without recurrence(n=39) | p Value |
|---|
| Sex (male) | 9 (64.3%) | 24 (61.5%) | 0.86 |
| Age (mean±S.D.) | 69.3±10.6 | 63.4±16.2 | 0.22 |
| Serum albumin concentration (g/dL) | 2.7±0.6 | 2.8±1.1 | 0.70 |
| WBC count (103 cells/mm3) | 6.9±4.3 | 8.2±6.3 | 0.49 |
| WBC count (<103 cells/mm3) | 1 (7.1%) | 3 (7.7%) | 0.95 |
| CRP value (mg/dL) | 6.4±8.1 | 4.3±4.9 | 0.41 |
WBC: white blood cell, CRP: C-reactive protein.
Table 7. Effect of the Starting Day of Drugs Associated with CDI after CDI Therapy on Recurrence
| Factors | Starting days of drugs | p Value |
|---|
| Patients with recurrence(n=14) | Patients without recurrence(n=39) |
|---|
| Drugs associated with CDI | 3.7±4.0 | 9.4±9.5 | <0.05 |
| Anti-infective agents | 5.8±5.4 | 8.3±8.1 | 0.42 |
| Antibiotics | 5.8±5.4 | 8.3±7.9 | 0.42 |
| Gastric acid-suppressive agents | 2.1±4.5 | 6.8±8.2 | 0.19 |
| Anticancer agents | 4.0±1.4 | 23.5±5.4 | <0.01 |
| Immunosuppressive agents | 1 | 4 | N.D. |
| Steroids | 0.5±0.5 | 19.3±6.5 | <0.01 |
CDI: Clostridium difficile infection, N.D.: not detected.
DISCUSSION
A meta-analysis indicated that clindamycin, fluoroquinolones, cephalosporins, monobactams, and carbapenems exhibited the strongest effects on CDI, while penicillins, macrolides, and sulfonamides/trimethoprim had weaker effects.18,19) Furthermore, a meta-analysis previously revealed a 65% increase in the incidence of CDI among proton pump inhibitor users.3) Another meta-analysis detected a strong correlation between proton-pump inhibitor use and CDI, while a weaker correlation was observed with H2 receptor antagonists.20) In the present study, 78 out of 98 toxin-positive C. difficile patients were administered third generation cephalosporins, carbapenems, and/or fluoroquinolones (Fig. 1), while 77 out of 98 patients were administered gastric acid-suppressive agents (Fig. 1). These results were consistent with the previous findings.
In the present study, no significant difference was observed in the cure rate at each dosage of vancomycin (Table 2). Fekety et al. reported that a daily dose of 500 mg was as effective as that of 2000 mg.21) Since the administration of a daily dose of 2000 mg is more expensive, that of 500 mg is preferred when vancomycin is used to treat CDI, unless the patient is critically ill. Furthermore, 157 C. difficile isolates in Japan, which were investigated by Kunishima et al., were susceptible to both metronidazole and vancomycin. The MIC50, MIC90, and MIC range for metronidazole, were 0.25, 0.5, and 0.06–1 µg/mL, respectively,22) while those for vancomycin, were 0.5, 1, and 0.12–2 µg/mL, respectively.22) Another previous study also showed that all 73 isolates were susceptible to both metronidazole and vancomycin.23) Thus, C. difficile isolates in Japan appear to be susceptible to both agents. In the present study, no significant difference was observed in the cure rate between vancomycin and metronidazole (Tables 2, 3). Since vancomycin is more expensive, metronidazole may be selected for an initial episode of CDI. The typical treatment for CDI has been to stop antibiotics being given for other purposes and immediately start treatment with metronidazole or vancomycin.24) Patients who remain on antibiotics while undergoing treatment with CDI have a higher likelihood of treatment failure with metronidazole.25) In this study, patients administered antibiotics and gastric acid-suppressive agents during CDI therapy were at significant higher risk of failing with the treatment (Table 5). These results suggested that gastric acid-suppressive agents should be stopped during CDI therapy. Furthermore, 90-d mortality was significantly lower in the success group than in the failure group (Table 4), indicating that overall condition of patients affect therapeutic efficacy. Thus, it is likely that the worse the general status of a patient with CDI is, the lower efficacy of the CDI therapy is.
