2014 年 37 巻 2 号 p. 239-247
2014 年 37 巻 2 号 p. 239-247
In the present study, novel ultradeformable liposomes (menthosomes; MTS), deformable liposomes (transfersomes; TFS) and conventional liposomes (CLP) were compared in their potential for transdermal delivery of meloxicam (MX). MTS, TFS and CLP were investigated for size, size distribution, zeta potential, elasticity, entrapment efficiency and stability. In vitro skin permeation using hairless mice skin was evaluated. Vesicular morphology was observed under freeze-fractured transmission electron microscopy (FF-TEM). Intrinsic thermal properties were performed using differential scanning calorimetry (DSC) and X-ray diffraction. The skin permeation mechanism was characterized using confocal laser scanning microscopy (CLSM). The results indicated that the difference in physicochemical characteristics of MTS, TFS and CLP affected the skin permeability. MTS and TFS showed higher flux of MX than CLP. CLSM image showed deformable vesicles mechanism for delivery of MX across the hairless mice skin. Our study suggested that ultradeformable and deformable liposomes (MTS and TFS) had a potential to use as transdermal drug delivery carriers for MX.
Meloxicam (MX), a cyclooxygenase-2 inhibitor non-steroidal anti-inflammatory drug (NSAID), is used to treat rheumatoid arthritis, osteoarthritis and other joint diseases. The injectable and oral administrations of NSAID drugs are not appropriate for needle-phobia and peptic ulcer patients. Moreover, the major limitation of MX is its low aqueous solubility (0.012 mg/mL in water and 0.086×10−2 mg/mL in 0.1 M HCl)1) with log P 1.91 and 0.07 at pH 5.0 and 7.4, respectively. MX delays its absorption from gastrointestinal tract (GI) and its prolonged use is associated with the incidence of GI side effects (bellyache, indigestion, ulceration and bleeding).2) If MX could be delivered without incidence of these limitations, MX administration would become safer and more acceptable. MX is suitable for development as a transdermal delivery candidate.
Transdermal drug delivery or skin delivery has become a global priority because the conventional drug administration is associated with numerous limitations. Liposomes are one of potential strategies that utilize for skin delivery of hydrophilic drugs,3) lipophilic drugs,4) protein5) and macromolecule.6) Although last decades, some design of conventional liposomes are of little or no value as carriers for transdermal drug delivery, but rather remain confined to the upper layer of the stratum corneum. However, recent approaches in vesicular modulating drug delivery through skin have resulted in many designs of novel vesicular carriers e.g., deformable liposomes (transfersomes),7) niosomes,8) ethosomes,9) invasomes,10) flexosomes11) and menthosomes.12) Transfersomes are the first generation of elastic vesicles introduced by Cevc and Blune7) and consist mainly of phospholipids and an edge activator or a single-chain surfactant which having a high radius of curvature that increases deformability of the bilayers.13) Niosomes are the second generation of elastic vesicles introduced by van den Bergh et al.8) and compose of the optimal ration of non-ionic surfactant and cholesterol. Ethosomes are another novel vesicular carriers, developed and introduced by Touitou et al.9) and incorporate ethanol into vesicle bilayer. Invasomes and flexible liposomes (Flexosomes) are introduced by Verma10) and Song and Kim11), respectively. The combination of penetration enhancers was observed in these last two types of vesicles. All special designed vesicular carriers enhanced skin delivery of various hydrophilic and lipophilic drugs.
Conventional liposomes and transfersomes were investigated as a carrier for skin delivery of a model drug, MX in our previous study.14) Moreover, the latest deformable liposomes, menthosomes was developed and introduced by our group.12) Menthosomes, novel ultradeformable carriers consist of phospholipids, menthol and edge activator. Menthosomes was optimized using a nonlinear response-surface method incorporating thin-plate spline interpolation (RSM-S). The accuracy and reliability of the optimal formulation obtained from RSM-S was also confirmed in several intensive studies.15,16) The result showed that the estimated values by the computer programs were very close to experimental values.12) To deliver MX into deep layer of the skin, the most potential liposomal carrier for transdermal delivery was required.
