2014 年 37 巻 4 号 p. 511-520
2014 年 37 巻 4 号 p. 511-520
Midkine (MK) and pleiotrophin (PTN) belong to the subfamily of heparin binding growth factors. They have ca. 50% structural homology, with similar C- and N-domains as well as comparable binding affinity to heparin, glycoproteins and proteoglycans. Both MK and PTN have diverse functions, such as mitogenicity, inflammation, angiogenesis, oncogenesis and stem cell self-renewal. The high expression of MK and PTN in many kinds of cancers makes them excellent as cancer biomarkers and targets for anticancer drug development. In addition, the important roles of MK and PTN in the regeneration of tissues, such as myocardium, cartilage, neuron, muscle, and bone, make them attractive candidates for the treatment of degenerative diseases such as myocardiac and cerebral infarction, Alzheimer’s disease, Parkinson’s disease and skeletal muscle injury. As a result, there has been a growing interest in the mechanisms of MK and PTN function, including the diverse receptors on the cell membrane and complex signal pathways in the cytoplasm. This work reviews the structures of MK and PTN, as well as the receptors and the intracellular signal pathways involving MK and PTN which will pave the way for future development of MK and PTN therapeutics.
Midkine (MK), a heparin binding growth factor,1) was first identified from the cDNA library of mouse embryonal carcinoma cell line HM-1 stimulated with retinoic acid.2) Pleiotrophin (PTN), along with MK, comprised a new family of heparin-binding growth factors, and was first isolated from perinatal rat brain using heparin sepharose.3) MK and PTN have ca. 50% homology in their amino acid sequences and contain similar conserved C- and N-domains yet are distinctly different from other heparin-binding factors. Mouse models with either MK or PTN genes knocked out exhibited similarly moderate abnormalities4–6) while those with both MK and PTN simultaneously knocked out displayed severe abnormalities.7–10) These results indicate that the functions of MK and PTN are overlapped. Indeed, MK and PTN both have similar functions that include mitogenicity, cellular survival, oncogenicity, inflammation, differentiation and stem cell renewal (Fig. 1). The mitogenicity and cellular survival functions of MK and PTN are mainly reflected by promoting cell proliferation not only for normal cells coming from epithelium, endothelium and fibroblast tissues but also for carcinoma cell lines developing from different tissues.11–13) In addition, MK and PTN play an important role in cell differentiation and embryo development.14) During embryo development, particularly in the nervous system, MK and PTN mRNA are strongly expressed.1) MK is first highly expressed in the mid-gestational period and is followed by increased PTN expression which reaches the maximum level around birth.15) Both MK and PTN expression are localized in the radial glial processes of the embryonic brain along which neural stem