2014 年 37 巻 2 号 p. 262-267
2014 年 37 巻 2 号 p. 262-267
Long-term peritoneal dialysis (PD) frequently produces morphological and functional changes of the peritoneum, which makes continuation of PD difficult. Moreover, the progression of peritoneal injury causes complications and poor prognosis. Since therapeutic treatments for peritoneal injury during PD have yet to be established, it is important to diagnose peritoneal injury as early as possible. The aim of this study was to develop a method of monitoring peritoneal function to diagnose peritoneal injury. Model rats of peritoneal injury were prepared by intraperitoneal injection of methylglyoxal (MGO) for five consecutive days. Then, marker substances of various molecular weights (phenolsulfonphthalein, fluorescein isothiocyanate-dextran (FD)-10, FD-40, FD-70, FD-2000 or tetramethylrhodamine-dextran (RD)-10) were injected into the peritoneal cavity. At 120 min after injection, the remaining amounts of all marker substances were significantly decreased in the MGO-treated rats compared with those in the vehicle-treated rats. Molecular weight dependence of the peritoneal permeability was observed. A substance with a molecular weight of approximately 10000 was found to be suitable to diagnose peritoneal injury. Moreover, coadministration of RD-10 with FD-2000 enabled us to monitor enhanced peritoneal permeability and the transfer of water simultaneously, without the recovery of whole PD fluid, even in the case of different ultrafiltration volumes. We demonstrated the usefulness of administering substances to evaluate peritoneal permeability and the transfer of water simultaneously to diagnose peritoneal injury. This study should be valuable for safe and effective PD.
Peritoneal dialysis (PD) is one of the major treatments of end-stage renal diseases and a useful therapy for compensating for decline in some renal functions, such as the elimination of waste from the body. In particular, PD can preserve residual renal function and contribute to improving the quality of life of patients undergoing PD.1–3) Furthermore, in recent years, the strategy of “PD first,” which involves starting with PD and then switching to hemodialysis (HD) after utilizing residual renal function, has been anticipated to produce a good prognosis for patients. However, in spite of its merits, no more than 3.2% of chronic dialysis patients in Japan have received PD.4) A reason for this is that long-term PD frequently produces morphological and functional changes of the peritoneum, which leads to discontinuation of PD.5–9) Moreover, progression of peritoneal injury causes serious complications, such as encapsulating peritoneal sclerosis (EPS).10) Since therapeutic treatments for peritoneal injury and EPS have yet to be established,11,12) patients are forced to switch from PD to HD when peritoneal injury occurs. Therefore, it is necessary to evaluate peritoneal injury as early as possible.
We have already reported that pharmacokinetic analysis of peritoneal hyperpermeability of a marker substance, phenolsulfonphthalein (PSP), might be a potent method for diagnosing peritoneal injury.13) Unfortunately, PSP was well absorbed not only in peritoneal injury rats but also in healthy rats; consequently, it was not suitable as a diagnostic marker of peritoneal injury. On the other hand, we have already analyzed the absorption characteristics of the peritoneum and revealed that substances with a high molecular weight poorly permeated the peritoneum of intra-abdominal organs such as the liver, kidney and stomach14–16) in healthy rats. Here, we hypothesized that molecular weight-dependent absorption characteristics might change in peritoneal injury rats, and decided to investigate the optimum molecular weight associated with a large difference in absorption between healthy and peritoneal injury rats.
In this study, we focused on macromolecular substances and searched for the optimum marker that could be an alternative to PSP, utilizing the increased peritoneal permeability in peritoneal injury rats. Moreover, we developed a convenient diagnosis method for evaluating peritoneal injury using dual macromolecular markers, which enabled us to determine hyperpermeability with the correction of water transfer by measuring the concentration of each marker instead of the amount.
Methylglyoxal (MGO) and PSP were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). Fluorescein isothiocyanate-dextrans (FDs) were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Tetramethylrhodamine-dextran (RD) was obtained from Invitrogen (Carlsbad, CA, U.S.A.). Hematoxylin and eosin (H&E) were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Embedding medium Tissue-Tek® O.C.T. compound for frozen tissue specimens was obtained from Sakura Finetek Japan Co., Ltd. (Tokyo, Japan). PD fluid Dianeal-N PD-4 1.5 was obtained from Baxter Ltd. (Tokyo, Japan).
