2014 Volume 37 Issue 3 Pages 454-460
2014 Volume 37 Issue 3 Pages 454-460
Despite the increasing sales of black cohosh (the dried rhizome and root of Cimicifuga racemosa L.) in the world herbal market, these products have continuous adulteration issues. The botanical authenticity of the black cohosh products is the first important step for ensuring their quality, safety and efficacy. In this study, we genetically identified the botanical sources of 10 black cohosh products and 5 Cimicifuga Rhizome crude drugs of Japanese Pharmacopoeia grade, and analyzed the metabolic profiling of 25 black cohosh products using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Consequently, we found that C. dahurica and possibly C. foetida are misused as sources of the black cohosh products and in some cases, the extracts of black cohosh were adulterated with the plant materials of C. dahurica. We demonstrated that these three species can be distinguished by three marker compounds in a specific mass range. These results must be helpful in establishing regulations for the safe use of the black cohosh products.
Black cohosh (Cimicifuga racemosa (L.) NUTT.) is one of the most popular medicinal herbs in North America and Europe.1,2) It was historically used by Native Americans for a variety of maladies including rheumatism, malaria, and gynecological complaints and was adopted by early European settlers for treating menopausal symptoms.3) In modern phytotherapy, black cohosh extracts or materials are used in dietary supplements, non-prescription drugs and health food products to chiefly alleviate symptoms of menopause, such as hot flashes and other vasomotor symptoms.4–6)
Black cohosh is officially defined as the dried rhizome and root of Cimicifuga racemosa (L.) NUTT. of the family Ranunculaceae under the Latin name of “Cimicifugae Rhizoma” in the European Pharmacopoeia7) and the United States Pharmacopoeia, the National Formulary.8) On the other hand, some congeneric species native to Asia are also widely used as traditional medicines due to their antipyretic, analgestic and anti-inflammatory properties. For example, the rhizome of C. dahurica (TURCZ.) MAXIM., C. foetida L. and C. heracleifolia KOM. are prescribed as Sheng-ma with the Latin name of “Cimicifugae Rhizoma” in the Chinese Pharmacopoeia,9) and the Japanese Pharmacopoeia prescribes C. simplex (DC.) WORMSK. in addition to the above three species as the botanical origin of Cimicifugae Rhizoma (indicated Cimicifuga Rhizome (JP 16) in this study).10) This complicated situation causes a concern that black cohosh products are misbranded or adulterated with other Cimicifuga species. Indeed, an evaluation study of 11 black cohosh products in the U.S. market using HPLC reported that 4 of them contained Asian species.11) Moreover, the increased market demands along with the case reports of liver toxicity associated with products labeled as black cohosh have led to both suspicion and actual identification of the economic adulteration of commercial black cohosh supplies. Since most of all black cohosh products sold as health food products in Japan are imported from the U.S., evaluation and appropriate regulation should be required for them.
The chemistry of black cohosh has been studied since the 1960 s and ca. 60 triterpene glycosides, such as actein (1), 23-epi-26-deoxyactein (2) (Fig. 1) and ca. 30 polyphenols have been isolated as the most frequently encountered compounds.12–14) Most of these secondary metabolites are found not only in C. racemosa, but also in other Cimicifuga species and it makes the identification of the botanical source of black cohosh difficult. While numerous efforts have already been made to establish effective methods for the identification of the botanical source of black cohosh products with the suitable marker compounds, by LC-UV, LC-evaporative light scattering detection (ELSD), LC-MS, liquid chromatography-tandem mass spectrometry (LC-MS/MS), high performance TLC (HPTLC), GC-MS and high-field NMR analysis,15–19) only a few experiments have been performed for evaluating the authenticity of commercial products.11,17) In this study, 25 black cohosh products were genetically and chemically evaluated to identify their botanical source, and a discrimination method between the black cohosh and related species with limited number of marker compounds was established.