cells migrate and differentiate.15,16) In addition to the neural system, MK and PTN regulate the differentiation and morphogenesis of other organs during the vertebrate embryo development.16,17) Notably, while MK and PTN are scarcely expressed in the adult body, they are expressed in many tissues during regeneration after injury or trauma.14) Furthermore, MK and PTN are highly expressed in many cancers and play a key role in the cancer cell proliferation and metastasis, which make MK and PTN excellent as both cancer biomarkers and targets for new anti-cancer drug development.18) MK and PTN are also involved in inflammation (e.g. chemo-taxis of macrophages and neutrophils) which leads to vascular restenosis, interstitial nephritis, renal injury and rheumatoid arthritis.19) At present, the involvement of MK and PTN in stem cell proliferation and renewal20–22) further highlight their potential as regenerative therapeutics. For example, MK has been demonstrated to promote the recovery of ischemic injury in the heart and brain,23–28) attenuate the deposition of amyloid β-peptide plaques and progression of Alzheimer’s disease,29) facilitate liver regeneration30) as well as aid in the repair of bone fracture and muscle injury.31) PTN has also been shown to promote hematopoiesis of bone marrow.21,22)
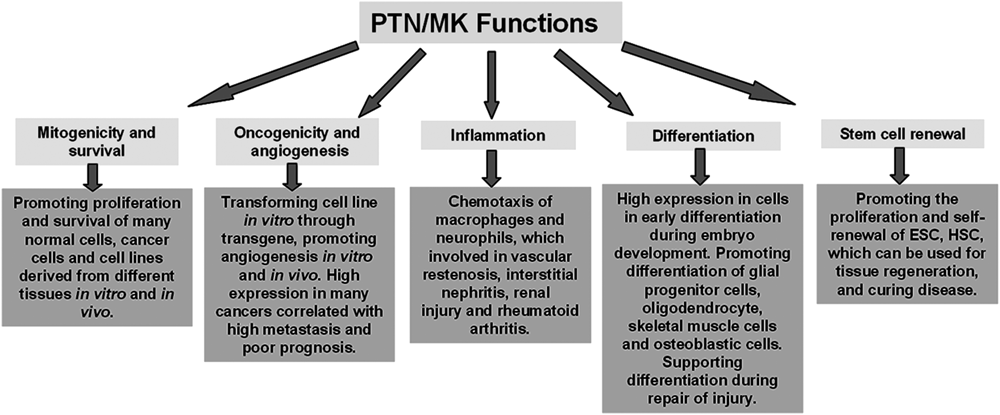
The diverse functions of MK and PTN are determined by the molecular mechanisms like the receptors on the cell membrane and intracellular signal pathways. To date, there have been key advances made on elucidating the functional mechanisms of MK and PTN, including diverse receptors and complicated intracellular signal pathways. This review seeks to provide an overview of the structures, membrane receptors and intracellular signal pathways of MK and PTN which we expect to serve as a guide for developing future insights and applications of MK and PTN.
MK is 13 kDa and has 143 amino acids32) while PTN is 18 kDa and has 168 amino acids.33) The amino acid sequences, conserved domains and three-dimensional structures are shown in Fig. 2. MK and PTN have ca. 50% sequence homology and the basic amino acids which are important for the structure and function are conserved. In particular, MK and PTN have similar conserved N- and C-terminal domains, with three and two disulfide bonds respectively and a flexible hinge linking each other.15)
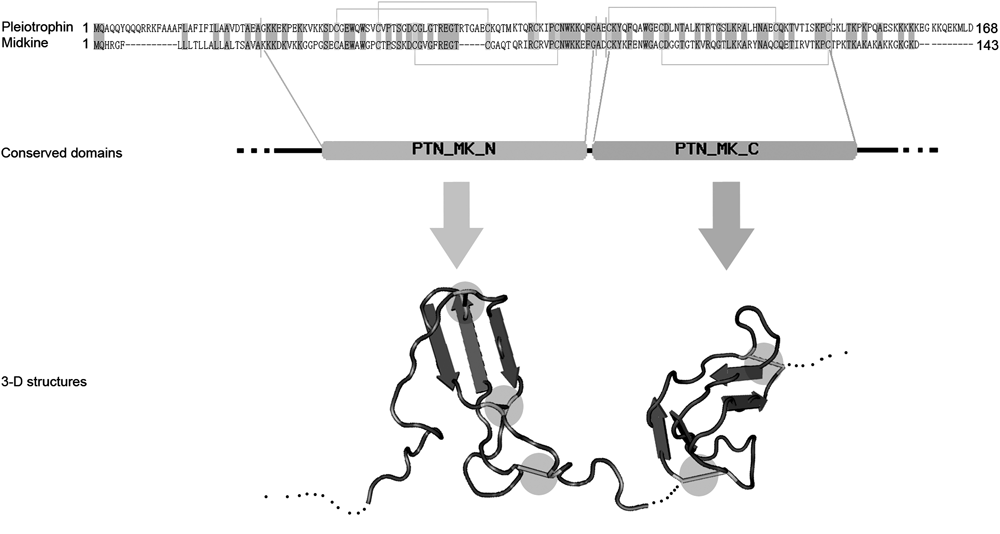
MK and PTN have ca. 50% homology (homological sequence is noted with gray), and conserved disulfide bonds (noted with yellow line in sequence). MK and PTN have similar conserved domains, N- and C-terminal domain. Both N- and C-terminal conserved domains are composed of three antiparallel β-sheets and linked by a flexible hinge. The N- and C-terminal structures are maintained by three and two disulfide bonds (noted with yellow circle), respectively, while the N- and C-terminal tail are composed of random coils. (Color images were converted into gray scale.)