AnimalsMale Wistar rats (210–277 g) were housed in a cage in an air-conditioned room and maintained on a standard laboratory diet (MF, Oriental Yeast, Co., Ltd., Tokyo, Japan) and water ad libitum. All animal experiments in the present study conformed to the Guidelines for Animal Experimentation of Nagasaki University.
Preparation of Peritoneal Injury RatsPeritoneal injury rats were established following the protocol reported previously.17) Briefly, 20 mL of 20 mM MGO dissolved in saline was injected intraperitoneally for five consecutive days. In a control experiment, saline was intraperitoneally injected in the same fashion.
Assessment of Peritoneal Injury with MarkersPeritoneal injury rats were anesthetized with sodium pentobarbital (60 mg/kg intramuscular injection). Then, the skin was cut and the abdominal wall exposed. The marker substance for evaluation of peritoneal injury was injected into the peritoneal cavity using a syringe with a 21 G×1 1/2″ needle (Nipro Co., Ltd., Osaka, Japan). The needle was then removed and the pinhole in the abdominal wall was closed with surgical adhesive (Aron Alpha A; Daiichi Sankyo Pharmaceutical Co., Ltd., Tokyo, Japan) to prevent leakage of the fluid. Conditions using a single marker and dual markers were as follows.
Single Marker: One milligram (100 µg/mL, 10 mL) of marker substance (PSP molecular weight (MW) 354), FD-10 (MW 10000), FD-40 (MW 40000), FD-70 (MW 70000) and FD-2000 (MW 2000000) dissolved in phosphate-buffered saline (PBS) was injected into the peritoneal cavity and then the whole residual solution was recovered 120 min after injection.
Dual Markers: FD-2000 (0.05 mg, 5 µg/mL) and RD-10 (MW 10000, 0.1 mg, 10 µg/mL) dissolved in 10 mL of PBS, PD fluids (PDFs) (Dianeal-N PD-4 1.5 PDF (containing 1.36% glucose)) or 2.5 PDF (containing 2.27% glucose) were injected into the peritoneal cavity. The entire residual solution was recovered 120 min after injection. To evaluate the time course of absorption of markers dissolved in 1.5 PDF, samples were collected from two separate groups (group 1 for 10, 60, 120 min and group 2 for 120, 240, 360 min after injection) to minimize the effect of volume loss. To evaluate the effect of injected volume, FD-2000 and RD-10 dissolved in 5 mL of 1.5 PDF were injected in the peritoneal cavity.
Quantification of MarkersThe obtained samples were centrifuged at 1600×g for 15 min and the supernatants were obtained. Quantification conditions using single marker and dual markers were as follows.
Single Marker: PSP in the sample solution was quantified according to the method reported by Hart and Schanker.18) Residual solution in the peritoneal cavity (100 µL) was diluted with 2 mL of 1 M NaOH, and immediately absorbance at 560 nm was measured using a spectrophotometer (UV-1600, Shimadzu Corp., Kyoto, Japan). The sample of FDs (100 µL) was diluted with 2 mL of PBS, and was then measured using a spectrofluorophotometer (RF-1500, Shimadzu Corp.) at excitation and emission wavelengths of 489 and 515 nm, respectively.
Dual Markers: For the quantification of FD-2000, the sample (80 µL) was diluted with 2 mL of PBS and then measured using the spectrofluorophotometer at excitation and emission wavelengths of 494 and 521 nm, respectively. For quantification of RD-10, the sample (160 µL) was diluted with 2 mL of PBS and then measured using the spectrofluorophotometer at excitation and emission wavelengths of 555 and 580 nm, respectively. In the experiment using PDFs, the amounts of samples and PBS were reduced by one-quarter, and the measurement was performed using a microcuvette.
