Abstract
Drug delivery systems represent an important strategy for cancer treatment. The targeted delivery of drugs is required for effective and safe cancer therapy. In cancer therapy, the target cells include cancer cells and immunocompetent cells such as antigen presenting cells. Anticancer drugs utilized include small molecular drugs, proteins and nucleic acid medicines. In order to deliver these drugs into the target cells, various nanoparticles have been developed. However, the efficacy of the nanoparticulate system itself is generally insufficient for the safe and effective treatment of cancer. For example, polyethylene glycol (PEG)-modified (PEGylated) nanoparticles accumulate in cancerous tissues; however, the PEG moiety on the surface of the nanoparticles disturbs cellular uptake, which is known as the ‘PEG dilemma.’ Thus, additional strategies such as receptor-mediated targeting are necessary to improve the delivery and cellular uptake of nanoparticles. Among additional strategies, in this review we have focused on the combination of nanoparticles with various physical stimuli, such as electric pulse and ultrasound, to improve the targeted delivery of the nanoparticles.
1. INTRODUCTION
Beating cancer is a challenging task. Approaches toward cancer therapy include several strategies such as surgical excision, drug treatment, and immunomodulation. In spite of progress in the development of regimens of chemotherapy and molecular target drugs, the outcomes from cancer therapy are not yet enough. In most cases, the reason for the insufficient outcome of cancer therapy may be problems in drug delivery. Targeted delivery of anticancer drugs is an important issue that determines not only the efficacy but also the safety of cancer therapy. To deliver anticancer drugs selectively to cancer regions, the development of nanoparticulate systems is a promising approach.
Nanoparticles can deliver various materials including small molecular drugs, proteins and nucleic acid medicines. Nanoparticulate systems can also modulate the immune system. Various platforms for delivering drugs have been developed such as liposomes, polymeric micelles, dendrimers, etc. Targeting cancer and/or immunocompetent cells is a rational strategy in cancer therapy. However, disposition of nanoparticles is dependent on their physicochemical characteristics such as size and surface charge. Thus, it is important to regulate the physicochemical characteristics of nanoparticles.1) Interaction of nanoparticles with blood components also affects the biodistribution of the nanoparticles.2–4) Polyethylene glycol (PEG)-modification (PEGylation) of nanoparticles is useful to passively deliver the nanoparticles to cancerous tissues via an enhanced permeability and retention (EPR) effect.5) However, the PEG moiety on the surface of the nanoparticles disturbs cellular uptake, which is known as the ‘PEG dilemma.’6) Using receptor-mediated endocytosis, the disposition and cellular uptake of nanoparticles can be controlled.7,8) Moreover, microenvironment-responsive systems such as matrix metalloproteinase-cleavable PEG-lipids9,10) and acidic pH-responsive nanocarrier systems11) have been developed. Especially, combination of nanoparticles with various physical stimuli is a rational approach to improving the efficiency of cellular uptake of the nanoparticles. In this review, we focused on the physical stimuli-mediated delivery of nanoparticles and on applications of these systems toward cancer therapy.
2. PHYSICAL STIMULI-MEDIATED DELIVERY OF NANOPARTICLES
Various physical stimuli, such as electric pulse, ultrasound, hyperthermia and so on, have been used to improve the efficacy of nanoparticles. Table 1 summarizes the in vivo delivery of nanoparticles in combination with physical stimuli.