Six commercially available nonprescription drugs containing black cohosh extracts were purchased from pharmacies in Europe and 19 health food products containing black cohosh roots and/or dry extracts were purchased through the internet. The Cimicifuga Rhizome (JP 16) samples were purchased from different companies in Japan. These products are listed in Table 1.
| Sample ID | Classa) | Distributor country | Form | State |
|---|---|---|---|---|
| BC-1 | A | U.S.A. | Capsule | Powdered plant material |
| BC-2 | A | U.S.A. | Tablet | Dry extract |
| BC-3 | A | U.S.A. | Capsule | Powdered plant material |
| BC-4 | A | U.S.A. | Capsule | Powdered plant material and dry extract |
| BC-5 | A | U.S.A. | Tablet | Dry extract |
| BC-6 | A | U.S.A. | Capsule | Powdered plant material |
| BC-7 | A | U.S.A. | Capsule | Powdered plant material and dry extract |
| BC-8 | A | U.S.A. | Capsule | Dry extract |
| BC-9 | A | U.S.A. | Capsule | Powdered plant material and dry extract |
| BC-10 | A | U.S.A. | Capsule | Dry extract |
| BC-11 | A | U.S.A. | Capsule | Dry extract |
| BC-12 | A | U.S.A. | Capsule | Powdered plant material |
| BC-13 | A | U.S.A. | Capsule | Dry extract |
| BC-14 | A | U.S.A. | Tablet | Dry extract |
| BC-15 | A | U.S.A. | Capsule | Powdered plant material |
| BC-16 | A | U.S.A. | Capsule | Powdered plant material and dry extract |
| BC-17 | A | U.S.A. | Tablet | Dry extract |
| BC-18 | A | U.S.A. | Tablet | Dry extract |
| BC-19 | A | U.S.A. | Capsule | Powdered plant material and dry extract |
| BC-M1 | B | Germany | Tablet | Dry extract |
| BC-M2 | B | Germany | Tablet | Dry extract |
| BC-M3 | B | Germany | Tablet | Dry extract |
| BC-M4 | B | Germany | Capsule | Dry extract |
| BC-M5 | B | Germany | Tablet | Dry extract |
| BC-M6 | B | Switzerland | Tablet | Dry extract |
| CR-1 | C | Japan | Cut crude drug | |
| CR-2 | C | Japan | Cut crude drug | |
| CR-3 | C | Japan | Cut crude drug | |
| CR-4 | C | Japan | Cut crude drug | |
| CR-5 | C | Japan | Cut crude drug |
a) A: health food products, B: nonprescription drugs, C: crude drugs.
Actein (1) and 23-epi-26-deoxyactein (2) used as authentic compounds were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). HPLC grade acetonitrile and Milli Q water were used for the LC-MS/MS analysis. All other reagents were of guaranteed grade.
Molecular IdentificationHealth food products BC-1, 3, 4, 6, 7, 9, 12, 15, 16, and 19 and Cimicifuga Rhizome (JP 16) samples CR-1 to 5 were analyzed as follows. The total DNA was extracted from 20–35 mg of powdered samples using a DNeasy™ Plant Mini Kit (QIAGEN, Germany). The partial region between the intergenic transcribed spacer (ITS) 1 and ITS2 was amplified via polymerase chain reaction (PCR) using KOD Plus DNA polymerase (Toyobo, Japan) with specific primers CS-1 (5′-GGA AGT AAA AGT CGT AAC AAG G-3′), L-CA2′ (5′-TCC TCC GCT TAT TGA TAT GC-3′) under the following conditions: 94°C for 2 min, 35 cycles of 98°C for 10 s, 55°C for 30 s and 68°C for 1 min, then 68°C for 2 min. Pl-CA1′ (5′-TAT CCG TTG CCG AGA GTC-3′) was used as a reverse primer instead of L-CA2′ for amplification of the ITS1 region. The plastidic trnL-F region was also amplified from the health food product BC-15 using the BIOTAQ™ HS DNA polymerase (Bioline, U.K.) with specific primers BC-trn-S5-race-CTA (5′-TCC TGA GCC AAA TCC TGT TCT A-3′), BC-trn-AS1 (5′-TCA GAC TAT GGA GTG ACT GAC TGA TTT GA-3′) under the following conditions: 95°C for 10 min, 40 cycles of 94°C for 30 s, 60°C for 1 min and 72°C for 1 min, then 72°C for 7 min. The resulting PCR products were detected by a Microchip Electrophoresis System MCE-202 MultiNA (Shimadzu, Japan), purified using a MinElute PCR Purification Kit (QIAGEN, Germany) and directly sequenced. Subcloning of the amplicon into plasmid vectors was performed as required using a Zero Blunt TOPO® PCR Cloning Kit (Invitrogen, U.S.A.) or a TOPO® TA Cloning Kit (Invitrogen).