The soluble structure of MK analyzed by magnetic resonance imaging (MRI) revealed that both the N- and C-terminal domains have three anti-parallel β-sheets. The flexible hairpin loop (Lys86, Lys87, Arg89) between the second and third β-sheet of C-terminal domain mediates the binding with heparin, and another heparin-binding site is located at the series of basic amino acids (Lys79, Arg81, Lys102) on the β-sheet.34) The MK dimer, formed with a fused heparin binding site at the interface of the C-terminal domain, is the active form in enhancing plasminogen activator activity, promoting neurite outgrowth and neuronal cell survival, which can be inactivated by heparin (>20 mer).34) The mutation assays of MK as well indicate that the C-terminal domain is important for its biological activity while the N-terminal domain plays a role in maintaining structural stability.35,36)
The soluble structure of PTN resolved by MRI was similar to MK and the binding of heparin similarly occurs at the β-sheet domains. This heparin-binding site is homologous with the thrombospondin type-I repeat (TSR-I) motif which is found in various extracellular proteins that mediate cell-to-extracellular matrix and cell-to-cell interactions.33) The peptide sequence, P (65–97), corresponding to the C-terminal TSR-I motif of PTN can also inhibit the mitogenic, tumorigenic and angiogenic activities through antagonizing the binding of PTN to heparin,37) indicating that, like MK, the C-terminal domain of PTN is critical for its bioactivity.37)
RPTPζ belongs to the receptor-type protein-tyrosine phosphatase family which plays an important regulative role in morphogenesis of neuronal system in vertebrates and invertebrates.38) Extracellular region of RPTPζ contains a carbonic anhydrase (CAH)-like domain, a FN-III-like domain that connected with two intracellular PTPase domains through a cysteine-free region.39) There exist three splice variants of RPTPζ: the full-length RPTPζ (RPTPζ-A); the short form of RPTPζ, in which most of the cysteine-free region is deleted (RPTPζ-B); and the secreted form (RPTPζ-S) that resembles the extracellular region of RPTPζ-A. All these three variants are expressed as chondroitin sulfate proteoglycans in the brain40) and have important function in neural system development, like axonal growth and neuronal migration, mitogenic and neurite out-growth-promoting activity.40,41) Using RPTPζ-S proteoglycan-sepharose, an 18 kDa protein was extracted from the rat brain tissue and was identified as PTN.42) RPTPζ-S has two binding sites for PTN, low (Kd=3 nM) and high (Kd=0.25 nM) binding site, and chondroitinase ABC digestion can decrease the binding affinities without changing the number of binding sites.42) MK also binds to RPTPζ with two affinity binding sites, low (Kd=3 nM) and high (Kd=0.58 nM) binding site.43) The binding of MK or PTN to RPTPζ can be inhibited by soluble PTN, some specific glycosaminoglycans and chondroitinase ABC digestion.43) C-Terminal domain of MK has the full binding activity to RPTPζ, and a mutation at Arg78 results in loss of the high affinity binding and reduces neuronal migration-inducing activity,43) indicating that RPTPζ is a functional receptor for MK and PTN, and the binding of MK and PTN to RPTPζ requires the chondroitin sulfate on the RPTPζ.