Table 1. Summary of
in Vivo Delivery of Nanoparticles with Physical Stimuli
| Physical stimuli | Cargos/drugs | Target tissues | Injection routes | Refs. |
|---|
| Electroporation | | | | |
| Electric pulse | −/pDNA | Liver | Direct injection or tail vein | 33) |
| Electric pulse | −/pDNA | Various tissues | Direct injection or tail vein | 34) |
| Electric pulse | Galactosylated polymers/pDNA | Liver | Tail vein | 60) |
| Electric pulse | −/pDNA | Tumor | Direct injection | 61) |
| Electric pulse | Liposome/pDNA | Tumor | Direct injection | 62) |
| Electric pulse (irreversible) | −/pDNA | Liver | Hepatic artery or portal vein | 14) |
| Sonoporation | | | | |
| Ultrasound+bubble liposome | −/doxorubicin | Tumor | Tail vein | 17) |
| Ultrasound+bubble liposome | −/pDNA | Artery | Femoral artery | 16) |
| Ultrasound+bubble liposome | Mannosylated liposome/pDNA | Liver or spleen | Tail vein | 20) |
| Ultrasound | Mannosylated bubble liposome/pDNA | Liver or spleen | Tail vein | 21) |
| Ultrasound | Mannosylated bubble liposome/siRNA | Liver | Tail vein | 63) |
| Hyperthermia | | | | |
| Heating | Thermosensitive liposome/doxorubicin | Tumor | Tail vein | 26) |
| High intensity focused ultrasound | Thermosensitive liposome/doxorubicin | Tumor | Tail vein | 28) |
| Others | | | | |
| Tissue pressure | −/pDNA | Kidney | Tail vein | 40) |
| Tissue suction | −/pDNA | Various tissues | Tail vein | 42) |
| Rubbing | −/pDNA | Stomach | Organ surface instillation | 43) |
Electroporation uses an electric pulse to make transient pores on the cellular membrane. By electroporation, both small molecules and macromolecules can permeate the cellular membrane both in vitro and in vivo.12,13) In general, the effect of electroporation is reversible. However, tissue damage often occurs by the electroporation. In the case of cancer treatment, such damage might be acceptable to induce tumor death. Moreover, it was reported that irreversible electroporation not only killed the tumor but also enhanced the transfection efficiency in a peripheral zone surrounding the tumor.14)
2.2 UltrasoundSonoporation creates transient pores on the cellular membrane by cavitation due to ultrasound exposure. Use of microbubbles enhances the ultrasound-mediated transfection of naked plasmid DNA (pDNA), lipoplex and polyplex in cultured cells.15) As a more pharmaceutically stable system, bubble liposomes (PEGylated liposomes containing an ultrasound imaging gas such as perfluoropropane) have been developed.16) The combination of bubble liposomes and ultrasound exposure with doxorubicin inhibited tumor growth in vivo.17) Tumor-homing peptide AG73-modified bubble liposomes improved in vitro drug and gene delivery to tumor cells.18,19)
As to a DNA vaccine, it is necessary to deliver pDNA to antigen-presenting cells. Since antigen-presenting cells express mannose receptors, the concomitant use of mannosylated lipoplex and bubble liposomes with ultrasound exposure can transfect the liver and spleen, in which antigen-presenting cells are abundant.20) As a more simple delivery system, a mannosylated PEGylated bubble lipoplex system selectively transfected antigen-presenting cells in vivo.21) DNA vaccination by the mannosylated PEGylated bubble lipoplex with ultrasound exposure suppressed melanoma growth and metastasis in vivo.22)
2.3 HyperthermiaTumors are generally sensitive to hyperthermia.23) The combination of hyperthermia with radiotherapy and chemotherapy improves cancer treatment.24) Hyperthermia targeted to the tumor region can improve the delivery of liposomes by enhancing vasculature permeability and interstitial fluid flow.25) Moreover, hyperthermia against solid tumors induced doxorubicin release from thermosensitive liposomes.26) As a hyperthermia-inducing method, high intensity focused ultrasound (HIFU) has been used for tumor ablation.27) The combination of HIFU with thermosensitive liposomes containing doxorubicin resulted in significant tumor regression.28) Magnetic nanoparticles also have been employed for hyperthermia-based therapy.29) In addition, the photothermic regulation of heat shock promoter-driven gene expression can be triggered by laser-induced carbon nanohorns.30) This system is potentially useful for hyperthermia-based therapy in the near future.