The aim of our present study was to compare the capability of novel ultradeformable liposomes, deformable liposomes and conventional liposomes to be used as transdermal drug delivery systems, and investigate the possible mechanisms on skin delivery of MX using these carriers. Menthosomes (MTS), transfersomes (TFS) and conventional liposomes (CLP) was formulated. The size, size distribution, zeta potential, morphology, elasticity, content drug in the formulation, entrapment efficiency, thermal properties, stability and in vitro skin permeation study was also evaluated for their physicochemical characteristics and permeability. Vesicular morphology was observed under a freeze-fractured transmission electron microscopy (FF-TEM). Intrinsic thermal properties were performed using differential scanning calorimetry (DSC) and X-ray diffractometer (XRD). Skin permeation mechanism was also performed using confocal laser scanning microscopy (CLSM).
Phosphatidylcholine (PC) from soybean (90%) was generously supplied by LIPOID GmbH (Cologne, Germany). Cholesterol (Chol) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Cetylpyridinium chloride (CPC) was purchased from MP Biomedicals (Illkirch, France). l-Menthol (MEN) was purchased from Tokyo Chemical Industry (Tokyo, Japan). MX was supplied from Fluka (Buchs, Switzerland). 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanineperchlorate (DiI) was purchased from Lambda Probes and Diagnostics (Graz, Austria). All other chemicals used were of reagent grade and purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Preparation of Meloxicam Loaded VesiclesMenthosomes, transfersomes and liposomes were prepared according to formulations obtained from our previous study.12) As shown in Table 1, MX loaded vesicles (menthosomes, transfersomes and liposomes) composed of a controlled amount of MX, PC, and various amounts of Chol as membrane stabilizer, and CPC and MEN as penetration enhancer were prepared. l-menthol was reported to improve the skin permeation of various drugs by increase of drug partition and diffusion.17,18) The quantities of MX and PC were fixed at 0.077 and 0.773% (w/v), respectively. Vesicle formulations were prepared by sonication method. Briefly, lipid component mixtures of PC, Chol, CPC, MEN and MX were dissolved in chloroform–methanol (2 : 1, v/v ratio). These lipid component mixtures were mixed. The solvent was evaporated under nitrogen gas stream. The lipid film was placed in a desiccator for at least 6 h to remove the remaining solvent. The dried lipid film was hydrated with 0.01 M acetate buffer solution (pH 5.5). Vesicles were subsequently sonicated for 2 cycles of 15 min. using a bath-type sonicator (5510J-DTH Branson Ultrasonics, CL, U.S.A.). The vesicle formulation was freshly prepared or stored in airtight container at 4°C prior to use.
| Formulation | Composition % (w/v) | |||||
|---|---|---|---|---|---|---|
| PC | Chol | CPC | MEN | MX | Buffer | |
| MTS | 0.77 | 0.04 | 0.10 | 0.07 | 0.08 | 100 |
| TFS | 0.77 | 0.04 | 0.10 | — | 0.08 | 100 |
| CLP | 0.77 | — | — | — | 0.08 | 100 |
The MX-suspension (SUS) was prepared by incorporating excess amount of MX higher than its solubility in acetate buffer solution (0.01 M, pH 5.5) and stirring over 24 h to ensure constant thermodynamic activity through the course of the skin permeation experiment. The thermodynamic activity of the MX in formulation could decrease and may result in a decrease permeation rate, therefore the excess amount of MX was added.
Measurement of Vesicles Size, Size Distribution, Zeta Potential and MorphologyAverage vesicle size, size distribution and zeta potential of the vesicle formulations and MX-suspension were measured by photon correlation spectroscopy (PCS) (Zetasizer Nano series, Malvern Instrument, UK). Twenty microliter of the vesicle formulations were diluted with 1480 µL of deionized water. All measurement samples were performed at room temperature, at least three independent samples were taken, and the particle size, size distribution and zeta potential were measured at least three times.
The morphology of the lipid vesicles was observed by freeze-fractured electron microscopy. A small drop of sample solution placed on a small copper block was rapidly frozen in nitrogen slash, which was freshly prepared just before its use by decompression in a vacuum chamber.19) The quenched sample was fractured in a freese-fractured apparatus JFD-9010 (JEOL, Tokyo, Japan). The fractured surface was rotary-shadowed with platinum-carbon at an angle of 10° and the shadowed surface was coated with carbon. The freeze-fractured replica obtained was washed with chloroform–methanol (4 : 1, v/v ratio) and observed with a transmission electron microscope JEM1400 (JEOL, Tokyo, Japan) equipped with a digital CCD camera (ES500W Erlangshen, Gatan, U.S.A.).