Kamboj et al. examined C. difficile isolates from 102 patients with repeated episodes of CDI. Almost all second episodes within 8 weeks of the index case were due to the same strain.26) Among the 20 recurrent cases examined, 16 cases (80%) were identified as cases of recurrence caused by the initial strain while the remaining 4 cases (20%) were identified as reinfection cases by different strains; therefore, Oka et al. suggested that the germination ability of C. difficile may be a potential risk factor for the recurrence of CDI.23) Cancer chemotherapy is a risk factor for CDI that is mediated by the antimicrobial activity of several chemotherapeutic agents10,27) and could also be related to the immunosuppressive effects of neutropenia.28,29) The risk of recurrent disease has been shown to be related to the host immune response.30,31) In this study, we demonstrated that the starting days of anticancer agents and steroids after CDI therapy were significantly longer in the patients without recurrence than in the patients with recurrence (Table 7). Thus, the recurrence of CDI may be caused by the reactivation of C. difficile with immunosuppressive effects. Furthermore, the clinical practice guidelines suggest that number of antimicrobial agents prescribed should be minimized in an attempt to reduce the risk of CDI.14) We showed that the starting days of drugs associated with CDI were significantly longer in the patients without recurrence than in the patients with recurrence (Table 7).
In conclusion, the results of the present study indicate that the total number of drugs associated with CDI should be minimized in order to reduce the risk of CDI, that not only antibiotics, but also gastric acid-suppressive agents should be discontinued during CDI therapy to increase the therapeutic efficacy, and that the use of anticancer agents and steroids should be delayed as long as possible after patients are cured by CDI therapy to prevent recurrence.
REFERENCES
- 1) McFarland LV. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat. Clin. Pract. Gastroenterol. Hepatol., 5, 40–48 (2008).
- 2) Aldeyab MA, Harbarth S, Vernaz N, Kearney MP, Scott MG, Funston C, Savage K, Kelly D, Aldiab MA, McElnay JC. Quasiexperimental study of the effects of antibiotic use, gastric acid-suppressive agents, and infection control practices on the incidence of Clostridium difficile-associated diarrhea in hospitalized patients. Antimicrob. Agents Chemother., 53, 2082–2088 (2009).
- 3) Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am. J. Gastroenterol., 107, 1001–1010 (2012).
- 4) Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J. Antimicrob. Chemother., 51, 1339–1350 (2003).
- 5) Vernaz N, Hill K, Leggeat S, Nathwani D, Philips G, Bonnabry P, Davey P. Temporal effects of antibiotic use and Clostridium difficile infections. J. Antimicrob. Chemother., 63, 1272–1275 (2009).
- 6) Owens RC Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin. Infect. Dis., 46 (Suppl. 1), S19–S31 (2008).
- 7) Pépin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S, Leblanc M, Rivard G, Bettez M, Primeau V, Nguyen M, Jacob CE, Lanthier L. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin. Infect. Dis., 41, 1254–1260 (2005).
- 8) Mertz D, Frei R, Plagge H, Battegay M, Widmer AF. Stronger correlation between antibiotic use and the incidence of Clostridium difficile determined by culture results instead of faecal toxin detection only. Eur. J. Clin. Microbiol. Infect. Dis., 29, 1575–1578 (2010).
- 9) Svenungsson B, Burman LG, Jalakas-Pörnull K, Lagergren A, Struwe J, Akerlund T. Epidemiology and molecular characterization of Clostridium difficile strains from patients with diarrhea: low disease incidence and evidence of limited cross-infection in a Swedish teaching hospital. J. Clin. Microbiol., 41, 4031–4037 (2003).
- 10) Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin. Infect. Dis., 17, 109–113 (1993).