2.4 RadiationAmong other physical stimuli-based therapies, boron neutron capture therapy (BNCT) is promising for the treatment of several tumors, including gliomas. Selective delivery of the boron-10 isotope to tumor cells is important for successful BNCT. Thus, various targeting systems for boron delivery have been developed. Boronated epidermal growth factor can be selectively delivered to epidermal growth factor receptor-positive tumors by intratumoral injection.31) Since folate receptors are often expressed on cancer cells, boron-containing folate receptor-targeted liposomes are also promising candidates for BNCT.32)
2.5 Other Physical StimuliThe in vivo transfection efficiency of naked pDNA is generally low due to rapid degradation and poor cellular uptake. Here, the combination of naked pDNA with several physical stimuli has been investigated. As mentioned above, physical stimuli such as electroporation33–35) and sonoporation36,37) have been used to enhance the transfection efficiency of naked pDNA. Hydrodynamics-based transfection, i.e., rapid intravenous large volume injection of naked pDNA, is an efficient method to deliver naked pDNA to the liver.38,39) As more mild physical stimuli, pressure-mediated transfections of intravenously delivered naked pDNA to the kidney, liver and spleen have been reported.40,41) Moreover, tissue suction using a device enables site-specific transfection by naked pDNA to the kidney, liver, spleen, and heart.42) On the other hand, rubbing stimuli against the gastric serosal surface greatly enhanced naked pDNA transfer.43) In addition, the concomitant use of an abrasive compound calcium carbonate with naked pDNA was effective to a similar extent as the rubbing stimuli.44) These physical stimuli would improve the efficacy of the nanoparticulate systems.
3. PERSPECTIVES
In the physical stimuli-mediated delivery of nanoparticles, the development of specialized devices is important to successfully increase the cellular uptake of the nanoparticles in clinical use. In the case of electroporation, the shape of the electrodes is an important factor, not only for efficacy but also for safety.45) As another example of a device, an implantable pneumatically actuated microsystem was developed to achieve renal pressure-mediated transfection.46)
As mechanisms of improved efficacy of the combination of nanoparticles with physical stimuli, both cellular uptake and intracellular disposition of the nanoparticles are important issues (Fig. 1). Especially, localization of the nanoparticles in endosomes/lysosomes can result in the degradation of contents in the nanoparticles. In the case of electroporation, nanoparticles directly enter cytosol through transient pores on the cellular membrane. Also, ultrasound exposure of bubble lipoplex increased cytosolic pDNA.47) It was reported that the concomitant use of TAT-PEG liposomes containing pDNA and bubble liposomes with ultrasound exposure promoted the endosomal escape of pDNA.48) Thus, in the case of bubble liposomes with ultrasound exposure, both transient pores (Fig. 1 (i)) and endosomal escape (Fig. 1 (ii)) might be involved in the transfer of pDNA into cytosol. On the other hand, in many cases the physical stimuli induce cellular actions such as changes in gene expression. This affects the efficacy of the nanoparticles. Especially, transcriptional activation (Fig. 1 (iii)) was involved in enhanced transgene expression by hydrodynamics-based transfection,49,50) ultrasound-mediated transfection,51) and pressure-mediated transfection.52) Thus, it is necessary to consider the effect of the physical stimuli on cellular functions in view of future clinical use.
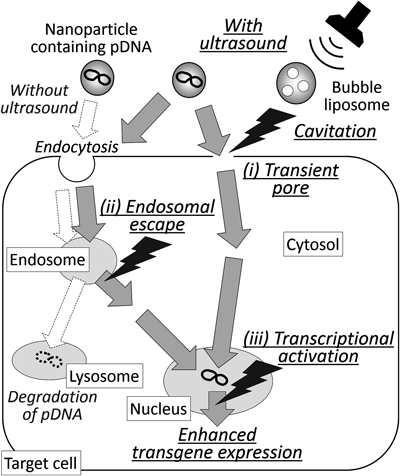
The combination of active targeting, such as utilization of receptor-mediated endocytosis, with physical stimuli would greatly improve target specificity. Moreover, the combination of several physical stimuli such as electric pulse with ultrasound53,54) could further enhance the efficacy of the nanoparticles. On the other hand, in the tissue suction-mediated transfection, the transgene expression was not enhanced in the case of the stomach.42) This is probably due to the poor biodistribution of pDNA in the stomach after intravenous injection. In contrast, naked pDNA instillation onto the stomach without the physical stimuli resulted in efficient transgene expression.55–57) As mentioned above, physical stimuli such as rubbing the stomach surface could enhance the transgene expression.43) Thus, appropriate selection of the administration routes would also be an important issue in the physical stimuli-mediated delivery of the nanoparticles.58) In general, endocytosis is the primary cellular uptake mechanism of nanoparticles. For example, in the case of naked pDNA instillation onto the stomach, the cellular uptake mechanism was macropinocytosis.59) Since endocytosed nanoparticles are generally degraded in lysosomes, the uptake mechanism greatly governs the intracellular fate of the nanoparticles. Therefore, appropriate selection of physical stimuli which change the uptake mechanism would be a useful strategy for developing effective cancer therapy using nanoparticles.