Histological StudyHistological analysis was performed by H&E staining. After recovery of the residual solution in the peritoneal cavity, the abdominal walls were cut and fixed with 4% paraformaldehyde. Then, they were embedded in O.C.T. compound and frozen at −80°C. They were sliced into 10 µm sections using a microtome (Retoratome REM-710; Yamato Kohki Industrial Co., Ltd., Saitama, Japan) and stained by H&E. Stained samples were observed using a microscope with a 10×objective lens.
Statistical AnalysisStatistical comparisons were performed by unpaired Student’s t-test for two groups (Figs. 2a, 3, 4, 6) or by two-way ANOVA followed by Tukey’s test for multiple comparisons (Fig. 5). Values of p<0.05 were considered to indicate statistical significance.
To confirm the preparation of the peritoneal injury model, the peritoneal injury caused by MGO treatment for five consecutive days was histologically assessed by H&E staining. Figure 1 shows photographs of the peritoneum of an intact rat, a saline-treated rat (vehicle) and an MGO-treated rat. The peritoneum of MGO-treated rat seemed to be thicker than those of intact rat and the vehicle-treated one (Fig. 1).
Exploring the Optimum Molecular Weight to Evaluate Peritoneal InjuryTo determine the optimum molecular weight of a marker that could be used to evaluate peritoneal injury, we administered several compounds (MW 354–2000000) into the peritoneal cavity of vehicle- or MGO-treated rats. The remaining amounts of PSP, FD-10, FD-40, FD-70 and FD-2000 were significantly decreased in the MGO-treated rats compared with those in the vehicle-treated ones (Fig. 2a). Moreover, the remaining amounts of the markers were dependent on their molecular weight. The ratio of the remaining amount of FD-10 in MGO-treated rat to that in saline-treated rat (MGO/vehicle ratio) was the lowest among the six markers (Fig. 2b).
Establishment of a Method for Evaluating Peritoneal Injury with Dual MarkersThe concentrations of FD-2000 and RD-10 were significantly decreased in the MGO-treated rats compared with those with the vehicle (Figs. 3a, b). The ratio of the concentration of RD-10 to FD-2000 (RD-10/FD-2000 ratio) also significantly decreased in the MGO-treated rats (Fig. 3c). Figure 4 shows the time courses of FD-2000 and RD-10 concentrations and the RD-10/FD-2000 ratio when the markers were dissolved in 1.5 PDF. At every point, the concentration of RD-10 and the RD-10/FD-2000 ratio in MGO-treated rats were significantly lower than those with vehicle (Fig. 4).
The recovery volume of peritoneal fluid 120 min after injection was significantly decreased in the MGO-treated rats, and was larger in the 2.5 PDF group than in the 1.5 PDF group, irrespective of the existence of peritoneal injury (Fig. 5a). Regardless of the concentration of glucose in PDFs, RD-10/FD-2000 ratios were similar in vehicle- and MGO-treated rats, and significantly decreased in the MGO-treated rats (Fig. 5b).
In the clinical use of PDFs, the initial volume of PDFs was shown to be dependent on patient conditions. To evaluate the effect of the initial volume of PDFs on the evaluation of peritoneal injury, we decreased the administered volume. Figure 6 shows the recovery volumes of peritoneal fluid and RD-10/FD-2000 ratios when the initial volumes of RD-10 and FD-2000 were halved. Both volume changes 120 min after injection and the RD-10/FD-2000 ratio were similar in vehicle- and MGO-treated rats. In addition, it was shown to be possible to evaluate peritoneal injury even when the initial volume was decreased (Fig. 6b).
In this study, we administered several compounds to the peritoneal cavity of peritoneal injury rats to determine the optimum molecular weight of a marker and to establish a method for the evaluation of peritoneal injury.
We prepared peritoneal injury rats treated by MGO. MGO is a glucose degradation product and its existence in PDF has been confirmed.19) Therefore, the MGO-treated model rats of peritoneal injury are thought to reflect the clinical condition. It has been reported that the treatment of MGO increased the gene expression of vascular endothelial growth factor, transforming growth factor beta, collagen 1 and matrix metalloproteinase-2, which are factors related to angiogenesis, fibrosis and cell filtration.20) In fact, MGO-treated rats exhibited morphological alterations of the peritoneum, such as angiogenesis, fibrosis and cell filtration.21) Figure 1c shows that the peritoneum of an MGO-treated rat was thickened compared with that with vehicle, confirming that peritoneal morphological alterations were caused by MGO treatment in the present study.