- 11) Blot E, Escande MC, Besson D, Barbut F, Granpeix C, Asselain B, Falcou MC, Pouillart P. Outbreak of Clostridium difficile-related diarrhoea in an adult oncology unit: risk factors and microbiological characteristics. J. Hosp. Infect., 53, 187–192 (2003).
- 12) Cudmore MA, Silva J Jr, Fekety R, Liepman MK, Kim KH. Clostridium difficile colitis associated with cancer chemotherapy. Arch. Intern. Med., 142, 333–335 (1982).
- 13) Sharma AK, Holder FE. Clostridium difficile diarrhea after use of tacrolimus following renal transplantation. Clin. Infect. Dis., 27, 1540–1541 (1998).
- 14) Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect. Control Hosp. Epidemiol., 31, 431–455 (2010).
- 15) Drekonja DM, Butler M, MacDonald R, Bliss D, Filice GA, Rector TS, Wilt TJ. Comparative effectiveness of Clostridium difficile treatments: a systematic review. Ann. Intern. Med., 155, 839–847 (2011).
- 16) Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin. Infect. Dis., 45, 302–307 (2007).
- 17) Monaghan T, Boswell T, Mahida YR. Recent advances in Clostridium difficile-associated disease. Gut, 57, 850–860 (2008).
- 18) Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob. Agents Chemother., 57, 2326–2332 (2013).
- 19) Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DD, Sferra TJ, Hernandez AV, Donskey CJ. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J. Antimicrob. Chemother., 68, 1951–1961 (2013).
- 20) Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am. J. Gastroenterol., 102, 2047–2056, quiz, 2057 (2007).
- 21) Fekety R, Silva J, Kauffman C, Buggy B, Deery HG. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. Am. J. Med., 86, 15–19 (1989).
- 22) Kunishima H, Chiba J, Saito M, Honda Y, Kaku M. Antimicrobial susceptibilities of Clostridium difficile isolated in Japan. J. Infect. Chemother., 19, 360–362 (2013).
- 23) Oka K, Osaki T, Hanawa T, Kurata S, Okazaki M, Manzoku T, Takahashi M, Tanaka M, Taguchi H, Watanabe T, Inamatsu T, Kamiya S. Molecular and microbiological characterization of Clostridium difficile isolates from single, relapse, and reinfection cases. J. Clin. Microbiol., 50, 915–921 (2012).
- 24) Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J. Gastroenterol., 15, 1554–1580 (2009).
- 25) Modena S, Gollamudi S, Friedenberg F. Continuation of antibiotics is associated with failure of metronidazole for Clostridium difficile-associated diarrhea. J. Clin. Gastroenterol., 40, 49–54 (2006).
- 26) Kamboj M, Khosa P, Kaltsas A, Babady NE, Son C, Sepkowitz KA. Relapse versus reinfection: surveillance of Clostridium difficile infection. Clin. Infect. Dis., 53, 1003–1006 (2011).
- 27) Morales Chamorro R, Serrano Blanch R, Méndez Vidal MJ, Gómez España MA, Rubio Pérez MJ, de la Haba Rodríguez JR, Aranda Aguilar E. Pseudomembranous colitis associated with chemotherapy with 5-fluorouracil. Clin. Transl. Oncol., 7, 258–261 (2005).
- 28) Bilgrami S, Feingold JM, Dorsky D, Edwards RL, Bona RD, Khan AM, Rodriguez-Pinero F, Clive J, Tutschka PJ. Incidence and outcome of Clostridium difficile infection following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant., 23, 1039–1042 (1999).
- 29) Gorschlüter M, Glasmacher A, Hahn C, Schakowski F, Ziske C, Molitor E, Marklein G, Sauerbruch T, Schmidt-Wolf IG. Clostridium difficile infection in patients with neutropenia. Clin. Infect. Dis., 33, 786–791 (2001).
- 30) Johal SS, Lambert CP, Hammond J, James PD, Borriello SP, Mahida YR. Colonic IgA producing cells and macrophages are reduced in recurrent and non-recurrent Clostridium difficile associated diarrhoea. J. Clin. Pathol., 57, 973–979 (2004).
- 31) Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet, 357, 189–193 (2001).