Measurement of Elasticity ValueThe elasticity value of lipid bilayers of the vesicles were direct proportion to JFlux×(rv/rp)2; according to the equation obtained from the previous study.20,21)
 | (1) |
where JFlux is the rate of penetration through a permeable barrier (mg·s−1·cm−2), rv is the size of the vesicles after extrusion (nm) and rp is the pore size of the barrier (nm). To measure JFlux, the vesicles were extruded through a polycarbonate membrane (Nuclepore, Whatman Inc., MA, U.S.A.) with a pore diameter of 50 nm (rp), at a pressure of 0.5 MPa. After 5 min of extrusion, the extrudate was weighed (JFlux), and the average vesicle diameter after extrusion (rv) was measured by PCS at room temperature.
Determination of MX-Entrapment EfficiencyThe concentration of MX in the vesicle formulation was determined by HPLC analysis after disruption of the vesicles with Triton® X-100 (0.1% w/v) at a 1 : 1 volume ratio and appropriate dilution with phosphate buffer solution (pH 7.4). The vesicle/Triton® X-100 solution was centrifuged at 10000 rpm at 4°C for 10 min. The supernatant was filtered with a 0.45 µm nylon syringe filter. The entrapment efficiencies of MX loaded in the formulation were calculated according to the following equation, respectively:
 | (2) |
where CL is the concentration of MX loaded in the formulation as described in the above methods and Ci is the initial concentration of MX added into the vesicle formulation.
DSC MeasurementDSC measurements were performed using a Thermo plus DSC-8230 instrument (Rigaku Co., Tokyo, Japan), heated from −20 to 80°C with heating scan at the rate of 1°C/min. The empty vesicles and vehicle or continues phase were separated by centrifugation, the sediment was used as sample for DCS measurement. The sample (10 mg) was weighed and placed in an aluminum pan (Rigaku Co., Tokyo, Japan). The transition temperature was determined as the peak of the endothermic transition peaks.
X-Ray Diffraction MeasurementX-Ray diffraction was performed using a monochromatic synchrotron beam at Station BL 6A in the Photon Factory (Ibaraki, Japan). The wavelength (λ) of the X-ray beam was 0.1506 nm. The reciprocal spacing (S) was calibrated from the spacing of silver behenate, 58.38 nm at 25°C. The value of S is given by S=n/d= (2/λ) sin (2θ/2) where 2θ is the scattering angle and d is a repeat distance. A capillary tube with a diameter of 1 mm containing prepared MX-free vesicles sample was sealed by gas burner and placed in a sample holder of the X-ray diffractometer. The X-ray diffraction profiles were recorded with a flat panel and pixel array detector. Moreover, BL40B2 in SPring-8 (Hyogo, Japan) was also utilized for X-ray diffraction measurement for vesicles.
In Vitro Skin Permeation StudyThe excised skin of hairless mice (Laboskin®, HOS: HR-1 Male, 7 weeks, Sankyo Labo Service Corporation, Inc., Tokyo, Japan) was used as a permeation membrane for the in vitro skin permeation study. A side-by-side diffusion cell with an available diffusion area of 0.95 cm2 was employed. The receiving chamber was filled with 3 mL of phosphate buffer solution (pH 7.4, 32°C) and the donor chamber was filled with 3 mL MX-vesicle formulation (MTS, TFS and CLP) and MX-suspension (SUS). At appropriate times, an aliquot of the receiver fluid was withdrawn and the same volume of fresh buffer solution was replaced to the receiver chamber. The concentration of MX in the aliquot was analyzed using a HPLC.
After the skin permeation study, the concentration of MX remaining in the skin was measured. The skin was isolated from the diffusion cell, and the residual vesicle suspension was removed from the skin surface. The skin was washed with distilled water and then dried with cotton swab. The full-thickness skin of treated area was cut into small pieces with scissors and fine forceps, and sonicated for 2 h with 1 mL phosphate buffer solution pH 7.4 to extract the MX (solubility of MX in buffer pH 7.0–8.0 was 26.6–155 mg/100 mL,1) over 20–130 times of MX in water). After centrifugation at 10000 rpm for 10 min, the clear supernatant was analysis by HPLC. Moreover, the receiver fluid at the end of incubation time was also analyzed using a PCS for mechanism studying.