Sequencing reactions of the purified PCR products were performed using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, U.S.A.) and each sequence was determined by an ABI Prism 3130 Avant genetic analyzer (Applied Biosystems, U.S.A.) equipped with a 50-cm capillary array. The obtained sequences were compared to the sequences in the GenBank to identify the source plant species (Table S1).
LC-MS/MS AnalysisA 0.25 mg/mL aliquot of actein and 0.20 mg/mL aliquot of 23-epi-26-deoxyactein were prepared as standard solutions by dissolving weighed amounts in methanol. The black cohosh products or Cimicifuga Rhizome (JP16) samples were ground into a fine powder and 400.0 mg was weighed into 10 mL flasks. Each sample was extracted by 9 mL of 75% methanol with sonication for 3 h in a water bath. The resulting suspension was filled to the final volume of 10.00 mL and centrifuged at 2000 rpm for 5 min to obtain the supernatant. Prior to the LC-MS/MS analysis, each extract was diluted 4-fold and filtered through a 0.45 µm Ultrafree-MC centrifugal filter unit (Millipore, U.S.A.).
The 10 µL of the filtrate was injected and LC-MS/MS analyses were carried out using a Prominence UFLC (Shimadzu, Japan)–LTQ Orbitrap XL (Thermo Fisher Scientific, U.S.A.) equipped with a Hypersil-GOLD (2.1×100 mm, 1.9 µm; Thermo Fisher Scientific) in the gradient mode at the flow rate of 0.2 mL/min and 40°C. The solvent system consisted of 0.1% acetic acid in deionized water (solvent A) and 0.1% acetic acid in acetonitrile (solvent B). The gradient profile was as follows: 5–70% B (30 min) to 100% B (35 min). Mass spectra were obtained in the electrospray ionization (ESI)-negative mode using the following parameters: ESI needle voltage, 3000 V; capillary temperature, 300°C. The samples were analyzed in the negative mode because peaks derived from the surfactants and diluents led to difficulty in detecting the metabolites in the positive mode.
Currently, the DNA sequencing is the major method for correct identification of medicinal plants.20) In this study, the botanical sources of the black cohosh health food products containing powdered plant materials (BC-1, 3, 4, 6, 7, 9, 12, 15, 16, 19 in Table 1) were initially identified by the sequence determination (Table S1). Comparison of the nucleotide sequences of the ITS1-ITS2 region (607 bp) revealed that BC-1, 3, 4, and 9 had identical sequences to C. racemosa retrieved from the GenBank Nucleotide Sequence Database (accession no. Z98296), however, the 604 bp sequences of BC-7 and 16 were identical to C. dahurica (Z98284). Since the nucleotide sequences of BC-6, 12 and 19 were hardly determined by direct sequencing, we performed subcloning to obtain their sequences. As a result, the sequences of BC-12 showed 99% identities to those of C. dahurica except for 35 bp insertion that possibly caused by PCR in ITS1 region, whereas very similar sequences to Senna alexandrina (KF815491) were obtained from the clones of BC-6 and 19 with 99% identities. Therefore, we amplified the partial sequences of the ITS1 region (207 bp) on BC-6, 12 and 19 and finally determined the botanical origin of BC-6, 12 and 19 as C. dahurica with >99% sequence identities. By using the specific primer set for the trnL-F region of black cohosh, the nucleotide sequence of BC-15 was identified to C. racemosa (>99% identities with GQ409514), though the fragment of Millet (Panimum miliaceum; accession no. FJ606748) was preferentially amplified by the PCR primer sets for the ITS region (Table 2, Table S1). Thus, at least 5 of 10 health food products contained undeclared C. dahurica. This result did not only agree with the previous report,11) but also revealed that C. dahurica is the major specie for the adulteration of black cohosh products. DNA identification could not be carried out for BC-2, 5, 8, 10, 11, 13, 14, 17, and 18, because they were only dry extracts.