REFERENCES
- 1) Fumoto S, Kawakami S, Ito Y, Shigeta K, Yamashita F, Hashida M. Enhanced hepatocyte-selective in vivo gene expression by stabilized galactosylated liposome/plasmid DNA complex using sodium chloride for complex formation. Mol. Ther., 10, 719–729 (2004).
- 2) Yoshikawa N, Sakamoto K, Mizuno S, Sakaguchi J, Miyamoto H, Mine T, Sasaki H, Fumoto S, Nishida K. Multiple components in serum contribute to hepatic transgene expression by lipoplex in mice. J. Gene Med., 13, 632–643 (2011).
- 3) Fumoto S, Kawakami S, Shigeta K, Higuchi Y, Yamashita F, Hashida M. Interaction with blood components plays a crucial role in asialoglycoprotein receptor-mediated in vivo gene transfer by galactosylated lipoplex. J. Pharmacol. Exp. Ther., 315, 484–493 (2005).
- 4) Nagayama S, Ogawara K, Minato K, Fukuoka Y, Takakura Y, Hashida M, Higaki K, Kimura T. Fetuin mediates hepatic uptake of negatively charged nanoparticles via scavenger receptor. Int. J. Pharm., 329, 192–198 (2007).
- 5) Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J. Control. Release, 164, 138–144 (2012).
- 6) Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv. Drug Deliv. Rev., 63, 152–160 (2011).
- 7) Kawakami S, Fumoto S, Nishikawa M, Yamashita F, Hashida M. In vivo gene delivery to the liver using novel galactosylated cationic liposomes. Pharm. Res., 17, 306–313 (2000).
- 8) Fumoto S, Nakadori F, Kawakami S, Nishikawa M, Yamashita F, Hashida M. Analysis of hepatic disposition of galactosylated cationic liposome/plasmid DNA complexes in perfused rat liver. Pharm. Res., 20, 1452–1459 (2003).
- 9) Terada T, Iwai M, Kawakami S, Yamashita F, Hashida M. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J. Control. Release, 111, 333–342 (2006).
- 10) Hatakeyama H, Akita H, Kogure K, Oishi M, Nagasaki Y, Kihira Y, Ueno M, Kobayashi H, Kikuchi H, Harashima H. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther., 14, 68–77 (2007).
- 11) Manchun S, Dass CR, Sriamornsak P. Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sci., 90, 381–387 (2012).
- 12) Frandsen SK, Gissel H, Hojman P, Tramm T, Eriksen J, Gehl J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res., 72, 1336–1341 (2012).
- 13) Yao C, Guo F, Li C, Sun C. Gene transfer and drug delivery with electric pulse generators. Curr. Drug Metab., 14, 319–323 (2013).
- 14) Au JT, Wong J, Mittra A, Carpenter S, Haddad D, Carson J, Jayaraman S, Monette S, Solomon SB, Ezell P, Fong Y. Irreversible electroporation is a surgical ablation technique that enhances gene transfer. Surgery, 150, 474–479 (2011).
- 15) Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther., 7, 2023–2027 (2000).
- 16) Suzuki R, Takizawa T, Negishi Y, Hagisawa K, Tanaka K, Sawamura K, Utoguchi N, Nishioka T, Maruyama K. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J. Control. Release, 117, 130–136 (2007).