CLSM Measurement of Hairless Mice SkinMechanisms and depth of skin permeation of the MX loaded vesicles (MTS, TFS and CLP) were investigated using CLSM. The MX loaded vesicles was prepared and labeled bilayer by DiI (PC : DiI; 100 : 1 M ratio). The labeled vesicles were applied on the hairless mice skin for 12 h. After removing the excess amount of vesicle formulation, the skin was washed with distilled water and then dried with cotton swab. The skin was sectioned into the pieces of 1 cm2 size and evaluated for depth of fluorescent probe penetration. The full skin thickness was optically scanned at the different increments through the z-axis of the CLSM (CLSM, Radiance 2100, Bio-Rad Laboratories, Inc., Hercules, CA, U.S.A.). Maximum excitation was performed by a 543 nm line of internal He–Neon laser and fluorescence emission were detected with long pass barrier filter 560 DCLSP.
Stability Study of MX Load Vesicles FormulationThe MX loaded vesicle formulation were kept in glass bottle with plastic plugs and stored at 4±1°C and 25±1°C (room temperature; RT) for 30 d to examine the stability of the formulation. Both the physical and the chemical stability of MX loaded vesicle formulation were evaluated. The physical stability was evaluated by size, size distribution and zeta potential determination. The chemical stability was evaluated by measuring MX remaining in the formulation by HPLC at 1, 7, 15 and 30 d. The amount entrapped after freshly preparations (day 1) was normalized to 100%.
HPLC Analysis of MeloxicamThe HPLC system consists of a SIL-20A autosampler, LC-20AT liquid chromatograph and SPD-20AUV detector (Shimadzu Corporation, Kyoto, Japan). The analytical column was YMC-Pack ODS-A (150 mm×4.6 mm i.d., S-5, YMC Co., Ltd., Kyoto, Japan), and the mobile phase was composed of acetate buffer solution (pH 4.6)/methanol (50 : 50, v/v). The flow rate was set at 0.8 mL/min, and the wavelength used was 272 nm. The calibration curve for MX was in the range of 1–100 µg/mL with a correlation coefficient of 0.999. The percentage recovery was found from 99.85–100.30%, and relative standard deviation for both intra-day and inter-day was less than 2%.
Data AnalysisThe data were reported as mean±S.D. (n=3–6) and statistical analysis of the data was carried out using one way ANOVA followed by an LSD post hoc test. A p-value of less than 0.05 was considered to be significant.
Ethics in the Animal StudyThis animal study was performed at Hoshi University and complied with the regulations of the committee on Ethics in the Care and Use of Laboratory Animals.
The physicochemical characteristics of MTS, TFS and CLP are shown in Table 2. The incorporation of different component (Chol, CPC and/or MEN) in the liposome systems affected the size, zeta potential, elasticity, drug content, entrapment efficiency and transition temperature of the vesicle formulation. The physicochemical characteristics of the liposomes and their analogues are an important factor that differentiates the liposomes system from the other lipid dispersed systems, especially the bilayer and their elasticity.
| Formulation | Size (nm) | PDI | Zeta potential (mV) | Elasticity (mg·s−1·cm−2) | Content drug (µg/mL) | EE (%) | Tg (C°) |
|---|---|---|---|---|---|---|---|
| MTS | 65±2.7 | 0.3±0.02 | 49.7±2.1 | 150.2±7.3 | 634.8±14.8 | 65.2±1.5 | 0.41 |
| TFS | 83±4.4 | 0.4±0.02 | 43.8±1.6 | 82.4±2.3 | 860.8±14.1 | 88.5±1.4 | 1.72 |
| CLP | 98±5.1 | 0.3±0.02 | 4.4±1.7 | 23.8±1.1 | 230.4±31.3 | 23.7±3.2 | 2.08 |
The vesicle size were in the nano-size range of 60–100 nm with the size distribution (polydispersity index; PDI) of 0.3–0.4 suggesting that sonication method can prepare a nano-size vesicle. While, MX-SUS were in the micro-size range of 20–30 µm. Deformable liposomes (MTS and TFS) had smaller vesicle sizes compared to conventional liposomes (CLP) ranked as follows: MTS<TFS<CLP. The vesicle size decreased as the liposome component such as Chol and CPC was incorporated in the vesicles. The previous study22) showed that incorporation of 10–15% Chol increase the vesicle size as Chol can increase the net repulsion force and reduce the van der Waals attraction force between the lipid bilayer of liposomal systems. The incorporation of CPC can achieve higher curvature, thus deformable liposomes resulting in decrease in vesicle size compared to conventional liposomes.