3.2. Anaplastic Lymphoma Kinase (ALK)The receptor tyrosine kinase ALK was initially identified because of its involvement in anaplastic large cell lymphoma (ALCL), in which ALK fuses with cell cycle-regulated nuclear protein nucleophosmin (NPM) forming a new oncogene NPM-ALK through chromosome translocation.44,45) In ALCL, the NPM mediates the dimerization of NPM-ALK and results in the loss of control and continuous activation of the intracellular kinase activity of ALK.11,44,45) Using immobilized PTN as bait, a phage insert with homologous amino acid sequence of the extracellular domain (ECD) of ALK was isolated from a phage display of human cDNA library. Cell-free assay and radio ligand-receptor binding assay in intact cells shows that the binding affinity of PTN to ALK ECD is 32±9 pM.11) Comparably, MK binds to ALK with an apparent Kd of 170 pM on intact cells transfected with ALK, and the binding of MK can be inhibited by PTN and by monoclonal antibodies against the extracellular ligand-binding domain of ALK.13) Many kinds of cancer cells and normal cells that expressed ALK have proliferation and survival activity after MK or PTN stimulation,11–13) indicating that ALK is an important functional receptor for MK and PTN. Despite the above results, some other researchers remain skeptical regarding the role of ALK as a receptor for PTN, and state that ALK is still an orphan receptor in vertebrate.46–50) Perez-Pinera’s study indicates that ALK is not a direct receptor of PTN; instead, its activity is regulated by RPTPζ.51) In this case, ALK auto-activates itself through its own kinase activity, and ALK activity level is negatively regulated by the phosphatase activity of RPTPζ.51) Once PTN binds to RPTPζ and results in dimerization of RPTPζ, PTN inhibits the phosphatase activity of RPTPζ, further resulting in higher levels of ALK activity.51)
3.3. IntegrinsIntegrins are a family of diverse glycoproteins that canonically form heterodimeric receptors (α and β subunits) for cell-substratum and cell–cell adhesion and play an important role in morphogenesis, inflammation, cancer metastasis and stem cell self-renewal.52) Integrin β1 is a MK-binding protein extracted from 13-d-old mouse embryos through MK-agarose column, while the hetero-α-subunits capable of binding to MK are α4 and α6. MK induces migration of osteoblastic cells and neurite outgrowth of embryonic neurons through the α4β1- and α6β1-integrins which can be inhibited by anti-α4 antibody and anti-α6 antibody respectively.53) Unlike MK, PTN interacts with integrin ανβ3, and the binding is cell adhesion motif-RGD independent.54) A synthetic peptide corresponding to the β3 extracellular domain (177CYDMKTTC184) inhibits PTN-ανβ3 interaction and totally abolishes PTN-induced endothelial cell migration.54)
3.4. Neuroglycan C (NGC)NGC, membrane-bound chondroitin-sulfated proteoglycan, is specifically expressed in the nervous system and promotes neurite outgrowth of neurons in cultures prepared from the fetal rat cerebral cortex.55) Both MK and PTN bind to the acidic amino cluster box domain of NGC without being affected by chondroitinase ABC treatment, while MK also binds to the chondroitin sulfate attachment domain, which is sensitive to chondroitinase ABC digestion.56,57) Substrate coated with MK or PTN promotes cell attachment and process extension in the undifferentiated bipolar CG-4 cells, and this function can be inhibited by monoclonal anti-NGC antibody or a glutathione S-transferase-NGC fusion protein.56,58)
3.5. Low-Density Lipoprotein (LDL) Receptor Related Protein (LRP)LRP, belonging to the LDL receptor superfamily, is a large endocytic receptor that serves to internalize ligands into lysosome for degradation and metabolism. LRP is also known as a cell membrane signal receptor for activating intracellular signal-pathways in cell migration as well as proliferation.59) LRP, as a receptor of MK, is isolated from 13-d-old rat embryo by MK-affinity chromatography with a Kd of 3.5 nM.60) MK promotes neuron survival through LRP, and this function can be inhibited by a natural antagonist of LRP, Receptor associated protein (RAP). MK and LRP are also involved in skeletal muscle regeneration process with similar expression spectrum during the regenerative period of rat skeletal muscle injured by injection of bupivacaine.31) However, some in vitro experiments show that MK promotes anchorage-independent cell growth of many cancer cells61) through LRP induced internalization of MK, in which MK interacts with a cytoplasm-nucleus transport protein, nucleolin that loads MK to the cell nucleus to promote gene expression.61,62) MK also has weak affinity with another LDL receptor family member, Brushin/megalin, whose function is uncertain.60)