| Sample ID | State | ITS1-ITS2 region | ITS1 region | trnL-F region |
|---|---|---|---|---|
| BC-1 | Powdered plant material | C. racemosa | ― | ― |
| BC-3 | Powdered plant material | C. racemosa | ― | ― |
| BC-4 | Powdered plant material and dry extract | C. racemosa | ― | ― |
| BC-6 | Powdered plant material | Senna alexandrina | C. dahurica | ― |
| BC-7 | Powdered plant material and dry extract | C. dahurica | ― | ― |
| BC-9 | Powdered plant material and dry extract | C. racemosa | ― | ― |
| BC-12 | Powdered plant material | C. dahurica | C. dahurica | ― |
| BC-15 | Powdered plant material | Panimum miliaceum | P. miliaceum | C. racemosa |
| BC-16 | Powdered plant material and dry extract | C. dahurica | ― | ― |
| BC-19 | Powdered plant material and dry extract | Senna alexandrina | C. dahurica | ― |
―, not sequenced
The Japanese Pharmacopoeia defines the botanical origin of Cimicifuga Rhizome as the dried rhizome of C. simplex, C. dahurica, C. foetida, and C. heracleifolia.6) In 1997, Li et al. performed an anatomical study for the botanical origins of 13 commercial samples of Cimicifuga Rhizome collected in Japan and found that 4 of them were composed of C. heracleifolia, 2 were C. dahurica, 2 were C. foetida, and the other 5 were a mixture of C. dahurica and C. heracleifolia or C. foetida.21) Based on this report, several species had been expected for the botanical source of the Cimicifuga Rhizome (JP 16) samples obtained from the Japanese market in 2009. However, determination of the nucleotide sequences of the ITS1-ITS2 region by direct sequencing revealed that 4 samples (CR-1, 2, 4, 5) were composed of C. dahurica (>99% identities with GenBank accession no. Z98284) and the nucleotide sequences of CR-3 obtained by subcloning showed that it contained both C. dahurica (>99% identities with Z98284) and C. heracleifolia (>99% identities with Z98290) (Table 3, Table S1). This result indicated that C. dahurica is widely used for the botanical source of the crude drug at this time. On the basis of these DNA identification results, we used CR-4 as the standard of C. dahurica for the further LC-MS/MS analysis.
| Sample ID | State | ITS1–ITS2 region |
|---|---|---|
| CR-1 | Dried plant material | C. dahurica |
| CR-2 | Dried plant material | C. dahurica |
| CR-3 | Dried plant material | C. dahurica and C. heracleifolia |
| CR-4 | Dried plant material | C. dahurica |
| CR-5 | Dried plant material | C. dahurica |
According to the European Pharmacopoeia, commercial black cohosh drugs must be standardized to actein (1) and 23-epi-26-deoxyactein (2) (Fig. 1). All 6 nonprescription drugs purchased in Europe were extracted by 75% methanol and examined for these compounds by LC-MS/MS analysis.
Due to acetate attachment ([M+CH3COO]−) for the referented actein (1) and 23-epi-26-deoxyactein (2) (Fig. 2a), m/z 735.4 and m/z 719.4 were monitored to identify these standard compounds. The extract ion chromatograms (XIC) clearly showed that the black cohosh drug BC-M4 definitely contained both actein (1) and 23-epi-26-deoxyactein (2) (Fig. 2b) and all 6 drug samples had almost the same chromatograms. This result suggested that European nonprescription drugs are properly using the correct botanical source defined in the EP.

(a) Mixture of referential compounds. 1R, (26R)-actein; 1S, (26S)-actein; 2, 23-epi-26-deoxyactein. (b) BC-M4.
All the 19 health food products were extracted using 75% methanol and examined for standard compounds in the same way as the European nonprescription drugs. Samples were clearly classified into 4 different groups (Groups I to IV) by their chromatographic patterns at m/z 735.4 and m/z 719.4 (Fig. 3).
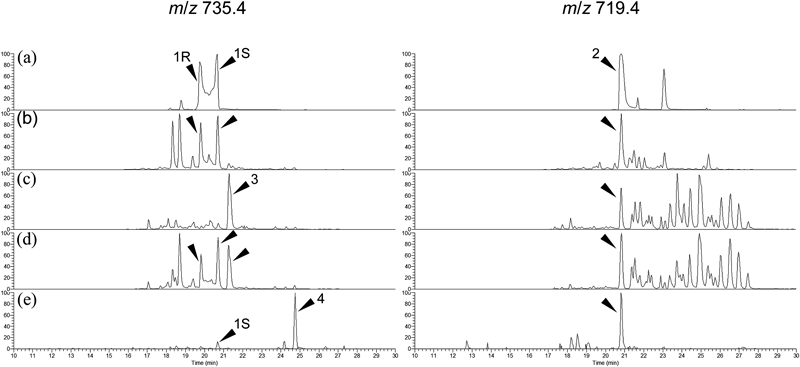
(a) Mixture of referential compounds. (b) BC-10 from Group I. (c) BC-6 from Group II. (d) BC-7 from Group III. (e) BC-18 from Group IV. Peaks: 1R, (26R)-actein; 1S, (26S)-actein; 2, 23-epi-26-deoxyactein; 3, compound X; 4, compound Y.