- 17) Ueno Y, Sonoda S, Suzuki R, Yokouchi M, Kawasoe Y, Tachibana K, Maruyama K, Sakamoto T, Komiya S. Combination of ultrasound and bubble liposome enhance the effect of doxorubicin and inhibit murine osteosarcoma growth. Cancer Biol. Ther., 12, 270–277 (2011).
- 18) Hamano N, Negishi Y, Omata D, Takahashi Y, Manandhar M, Suzuki R, Maruyama K, Nomizu M, Aramaki Y. Bubble liposomes and ultrasound enhance the antitumor effects of AG73 liposomes encapsulating antitumor agents. Mol. Pharm., 10, 774–779 (2013).
- 19) Negishi Y, Omata D, Iijima H, Takabayashi Y, Suzuki K, Endo Y, Suzuki R, Maruyama K, Nomizu M, Aramaki Y. Enhanced laminin-derived peptide AG73-mediated liposomal gene transfer by bubble liposomes and ultrasound. Mol. Pharm., 7, 217–226 (2010).
- 20) Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Enhanced transfection efficiency into macrophages and dendritic cells by a combination method using mannosylated lipoplexes and bubble liposomes with ultrasound exposure. Hum. Gene Ther., 21, 65–74 (2010).
- 21) Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Development of an ultrasound-responsive and mannose-modified gene carrier for DNA vaccine therapy. Biomaterials, 31, 7813–7826 (2010).
- 22) Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Suppression of melanoma growth and metastasis by DNA vaccination using an ultrasound-responsive and mannose-modified gene carrier. Mol. Pharm., 8, 543–554 (2011).
- 23) Dickson JA. Hyperthermia in the treatment of cancer. Lancet, 313, 202–205 (1979).
- 24) Moyer HR, Delman KA. The role of hyperthermia in optimizing tumor response to regional therapy. Int. J. Hyperthermia, 24, 251–261 (2008).
- 25) Li L, ten Hagen TL, Bolkestein M, Gasselhuber A, Yatvin J, van Rhoon GC, Eggermont AM, Haemmerich D, Koning GA. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J. Control. Release, 167, 130–137 (2013).
- 26) Li L, ten Hagen TL, Hossann M, Süss R, van Rhoon GC, Eggermont AM, Haemmerich D, Koning GA. Mild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacy. J. Control. Release, 168, 142–150 (2013).
- 27) Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int. J. Hyperthermia, 23, 89–104 (2007).
- 28) Park SM, Kim MS, Park SJ, Park ES, Choi KS, Kim YS, Kim HR. Novel temperature-triggered liposome with high stability: Formulation, in vitro evaluation, and in vivo study combined with high-intensity focused ultrasound (HIFU). J. Control. Release, 170, 373–379 (2013).
- 29) Kumar CS, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev., 63, 789–808 (2011).
- 30) Miyako E, Deguchi T, Nakajima Y, Yudasaka M, Hagihara Y, Horie M, Shichiri M, Higuchi Y, Yamashita F, Hashida M, Shigeri Y, Yoshida Y, Iijima S. Photothermic regulation of gene expression triggered by laser-induced carbon nanohorns. Proc. Natl. Acad. Sci. U.S.A., 109, 7523–7528 (2012).
- 31) Yang W, Barth RF, Adams DM, Soloway AH. Intratumoral delivery of boronated epidermal growth factor for neutron capture therapy of brain tumors. Cancer Res., 57, 4333–4339 (1997).
- 32) Pan XQ, Wang H, Shukla S, Sekido M, Adams DM, Tjarks W, Barth RF, Lee RJ. Boron-containing folate receptor-targeted liposomes as potential delivery agents for neutron capture therapy. Bioconj. Chem., 13, 435–442 (2002).
- 33) Liu F, Huang L. Electric gene transfer to the liver following systemic administration of plasmid DNA. Gene Ther., 9, 1116–1119 (2002).