3.6. Syndecan (SDC)SDC family members, SDC-1, 2, 3, 4, containing similar trans-membrane and intracellular domains while different extracellular domains, are heparin sulfate proteoglycans which interact with diverse ECM and cytokines.63) A 200 kDa protein extracted from the cultured rat brain neurons and rat brain tissue using PTN-affinity column was identified as SDC-3 by immunochemical analysis and partial peptide sequencing.64) PTN binds to SDC-3 with a Kd of 0.6 nM, and anti-SDC-3 antibody has an inhibitory effect on PTN-induced neurite outgrowth.64) SDC-4 purified from human endothelium-like EA.hy926 cells can bind to MK (Kd=0.5 nM), and the binding affinity can be destroyed by heparitinase but not chondroitinase ABC treatment or inhibited by heparin sulfate but not chondroitin sulfate, which means the heparin sulfate (HS) chains of the SDC-4 are responsible for its binding with MK.65)
3.7. NotchThe notch receptors, Notch1, 2, 3, 4, are a family of single-pass transmembrane receptor proteins, which play an important role in cell–cell communication through their well-known DSL (Delta/Serrate/LAG-2) family ligands that are also single-pass transmembrane proteins. MK as a ligand of notch2 was proved by the yeast 2-hybrid assay and Co-immunoprecipitation (Co-IP) assay.66,67) The C-terminal of MK (MKC), while not N-terminal of MK (MKN) have strong affinity with the N-terminal, the extracellular domain (ECD), of the notch2 receptor.66,67) Although there is no direct evidence show that the notch receptor have physical affinity with the PTN, in the PTN promoted hematopoietic stem cell self-renewal, notch receptor was found to be active during this process.21)
3.8. Multi-molecular ComplexSome research indicates that the receptor of MK and PTN is not simple single- or bi-molecular but multi-molecular complex. In the neuron survival assay induced by MK, RPTPζ was found as the MK receptor mediates the MK-induced neuron survival, and meanwhile MK also binds to extracellular domain of LRP6 and LRP8 (apoER2) who have been identified as functional receptor binding with Wnt and reelin respectively.68) In addition, MK functional receptors, both α4β1 and α6β1, interact with the ectodomain of LRP6 which also binds to RPTPζ.53) Integrin ανβ3, a PTN receptor that mediates endothelial cell migration also interacts with RPTPζ.54) Furthermore, RPTPζ also interacts with ALK on the membrane and negatively modulates ALK activation by dephosphorylating tyrosine on intracellular domain of ALK.51) This indicates that, in some specific conditions, the MK and PTN receptor may be a multi-molecular complex combined from several receptors mentioned above.
3.9. OthersBeside the receptors discussed above, other receptors of MK and PTN have been found. In human pediatric rhabdoid tumor kidney-derived G401 cells, a protein or complex (200 kDa) binds to MK (Kd=0.07±0.01 nM)69) without being affected by heparinase and chondroitinase ABC treatment70) and the MK binding further stimulates tyrosine phosphorylation of several intracellular proteins.70) MK also has affinity with membrane-expressed nucleolin that mediates the internalization of MK on T-lymphocytes.71) Glypican-2 is a membrane heparin sulfate proteoglycan (HSPG) whose heparin sulfate chain mediates the binding to MK, and the ligation of MK with cell-surface glypican-2 induces cell adhesion and neurite outgrowth.72) In addition, there are two proteoglycans, versican and neural cell adhesion molecule (NCAM), were isolated from mouse embryos73) and rat embryonic brain tissue60) respectively using MK-affinity column. Versican also binds to PTN, and the binding of MK and PTN to versican can both be inhibited by chondroitinase treatment, heparin and chondroitin sulfate.73) However, the induced functions of MK or PTN binding to versican or NCAM are not known.
MK and PTN have affinity for diverse functional receptors and several other receptors with unknown functions (shown in Fig. 3). Many of these receptors are membrane proteoglycans whose heparin sulfate chains or chondroitin sulfate chains are important for binding and biological functions of MK and PTN, while others are not proteoglycans and their interactions with MK and PTN are not sensitive to heparinase and chondroitinase treatment, indicating that MK and PTN have several active sites in their structures, and the different active sites along with the diverse receptors are the foundation for the diverse biological functions of MK and PTN.