The zeta potential of MTS, TFS and CLP were in positive charge range of 4–50 mV (Table 2). Deformable liposomes (MTS and TFS) also had higher positive zeta potential compared to CLP ranked as follows: MTS>TFS>CLP. On the other hand, the charge of MX-SUS was −20 mV. Under experimental condition of pH 5.5 which is lower than the isoelectric point (pI) of PC around 6, and higher than the pI of MX was 2.6, PC carries the net positive charge and MX is the negatively charge form, respectively. Moreover, the incorporation of CPC, a cationic surfactant also affected the net positive charge of the formulation. Although, the vesicle formulation were composed of neutral charge material e.g., Chol, positive charge material e.g., PC and CPC, and negative charge material e.g., MX, the net charge was positive zeta potential vesicles. Therefore, the net charge of the vesicle was affected by the total net charge of the vesicle component.
The morphology of menthosomes was further observed by freeze-fractured transmission electron microscopy. Small size, spherical shape and smooth surface vesicle of MX were observed (Fig. 1).

The elasticity was ranked as follows: MTS>TFS>CLP, penetration enhancers such as MEN and CPC may a factor that affected the elasticity of vesicles by insertion into the bilayer. The incorporation of Chol, CPC and/or MEN affected the elasticity of vesicle bilayers. Chol can increase rigidity and packing density of PC molecules, thus to decrease elasticity of vesicle bilayers.23,24) In contrast, the incorporation of edge activator e.g., CPC which have a high radius of curvature can increase deformability of the vesicle bilayers. MEN can decrease the orderly lipid microstructure by insertion and thus increase the fluidity or elasticity of vesicle bilayer.25,26)
The content drug in the formulation and entrapment efficiency of MTS, TFS and CLP were determined by analysis of total drugs presented in the formulation. The entrapment efficiency of MX in the vesicles varied in the range of 20–80% with the content drug in the formulation varied in the range of 230–860 µg/mL (Table 2). The solubility of MX in acetate buffer solution (pH 5.5) is 8 µg/mL, indicating that MX solubility was quite small in acetate buffer solution compared to MX in vesicle formulation. The results indicated that the entrapment efficiencies for deformable liposomes incorporating the mixture of CPC and/or MEN, were higher than that incorporating PC alone. The beneficial role of edge activators within vesicle bilayers are well recognized as the intrinsic properties of CPC led to increase solubility of MX in vesicle bilayers. Consistency with the previous study,27) as sodium stearate (edge activator) was incorporated into the phosphatidylethanolamine vesicles, the entrapment efficiency of the drug was significantly increased. However, incorporating MEN decreased the entrapment efficiencies of MX in MTS compared to TFS, as MEN may compete with MX and/or CPC to solubilize in the vesicle bilayers. Although, some vesicle component might form micelle and MX could be entrapped in micelle, however our pre-formulation study indicated that the vesicle component under this experiment was chosen to differentiate the liposome from micelle or mixed micelle, using turbidity evaluation.
The DSC thermograms of the MTS, TFS and CLP at various basic components are shown in Fig. 2A MTS, TFS and CLP underwent a single endothermic transition temperature of lipid bilayer at 0.41, 1.72 and 2.08°C, respectively (Table 2). With further increase in CPC and MEN, transition temperature shifted to a lower temperature as shown in thermograms (Fig. 2A). MEN and CPC may affect the lipid bilayer by increase in fluidity of lamellar structure of lipid bilayer.