- 34) Thanaketpaisarn O, Nishikawa M, Yamashita F, Hashida M. Tissue-specific characteristics of in vivo electric gene: transfer by tissue and intravenous injection of plasmid DNA. Pharm. Res., 22, 883–891 (2005).
- 35) Chabot S, Rosazza C, Golzio M, Zumbusch A, Teissié J, Rols MP. Nucleic acids electro-transfer: from bench to bedside. Curr. Drug Metab., 14, 300–308 (2013).
- 36) Suzuki R, Takizawa T, Negishi Y, Utoguchi N, Sawamura K, Tanaka K, Namai E, Oda Y, Matsumura Y, Maruyama K. Tumor specific ultrasound enhanced gene transfer in vivo with novel liposomal bubbles. J. Control. Release, 125, 137–144 (2008).
- 37) Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R, Hirata K, Taira Y, Utoguchi N, Negishi Y, Nakagawa S, Maruyama K. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J. Control. Release, 142, 245–250 (2010).
- 38) Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther., 6, 1258–1266 (1999).
- 39) Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum. Gene Ther., 10, 1735–1737 (1999).
- 40) Mukai H, Kawakami S, Hashida M. Renal press-mediated transfection method for plasmid DNA and siRNA to the kidney. Biochem. Biophys. Res. Commun., 372, 383–387 (2008).
- 41) Mukai H, Kawakami S, Kamiya Y, Ma F, Takahashi H, Satake K, Terao K, Kotera H, Yamashita F, Hashida M. Pressure-mediated transfection of murine spleen and liver. Hum. Gene Ther., 20, 1157–1167 (2009).
- 42) Shimizu K, Kawakami S, Hayashi K, Kinoshita H, Kuwahara K, Nakao K, Hashida M, Konishi S. In vivo site-specific transfection of naked plasmid DNA and siRNAs in mice by using a tissue suction device. PLoS ONE, 7, e41319 (2012).
- 43) Mine T, Ishii H, Nakajima S, Yoshikawa N, Miyamoto H, Nakashima M, Nakamura J, Fumoto S, Nishida K. Rubbing gastric serosal surface enhances naked plasmid DNA transfer in rats and mice. Biol. Pharm. Bull., 34, 1514–1517 (2011).
- 44) Fumoto S, Nakajima S, Mine T, Yoshikawa N, Kitahara T, Sasaki H, Miyamoto H, Nishida K. Efficient in vivo gene transfer by intraperitoneal injection of plasmid DNA and calcium carbonate microflowers in mice. Mol. Pharm., 9, 1962–1970 (2012).
- 45) Suzuki T, Shin BC, Fujikura K, Matsuzaki T, Takata K. Direct gene transfer into rat liver cells by in vivo electroporation. FEBS Lett., 425, 436–440 (1998).
- 46) Shimizu K, Kawakami S, Hayashi K, Mori Y, Hashida M, Konishi S. Implantable pneumatically actuated microsystem for renal pressure-mediated transfection in mice. J. Control. Release, 159, 85–91 (2012).
- 47) Un K, Kawakami S, Yoshida M, Higuchi Y, Suzuki R, Maruyama K, Yamashita F, Hashida M. The elucidation of gene transferring mechanism by ultrasound-responsive unmodified and mannose-modified lipoplexes. Biomaterials, 32, 4659–4669 (2011).
- 48) Omata D, Negishi Y, Hagiwara S, Yamamura S, Endo-Takahashi Y, Suzuki R, Maruyama K, Nomizu M, Aramaki Y. Bubble liposomes and ultrasound promoted endosomal escape of TAT-PEG liposomes as gene delivery carriers. Mol. Pharm., 8, 2416–2423 (2011).
- 49) Nishikawa M, Nakayama A, Takahashi Y, Fukuhara Y, Takakura Y. Reactivation of silenced transgene expression in mouse liver by rapid, large-volume injection of isotonic solution. Hum. Gene Ther., 19, 1009–1020 (2008).