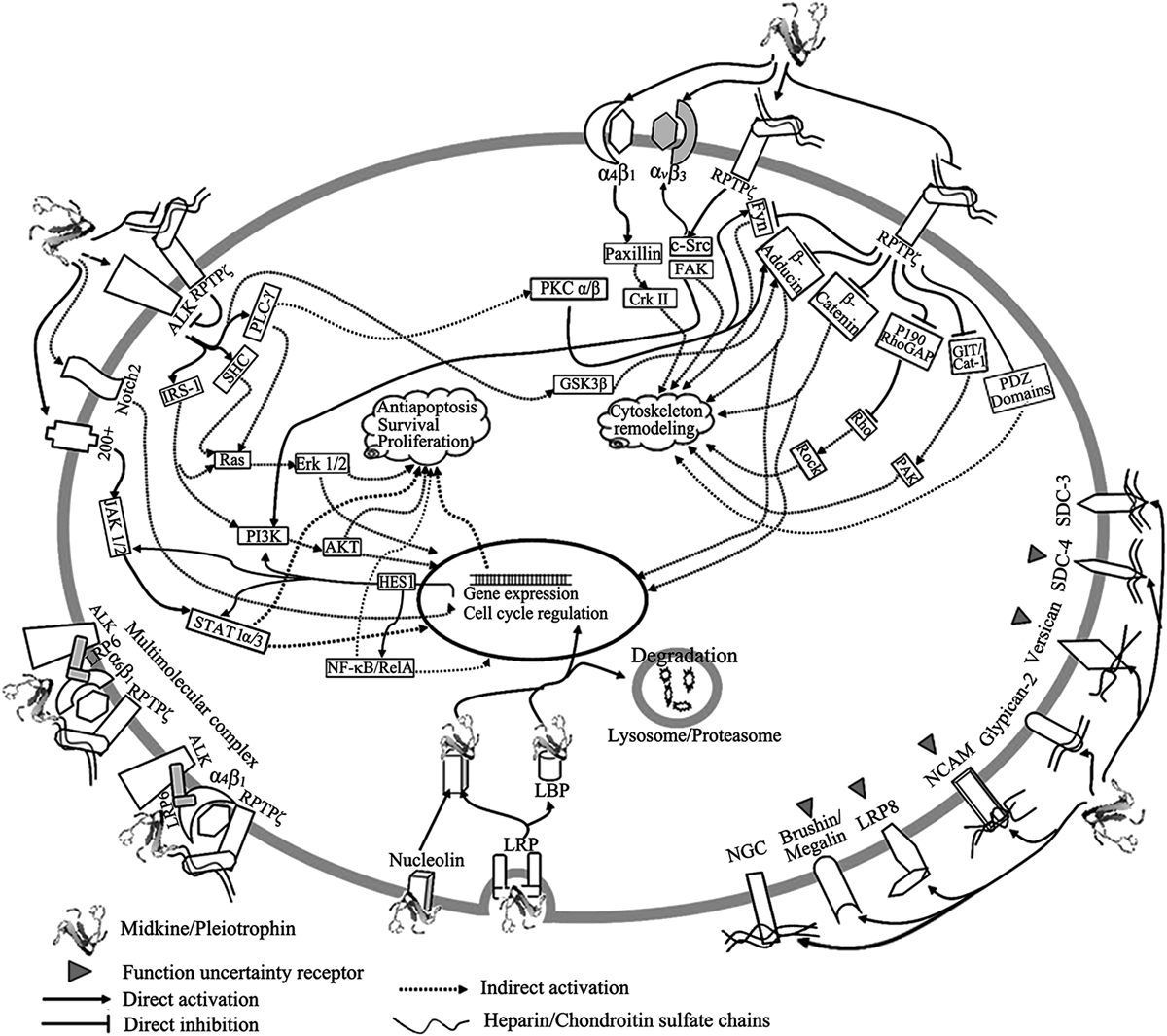
MK and PTN have diverse receptors and some of them can assembly as multi-molecular complex. The intracellular signal pathways are complicated and can be classified as cell skeletal remodeling associated pathways and proliferation, survival and anti-apoptosis associated pathways, both of which can interfere with each other through crosstalk.
Corresponding to the diverse receptors of MK and PTN, the intracellular signal pathways of MK and PTN are likewise complex. Based on the function, the intracellular signal pathways of MK and PTN can be categorized as cell skeletal remodeling associated pathways or as proliferation, survival and anti-apoptosis associated pathways (Fig. 3).