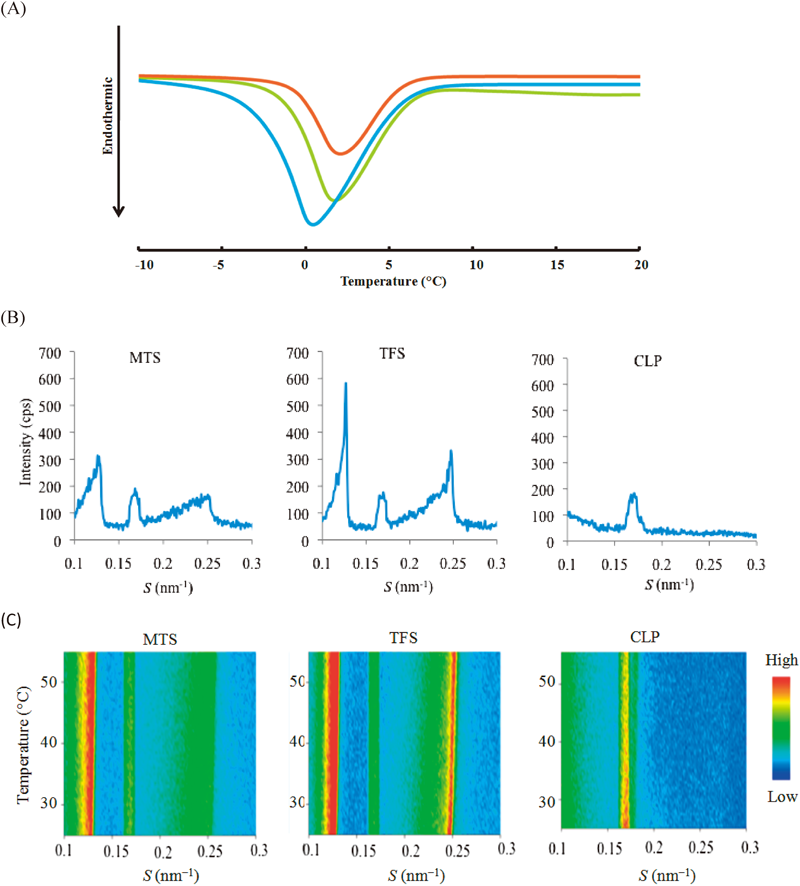
 ) MTS, (
) MTS, ( ) TFS and (
) TFS and ( ) CLP, (B) X-Ray Diffraction Profiles (C) the Thermal Scanning of MTS, TFS and CLP
) CLP, (B) X-Ray Diffraction Profiles (C) the Thermal Scanning of MTS, TFS and CLPThe diffraction profiles estimated by X-ray diffraction are shown in Fig. 2B. The results indicated that MTS, TFS and CLP vesicular were significantly different in membrane microstructure depended on of each basic component. PC, Chol and CPC in each vesicular bilayers. In small angle X-ray scattering, refereing to the diffraction of CLP, PC formed lamellar structure of 5.88 nm. Addition of cholesterol, CPC and MEN in the vesicle induced another lamellar structure (8.33 nm and 4.00 nm). Incorporating of MEN led to decrease in lamellar intensity of 8.33 nm and 4.00 nm. In thermal scanning study, peak position, integrated intensity and full-width at half-maximum was almost constant as shown in Fig. 2C. It means that no structural change was occurred in those three vesicles in the range of 25°C to 55°C. On the other hand, the diffraction was not observed in wide angle region (data not shown). The effect of MEN on the hydrocarbon chain packing and lamellar structure of lipid microstructure in the MTS bilayers was also examined using X-ray diffraction. The integrated intensities of the diffraction peaks for the MTS bilayers of CPC and Chol at 0.13 nm−1 and 0.25 nm−1, respectively were also reduced by the addition of MEN. MEN may disturbed the tightly packed hydrocarbon chains in vesicle bilayers which l-MEN specifically interacts with orthorhombic hydrocarbon chain packing.25,28) It was suggested that those lamellar were liquid-crystal state. Our study suggested that penetration enhancers contributed to decrease in orderly lipid microstructure and thus increased the fluidity. These results indicated that the change in lamellar structure of lipid bilayer of vesicles of diffraction profiles were consistent with the thermograms investigated by DSC.
Skin Permeation StudyFigure 3 shows (A) skin permeation profile, (B) steady-state flux and (C) MX deposit in the skin after skin permeation study. The cumulative amount per area of MX in each vesicle formulation increased linearly with lag time before 2 h. This linear accumulation was also observed for other formulations (Fig. 3A).

Each value represents the mean±S.D. (n=3), * p<0.05, ** p<0.01 compare to SUS.
The steady-state flux of MX through hairless mice skin from each formulation was determined as the slope of the linear portion of the plot and could be ranked as follows: MTS>TFS>CLP>SUS. The steady-state flux of MX in MTS, TFS and CLP was significantly higher than in SUS (p<0.05). Moreover, the steady-state flux of MX in deformable vesicles such as MTS and TFS was also significantly higher than in rigid vesicles such as CLP (p<0.05) (Fig. 3B).