BC-1, 2, 3, 4, 8, 9, 10, 11, 13, 14, 15, and 17 of Group I showed chromatographic patterns similar to the European nonprescription drugs and contained both actein (1) and 23-epi-26-deoxyactein (2). Since BC-1, 3, 4, 9, and 15 were identified as C. racemosa at the DNA level, the BC samples belonging to Group I were considered to be prepared from the correct botanical source.
BC-6 and 12 of Group II did not contain the actual peak for actein in XIC of m/z 735.4, but a more hydrophobic compound (3) (hereafter, written as compound X) with the same molecular formula of actein and 23-epi-26-deoxyactein (2) was detected (Fig. 3). Since these samples consisted only of powdered plant material (Table 1) and it was genetically identified to C. dahurica (Table 2), it was clearly revealed that these samples were prepared from an incorrect source by mistake or intent and that the chromatographic pattern of Group II represented that of C. dahurica. Compound X (3) at 21.2 min was predicted to be an isomer of actein based on the MS/MS fragmentation, but more investigation is needed for the final identification.
BC-7, 16, and 19 of Group III contained the identical peaks of actein (1) and 23-epi-26-deoxyactein (2) as well as the peak of compound X (3) (Fig. 3). This chromatographic pattern overlapped with the ones of Group I and Group II. Taken together with the results of the DNA identification that determined the plant material of BC-7, 16, and 19 to be C. dahurica, it was suggested that the BC samples in Group III consisted of powdered materials of C. dahurica and an extract prepared from C. racemosa.
BC-5 and 18 were classified to Group IV that did not contain the actual peak for actein in the XIC of m/z 735.4, but another hydrophobic compound (4) (hereafter, written as compound Y) with same molecular formula of actein and 23-epi-26-deoxyactein (2) in a small quantity was detected (Fig. 3). Compound Y (4) at 24.7 min was proposed to be acetylacteol 3-O-arabinoside (Fig. 1) based on the mass spectra and retention time compared to those of the authentic actein (acetylacteol 3-O-xyloside) and published data,17,22) indicating that BC-5 and 18 were also derived from Cimicifuga species. Since acetylacteol 3-O-arabinoside has been isolated only from C. foetida, C. foetida is one possible botanical source of Group IV.
LC-MS/MS Analysis of Cimicifuga Rhizome (JP16) SamplesTo obtain the standard chromatogram of C. dahurica, the 75% methanolic extract of CR-4 was analyzed by LC-MS/MS. As expected, its chromatogram was almost identical to that of Group II. Thus, there was no identical peak for actein (1), but compound X (3) and 23-epi-26-deoxyactein (2) were detected (Fig. 4). This result supported our hypothesis that the source of the BC samples belonging to Group II was C. dahurica, while Group III was mixture of C. racemosa and C. dahurica.

In this study, the botanical sources of 10 black cohosh products and 5 Cimicifuga Rhizome (JP16) samples were genetically identified by DNA analyses, and 6 European nonprescription drugs and 19 health food products of black cohosh were analyzed by LC-MS/MS and the chromatogram patterns were compared to that of a Cimicifuga Rhizome (JP 16) sample derived from C. dahurica. Since most health food products not only consist of plant materials, but also contain various excipient and the plant DNAs are susceptible to degradation after processing the commercial products, optimization of a PCR condition (target genes, length of amplicons, primer pairs, etc.) is often necessary for successful application and sequencing of the DNAs from health food products. During the DNA analyses in this study, the nucleotide sequences from the unlabeled species, Senna alexandrina, were amplified in the ITS1-ITS2 region from BC-6 and 19, and the nucleotide sequences of C. dahurica were confirmed by sequencing of their short ITS1 amplicons. It was considered that a small volume of other plant materials could be contaminated during the black cohosh production, because their manufacturers dealt in various health food products including Senna. The fact that any compounds derived from Senna were not detected by LC-MS also supported the limited contamination. The results of the DNA analyses conclusively showed that half of the black cohosh products containing plant materials were derived from C. dahurica instead of C. racemosa (Table 2).