- 50) Takiguchi N, Takahashi Y, Nishikawa M, Matsui Y, Fukuhara Y, Oushiki D, Kiyose K, Hanaoka K, Nagano T, Takakura Y. Positive correlation between the generation of reactive oxygen species and activation/reactivation of transgene expression after hydrodynamic injections into mice. Pharm. Res., 28, 702–711 (2011).
- 51) Un K, Kawakami S, Higuchi Y, Suzuki R, Maruyama K, Yamashita F, Hashida M. Involvement of activated transcriptional process in efficient gene transfection using unmodified and mannose-modified bubble lipoplexes with ultrasound exposure. J. Control. Release, 156, 355–363 (2011).
- 52) Mukai H, Kawakami S, Takahashi H, Satake K, Yamashita F, Hashida M. Key physiological phenomena governing transgene expression based on tissue pressure-mediated transfection in mice. Biol. Pharm. Bull., 33, 1627–1632 (2010).
- 53) Yamashita Y, Shimada M, Tachibana K, Harimoto N, Tsujita E, Shirabe K, Miyazaki J, Sugimachi K. In vivo gene transfer into muscle via electro-sonoporation. Hum. Gene Ther., 13, 2079–2084 (2002).
- 54) Yamashita Y, Shimada M, Minagawa R, Tsujita E, Harimoto N, Tanaka S, Shirabe K, Miyazaki J, Maehara Y. Muscle-targeted interleukin-12 gene therapy of orthotopic hepatocellular carcinoma in mice using in vivo electrosonoporation. Mol. Cancer Ther., 3, 1177–1182 (2004).
- 55) Nakamura J, Fumoto S, Shoji K, Kodama Y, Nishi J, Nakashima M, Sasaki H, Nishida K. Stomach-selective gene transfer following the administration of naked plasmid DNA onto the gastric serosal surface in mice. Biol. Pharm. Bull., 29, 2082–2086 (2006).
- 56) Nishi J, Fumoto S, Ishii H, Kodama Y, Nakashima M, Sasaki H, Nakamura J, Nishida K. Improved stomach selectivity of gene expression following microinstillation of plasmid DNA onto the gastric serosal surface in mice. Eur. J. Pharm. Biopharm., 69, 633–639 (2008).
- 57) Fumoto S, Nishi J, Nakamura J, Nishida K. Gene therapy for gastric diseases. Curr. Gene Ther., 8, 187–200 (2008).
- 58) ) Fumoto S, Kawakami S, Hashida M, Nishida K. Targeted Gene Delivery: Importance of Administration Routes. NOVEL GENE THERAPY APPROACHES (Wei M, Good D eds.) Intech, Croatia, pp. 3–31 (2013).
- 59) Fumoto S, Nishi J, Ishii H, Wang X, Miyamoto H, Yoshikawa N, Nakashima M, Nakamura J, Nishida K. Rac-mediated macropinocytosis is a critical route for naked plasmid DNA transfer in mice. Mol. Pharm., 6, 1170–1179 (2009).
- 60) Sakai M, Nishikawa M, Thanaketpaisarn O, Yamashita F, Hashida M. Hepatocyte-targeted gene transfer by combination of vascularly delivered plasmid DNA and in vivo electroporation. Gene Ther., 12, 607–616 (2005).
- 61) Kishida T, Asada H, Satoh E, Tanaka S, Shinya M, Hirai H, Iwai M, Tahara H, Imanishi J, Mazda O. In vivo electroporation-mediated transfer of interleukin-12 and interleukin-18 genes induces significant antitumor effects against melanoma in mice. Gene Ther., 8, 1234–1240 (2001).
- 62) Wells JM, Li LH, Sen A, Jahreis GP, Hui SW. Electroporation-enhanced gene delivery in mammary tumors. Gene Ther., 7, 541–547 (2000).
- 63) Un K, Kawakami S, Yoshida M, Higuchi Y, Suzuki R, Maruyama K, Yamashita F, Hashida M. Efficient suppression of murine intracellular adhesion molecule-1 using ultrasound-responsive and mannose-modified lipoplexes inhibits acute hepatic inflammation. Hepatology, 56, 259–269 (2012).