The MX deposit in the skin was determined after skin permeation study. MX suspension (SUS) was used as a control for MX deposit in the skin. MTS and TFS gave a slightly higher MX deposit in the skin than SUS. On the other hand, CLP gave a significantly higher MX deposit in the skin than control. The MX deposit in the skin was ranked as follows: CLP>MTS>TFS>SUS (Fig. 3C).
The skin permeation results indicated that MX in high elasticity value vesicles (MTS and TFS) had a significantly higher MX flux than low elasticity value vesicles (CLP). In contrast, CLP had a significantly higher MX deposited in the skin than MTS and TFS. These results may be explained by the effect of intrinsic characteristics of each liposome systems. The present study was consistent with the previous study29) that conventional liposomes are of little or no value as transdermal drug delivery carriers compared to deformable liposomes because they remain confined to upper layers of the stratum corneum. The thermodynamic activity of MX in formulation may be a factor affecting the skin permeation. An increase in content of MX in the formulation resulted in further increase MX permeated the skin. The content of MX in MTS and TFS were significantly higher than CLP, indicating that the thermodynamic activity of MX in MTS and TFS formulation was important for its promotion of MX permeation flux through the skin. The positive charge of elastic vesicles (MTS and TFS) might affect the skin permeability and skin deposit30) as positive charge of vesicle interacted with negative charge of skin, thus MTS showed higher skin deposit than TFS because MTS has a greater positive charge than TFS. Table 2 shows the significant difference of elasticity between MTS and TFS formulation. However, no significant difference between MTS and TFS formulation was observed in the cumulative amount per area and skin permeation flux. Elasticity is a factor influencing skin permeability, however the skin permeability is still depended on total effect of all physicochemical characteristics of vesicle formulation. The elastic vesicles were smaller size, higher zeta potential, higher elasticity, higher entrapment efficiencies and lower transition temperature, providing that MTS and TFS could penetrate through the skin easier than CLP.
It is known that the lipid structure of stratum corneum form orthorhombic and hexagonal hydrocarbon chain packing. Therefore, the change in the apparent abundance ratio of hexagonal/orthorhombic lipid hydrocarbon chain packing (RH/O) value might affect the barrier function ability of the stratum corneum of MX permeation because of the possibility of decreasing dense barrier formed by lipid hydrocarbon chain packing. MEN was reported to affect the lipid arrangement in the stratum corneum. As MEN was incorporated in the liposomes, a significant decrease of the RH/O value was observed. The observed decrease in RH/O value suggested that treatment with MEN preferentially altered the lipid hexagonal hydrocarbon chain packing.31) However, based on the skin permeation study suggested that liposome systems with an excellent design such as small; size, high; zeta potential, elasticity, content drug and entrapment efficiencies and low; transition temperature also provided the potential and useful liposome carriers for transdermal drug delivery.
Skin Distribution of DiI-Labeled VesiclesAccording to CLSM observation, the vesicle-DiI penetrated into the skin was investigated for supporting the predicted mechanism of action of our liposome systems. Figure 4 illustrated the labeled-vesicle formulations penetrated into full-thickness skin. MTS-DiI, TFS-DiI and CLP-DiI penetrated into deeper layers of the epidermis up to 40 µm, 30 µm and 15 µm, respectively. The result indicated that the low elasticity value vesicles had also low permeability, could not penetrate into the deep layer of the epidermis and only remained to the upper layer of the stratum corneum. On the other hand, the high elasticity value vesicles showed effective permeability up to viable epidermis as high fluorescence intensity in the skin between 15–40 µm (viable epidermis layer) was observed. The obtained results indicated that high elastic value vesicle penetrated across the skin greater than low elasticity vesicles as same as the previous study.5)

The liposome systems were able to promote skin permeation of an active drug by a variety of mechanisms: (a) the free drug mechanism, (b) the penetration-enhancing process of the liposome components, (c) vesicle adsorption to and/or fusion with the stratum corneum and (d) intact vesicle penetration into and through the intact skin and the localization at the site of action.32) Furthermore, one of the most important factors during the skin permeation of the drugs was osmotic gradient. Deformable liposomes were reported to penetrate intact skin, carrying therapeutic concentrations of drugs, but only when applied under non-occluded conditions.7) Deformable liposomes tended to penetrate skin barrier and migrate into the water-rich deeper strata to secure its adequate hydration. The skin permeation of the MX in SUS was significantly lower than the MX-loaded vesicle formulation suggested that the free-drug mechanism was significantly smaller or had no more effect than the mechanism of vesicle. Comparing the liposome component such as CPC and MEN which acts as penetration enhancers may produce an enhancing effect and may penetrate deep into the skin and/or fuse and mix with skin lipids to loosen their barrier structure.33) On the other hand, the liposome systems may adsorb to the stratum corneum surface with subsequent transfer of MX directly from the vesicles to skin, or the liposome systems may fuse and/or mix with the stratum corneum lipid matrix, increasing MX partitioning into the skin (Fig. 4). Moreover, the result obtained from the analyzing of receiver fluid indicated that no intact vesicle was observed. Accordingly, the skin permeation mechanism which was observed in our study was (1) the penetration-enhancing process of the liposome components (MEN and/or CPC) and (2) the vesicle adsorption to and/or fusion with the stratum corneum.