4.1. Cell Skeletal Remodeling Associated Pathways. PTN/RPTPζ PathwayThe PTN/RPTPζ pathway is an important function mode for PTN, in which RPTPζ as a receptor tyrosine phosphatase negatively regulates the tyrosine phosphorylation level of its intracellular substrates in homeostasis.74) The PTN binding to RPTPζ dimerizes the RPTPζ and blocks the phosphatase activity of the RPTPζ, and then the intracellular substrates activate with high tyrosine phosphorylation level.74) RPTPζ is also a receptor for MK,43) but there is no report so far that MK functions through this mode.
In glioblastoma U373-MG cells, the intracellular active-site domain of RPTPζ binds to β-catenin and reduces its tyrosine phosphorylation level, while PTN sharply increases tyrosine phosphorylation of β-catenin through inactivating the catalytic activity of RPTPζ.75) In normal conditions, β-catenin as an adaptor molecular stabilizes the cytoskeleton through its interaction with cell adhesion molecular (CAM), cadherin and intracellular cytoskeletal protein actin, while the tyrosine phosphorylation of β-catenin deteriorates its binding with cadherin and actin, which mainly contributes the loss of contact inhibition, breaking down of the cell adhesion and remodeling of the cytoskeleton in PTN transformed cells.75) The released β-catenin also goes into the nucleus and functions as a transcription factor for gene regulation.75) G protein-coupled receptor kinase-interactor 1/Cool-associated, tyrosine-phosphorylated 1 (GIT1/Cat-1) is another RPTPζ substrate which was uncovered using the yeast two hybrid assay and confirmed in NIH3T3 cells and v-Src transformed 3Y1 cells.76) Immunohistochemical analyses reveals that RPTPζ and GIT1/Cat-1 are co-localized in the processes of pyramidal cells in the hippocampus and neocortex in rat brain, and PTN increases tyrosine phosphorylation of GIT1/Cat-1 in neuroblastoma B103 cells.76) The activated GIT1/Cat-1 may further activate p21-activated serine threonine kinase (PAK) which has important role in cytoskeleton turnover and remodeling, and then promote neurite outgrowth and neuron migration.76) β-Adducin is another intracellular substrate of RPTPζ found out through yeast two hybrid method.77) β-Adducin localized on the membrane links the terminal of actin to spectrin and maintains the stability of cell membrane and cytoskeleton,77) and like β-catenin, phosphorylated β-adducin also enters the nucleus and interacts with heterochromatin and centriole, which may function in chromatin stability and transcription regulation.78) β-Adducin interacts with intracellular domain of RPTPζ, and its tyrosine phosphorylation level rises sharply after PTN stimulation, which further contributes to the disruption of cytoskeleton, increasing plasticity and reducing homophilic cell–cell adhesion.77) β-Adducin is also regulated by protein kinase C (PKC) whose activation is upstream regulated by PTN, while the activated PKC induced threonine phosphorylation (713 and 726) of β-adducin.78) Delta/Notch-like epidermal growth factor (EGF)-related receptor (DNER) is a trans-membrane protein highly expressed in cerebellar Purkinje cells along with co-localization of RPTPζ.79) Normal DNER is endocytosed and inhibits the retinoic-acid-induced neurite outgrowth of Neuro-2A cells. PTN stimulation inhibits the RPTPζ phosphatase activity and increases the tyrosine phosphorylation level of DNER and suppresses the endocytosis of this protein.79) Phosphorylated DNER accumulates on the plasma membrane and induces neurite outgrowth, probably though Notch signal pathway which has important roles in cell–cell adhesion and cell differentiation.79) Fyn is a tyrosine kinase belonging to the Src family and involves in the cell plasticity, cell–cell adhesion, cell migration and cell proliferation. Fyn interacts with the intracellular active site of RPTPζ, and its tyrosine phosphorylation level increases as soon as PTN stimulates.80) Fyn can also be further activated though threonine phosphorylation which is carried out by PKC that was activated after PTN stimulation.80) P190 RhoGAP is a GTPase-activating protein (GAP) for Rho GTPase, and its activity is determined