We have already reported that the absorption of PSP increased in MGO-treated rats.13) However, PSP can barely be used to diagnose peritoneal injury because it is well absorbed in saline-treated rats. Therefore, we needed to determine the optimum molecular weight of a compound that is well absorbed only in peritoneal injury. In our previous study, we showed that the absorption of compounds from an organ’s surface depended on their molecular weight in healthy rats.14–16) Thus, we considered that absorption from the peritoneal cavity might also depend on molecular weight in peritoneal injury rats. To determine the optimum molecular weight of a marker, we injected PSP and FDs into the peritoneal cavity of rats and quantified the amounts of marker remaining in the peritoneal cavity 120 min after injection (Fig. 2). As a result, the remaining amounts of all tested marker substances were significantly decreased in MGO-treated rats. Above all, FD-10 was well absorbed in peritoneal injury rats but poorly absorbed in those treated with vehicle, suggesting that a compound with a molecular weight of approximately 10000 is suitable for the evaluation of peritoneal injury. The difference in absorption might have been due to peritoneal morphological changes caused by MGO treatment. However, the detailed mechanisms behind the differences in absorption are unclear; therefore, further studies investigating the mechanisms of enhanced absorption from the peritoneal cavity of MGO-treated rats are needed.
We revealed that a molecular weight of approximately 10000 is suitable for evaluation of peritoneal injury, but the recovery of whole PD effluent from patients on PD is difficult. Therefore, we proposed an evaluation method using the concentration of markers; such a method enabled us to assess peritoneal function by the recovery of a portion of PD effluent. However, a method of evaluation using the concentration of markers was inadequate because the concentration of the marker with a molecular weight of 10000 was changed by not only absorption but also ultrafiltration capacity. In general, the ultrafiltration capacity differs among individuals owing to differences in the treatment approaches, such as the type and volume of PDFs and the frequency of PD, as well as the intrinsic peritoneal permeability.22–26) Long-term PD and peritonitis are risk factors causing angiogenesis and then increasing the effective peritoneal surface area (EPSA).27–29) Increased EPSA plays a role in the hypertransport of water and is responsible for individual variation of ultrafiltration.30) To resolve this, we decided to use a marker with a molecular weight of 2000000 for evaluation of the transfer of water because it was poorly absorbed from the peritoneal cavity to the body in peritoneal injury rats and those treated with vehicle (Fig. 2).
After FD-2000 (MW 2000000) and RD-10 (MW 10000) were simultaneously administered, we performed the evaluation of peritoneal injury using the RD-10/FD-2000 ratio, which could correct for the transfer of water and diagnose peritoneal injury. The RD-10/FD-2000 ratio was significantly decreased in MGO-treated rats (Fig. 3). Collecting time of PD effluent could be set 60 min or later after injection because of large differences in RD-10/FD-2000 ratios between vehicle- and MGO-treated rats (Fig. 4c). Moreover, when the glucose concentration in PDFs or the dosage volume changed, the RD-10/FD-2000 ratio was almost constant and reflected the grade of peritoneal injury (Figs. 5, 6). Currently, the peritoneal equilibration test (PET) is widely used for the evaluation of peritoneal function. However, it requires 2.5 PDF of 2 L for a dwelling time of 4 h. Our evaluation method, using the RD-10/FD-2000 ratio, is superior to PET in terms of being able to be used under various conditions, such as different types and volumes of PDFs, as well as times of collecting PD effluent. Thus, patients can evaluate peritoneal function during the typical performance of PD. In case of PET, it is necessary to measure the concentrations of creatinine in the blood and PDF effluent and glucose in the PDF effluent. Both creatinine and glucose are endogenous substances. In contrast, our method uses exogenous substances; thus, the accuracy of our method may be superior to that of PET. Moreover, sensitivity of our method would be good because of the measurement of fluorescence. Furthermore, it would also be useful as a screening procedure to develop treatment methods for peritoneal injury in experimental animals. Although the degree of peritoneal injury is not clear in this experiment and it is necessary to reveal this and determine a cut-off value in order to develop a method for the early diagnosis of peritoneal injury, the RD-10/FD-2000 ratio could be used to diagnose peritoneal injury under conditions with individual differences in future clinical use. To apply our method in the clinical settings, it is necessary to evaluate safety of RD-10 and FD-2000. In this study, we indicated the importance of molecular weight of the markers (Fig. 2a). Therefore, it is possible to search other suitable markers which have molecular weight of approximately 10000 and 2000000 even if RD-10 and FD-2000 have toxicity.