Stability StudyFigure 5 shows (A) size, (B) size distribution, (C) zeta potential and (D) MX remaining of MTS, TFS and CLP for 30 d at 4°C and 25°C. Both physical and chemical stability was investigated. No sedimentation was found in any formulation after fresh preparation. After storage at 4°C for 30 d, no sedimentation was observed, and the size, size distribution, zeta potential and percentage of MX remaining in the formulation at 4°C for 30 d were not significantly different from the initial preparation. These results indicated a good physical and chemical stability of these liposome systems at 4°C.

 ) Day 1, (
) Day 1, ( ) Day 7, (
) Day 7, ( ) Day 15 and (
) Day 15 and ( ) Day 30 and 25°C; (
) Day 30 and 25°C; ( ) Day 1, (
) Day 1, ( ) Day 7, (
) Day 7, ( ) Day 15 and (
) Day 15 and ( ) Day 30
) Day 30Each value represents the mean±S.D. (n=3).
After storage at 25°C for 30 d, no sedimentation was observed in any formulation, and the size, size distribution, zeta potential and percentage of MX remaining in the formulation at 25°C for 30 d were slightly different from the initial preparation. The vesicles trend to change the size to larger size with lower percentage of MX remaining in the formulation when compared to storage at 4°C.
There was no difference of vesicle size, size distribution, zeta potential and MX remaining between liposome in donor chamber before skin permeation (fresh preparation) and after skin permeation, indicating that the liposome formulation was stable at 32°C at period of studies (12 h).
These liposome systems were stable both 4°C and 25°C at storage condition. However, the higher temperature is the factor affecting the physical and chemical instability of liposome systems.34) In our study, the recommended condition for storing MTS, TFS and CLP was 4°C and 25°C for 30 d.
The designing of liposome systems for transdermal MX delivery have been developed to overcome the stratum corneum barrier. Different design of each liposome systems (e.g., CLP, TFS, MTS) resulted in different physicochemical characteristics and skin permeability. It is important to investigate and compare their potential for transdermal delivery. Moreover, the factor affecting the skin permeation enhancement of liposome and the mechanism of action was investigated. Thus, the finding conclusions will provide essential fundamental information for developing novel liposomal systems for transdermal drug delivery.
MTS, a novel deformable vesicles for transdermal drug delivery was investigated and compared. The potential use of the novel MTS formulation significantly improved the MX permeation through the skin compared to TFS, CLP and SUS. Considering the skin permeation profile, flux, MX deposit in the skin, basic physicochemical characteristics and physical and chemical stability, MTS showed the feasibility to be a transdermal delivery of MX. CLSM image showed deformable vesicles mechanism to delivered MX across the skin. In our comparative study suggested that ultradeformable and deformable liposomes (MTS and TFS) were the most potential carriers for transdermal drug delivery for MX.
The authors gratefully acknowledge the Thailand Research Funds through the Golden Jubilee Ph.D. Program (Grant No. PHD/0141/2550) for financial support and thank the Faculty of Pharmacy and Graduate School, Silpakorn University, Nakhon Pathom, Thailand and Department of Pharmaceutics, Hoshi University, Tokyo, Japan for all facilities and support. The authors gratefully acknowledge to Prof. Yoshie Maitani and Dr. Kumi Kawano in Department of Drug Delivery Research, Hoshi University for helpful assistance of determination of liposome elasticity. The synchrotron X-ray scattering experiments were performed in a BL6A at the Photon Factory under approval of the Photon Factory Advisory Committee (2012G055) and BL40B2 at the SPring-8 with the approval of the SPring-8 Proposal Review Committee (2012A1303, 2012B1127).