The results of the LC-MS/MS analyses revealed that all drugs and 12 food products were properly derived from C. racemosa, 2 black cohosh products used C. dahurica instead of C. racemosa as their botanical source, 3 products containing the dry extract of C. racemosa were adulterated with the plant material of C. dahurica and 2 products were likely to be prepared from C. foetida (Table 4). Thus, approximately one-third of the black cohosh health food products examined in this study were misbranded or adulterated.
| Group | State | Botanical source of plant material | Detected compound in XIC | Sample ID | |
|---|---|---|---|---|---|
| m/z 735.4 | m/z 719.4 | ||||
| I | Dry extract | ― | Actein | 23-epi-26-Deoxyactein | BC-M1, M2, M3, M4, M5, M6 BC-2, 8, 10, 11, 13, 14, 17 |
| Powdered plant material | C. racemosa | Actein | 23-epi-26-Deoxyactein | BC-1, 3, 15 | |
| Powdered plant material and dry extract | C. racemosa | Actein | 23-epi-26-Deoxyactein | BC-4, 9 | |
| II | Powdered plant material | C. dahurica | Compound X | 23-epi-26-Deoxyactein | BC-6, 12 |
| III | Powdered plant material and dry extract | C. dahurica | Actein Compound X | 23-epi-26-Deoxyactein | BC-7, 16, 19 |
| IV | Dry extract | ― | Compound Y | 23-epi-26-Deoxyactein | BC-5, 18 |
―, DNA was not obtained.
The adulteration of black cohosh with other lower-priced species is a major problem and the correct identification of the botanical source is a very important step for regulation. Although 23-epi-26-deoxy-actein (2) is used frequently as a marker compound for the standardization of black cohosh, some reports showed that it can be found in many Cimicifuga species other than C. racemosa,16,17) and 23-epi-26-deoxy-actein (2) was indeed detected from Cimicifuga Rhizome (JP16) samples derived from C. dahurica in this study. Thus, 23-epi-26-deoxy-actein (2) does not seem to be a suitable marker compound to verify the identity of the botanical source of black cohosh products. Instead, our results clearly showed that monitoring a specific mass range is useful to evaluate the botanical source of black cohosh products because three compounds with the same formula can serve to distinguish authentic C. racemosa from C. dahurica and C. foetida candidates in the black cohosh products.
Besides identifying peak 1 as actein by comparing to a reference standard, its adducted ion at m/z 735.400 and fragments at m/z 675.37 [M−H]−, 657.36 [M−H2O]−, 591.35, and 525.32 [M−xylose]− were confirmed. Although only a product ion at m/z 675.37 or 675.36 was observed from compound X (3) and compound Y (4), their elemental composition of C37H56O11 was predicted. Among the three compounds with C37H56O11, other than actein, isolated from the Cimicifuga plants as shown in Fig. 1, cimiracemosides A and G are found from C. racemosa, together with actein, while the acetylacteol 3-O-arabinoside was isolated only from C. foetida and has a longer retention time than actein on ordinary reverse phase HPLC conditions.12,17,19,23) Considering together the fact that black cohosh products substituted by C. foetida have been found in the U.S. market, compound Y (4) was strongly suggested as acetylacteol 3-O-arabinoside. On the other hand, there is no report yet about a compound with C37H56O11 isolated from C. dahurica. Since the intensity of the product ions was too weak to obtain MSn fragments other than 675.4, compound X (3) and Y (4) could be uncovered compounds and more investigation is needed for their structure identification.
Because black cohosh is used as medicine in Europe, this herbal product might have to also be regulated as a medicine in our country. This should be a possible solution for the problem of the high adulteration rate of black cohosh health care products commercially available in Japan. In view of this, the presented LC-MS/MS analysis to identify the marker compounds may provide a powerful tool regarding quality control of the black cohosh drugs.
This work was supported by Grants from the Ministry of Health, Labour and Welfare of Japan and the Japan Human Sciences Foundation.