In conclusion, we discovered that a molecular weight of approximately 10000 is suitable for the diagnosis of peritoneal injury. Furthermore, the RD-10/FD-2000 ratio could be used to evaluate peritoneal injury regardless of the ultrafiltration volume. This study should contribute to a convenient diagnosis of peritoneal injury and lead to safe and effective PD.

Intact rat (a). Rat treated with saline for five consecutive days (Vehicle) (b). Rat treated with MGO for five consecutive days (c). Arrows indicate the peritoneum.
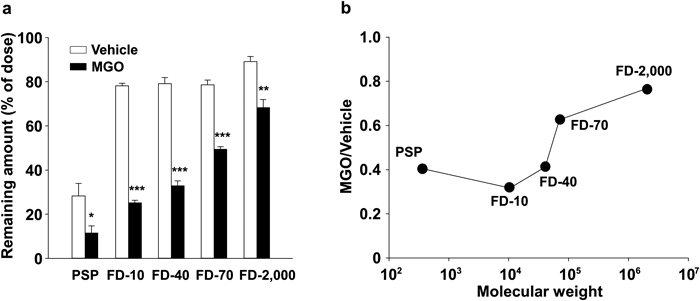
Remaining amounts of PSP, FD-10, FD-40, FD-70 and FD-2000 in the peritoneal cavity 120 min after intraperitoneal injection at a dose of 1 mg into rats treated with saline or MGO for five consecutive days (a). The ratio of the remaining amount of markers in MGO-treated rats to that in saline-treated rats (b). Each bar represents the mean+S.E. for at least four experiments. * p<0.05, ** p<0.01, *** p<0.001 compared with saline-treated rats.

The concentration of FD-2000 (a) and RD-10 (b) in the peritoneal cavity 120 min after intraperitoneal injection into rats treated with saline or MGO for five consecutive days. Initial concentrations of FD-2000 and RD-10 were 5 and 10 µg/mL, respectively. The evaluation of peritoneal injury using the ratio of RD-10 to FD-2000 (c). Each bar represents the mean+S.E. for four experiments. * p<0.05, *** p<0.001 compared with saline-treated rats.

Time courses of the concentrations of FD-2000 (a) and RD-10 (b) dissolved in PDFs in the peritoneal cavity 10, 60, 120, 240 and 360 min after intraperitoneal injection into rats treated with saline or MGO for five consecutive days. Initial concentrations of FD-2000 and RD-10 were 5 and 10 µg/mL, respectively. The evaluation of peritoneal injury using the ratio of RD-10 to FD-2000 (c). Each symbol represents the mean±S.E. for four experiments. * p<0.05, ** p<0.01, *** p<0.001 compared with saline-treated rats.

The residual solution in the peritoneal cavity 120 min after intraperitoneal injection to rats treated with saline or MGO for five consecutive days (a). The evaluation of peritoneal injury using the ratio of RD-10 to FD-2000 dissolved in PDFs (b). Each bar represents the mean+S.E. for at least three experiments. *** p<0.001 compared with saline-treated rats. ## p<0.01, ### p<0.001 compared with 1.5 PDFs.

The effect of dosage volumes on the residual solution in the peritoneal cavity 120 min after intraperitoneal injection in rats treated with saline or MGO for five consecutive days (a). The evaluation of peritoneal injury using the ratio of RD-10 to FD-2000 dissolved in PDFs (b). Each bar represents the mean+S.E. for at least three experiments. N.S. means not significant.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.