by phosphorylation level of Y1105, while the phosphorylation level of Y1105 is regulated by PTN/RPTPζ pathway.81) P190 RhoGAP activity regulated by PTN/RPTPζ pathway involves in hippocampus-dependent memory formation through downstream Rho/Rock path way which plays an important role in cell migration, axonal growth and synaptic plasticity.81) PSD-95/SAP90 family proteins along with RPTPζ are distributed in the dendrites of pyramidal neurons of the hippocampus and neocortex.82) PSD-95/SAP90 family proteins act as mediators of protein–protein interactions to form large synaptic macromolecular complexes and play important roles in the molecular organization and regulation of the synapses.82) C-terminal sequence of RPTPζ binds to the PSD-95/SAP90 family through the second PDZ (PSD-95/Dlg-A/Zo-1) domain, and regulates the synaptic function as postsynaptic macromolecular complexes with PSD-95/SAP90.82) membrane-associated guanylate kinase with inverted orientation (MAGI), as a scaffold molecular on the membrane, has many domains like PDZ which can interact with the cytoskeletal proteins, thereby provides a direct link between membrane signals and cell morphology.83) PDZ-2 domain of MAGI-3 can interacts with intracellular C-terminal domain of RPTPζ, and MAGI-3 can also interact with tyrosine phosphorylated P130, a substrate of RPTPζ, as a result MAGI-3 may serve as a scaffold molecular mediating the interaction of RPTPζ and its substrate P130, and the same molecular mechanism may be used for RPTPζ and its other intracellular substrates.83)
Src/FAK PathwaySrc/FAK pathway plays an important role in cytoskeleton remodeling and cell migration by regulating cadherin- and integrin-based cell-adhesive system.84) PTN binding to RPTPζ promotes migration and tube formation of the human umbilical vein endothelial cells (HUVEC) in vitro.85) Different from the function mode of PTN/RPTPζ pathway described above, PTN binds to RPTPζ and activates its phosphatase activity, and then the activated RPTPζ interacts with intracellular c-Src and activates c-Src through dephospharylating Tyr527 on it, and finally the activated c-Src binds to and activates focal adhesion kinase (FAK) which is important for cell migration.82)
Integrin/Paxillin PathwayIntegrin family proteins are important cell adhesion molecules (CAM) associated with cell–ECM and cell–cell adhesion, while Paxillin, a vital downstream intracellular signal protein of integrin, is a focal adhesion-associated, phosphotyrosine-containing protein which contains a number of motifs mediating protein–protein interaction and thus paxillin itself serves as a docking protein to recruit signaling molecules which regulate cell spreading and motility.86) MK increases the tyrosine phosphorylation level of paxillin through its binding to the integrin receptor α4β1, which promotes migration the osteoblast in vitro.53) The same signal pathway may be involved in the MK induced embryo neuron neurite outgrowth through α6β1.53) In contrast, PTN activates its receptor ανβ3 and induces migration of HUVEC by increasing tyrosine phosphorylation level of β3 which is RPTPζ and activated c-Src dependent.54) The possibility is that PTN activates c-Src through Src/FAK pathway, then the activated c-Src phosphorylates tyrosine on β3 of ανβ3.54)
4.2. Proliferation, Survival and Anti-apoptosis Associated Pathways. Mitogen-Activated Protein Kinase (MAPK) and Phosphatidyl Inositol 3-Kinase (PI3K)/AKT PathwaysMAPK and PI3K/AKT pathways are known to have significant roles in cell proliferation, survival and anti-apoptosis. Many kinds of cancer cells, cell lines and normal cells which have mitogenic or survival response after MK or PTN treatment are found having the activation of the MAPK and PI3K/AKT pathways. MK inhibits the serum starvation induced apoptosis of neuron through downregulating the activity of apoptotic protein caspase-3 in dose dependent manner.87) PTN promotes the proliferation of many kinds of ALK expressed cell lines: endothelial HUVEC, fibroblast NIH3T3, adrenal carcinoma SW-13, pancreatic cancer colo357, squamous cell ME-180, glioblastoma U87MG, U118MG and U138MG and breast cancer MDA-MB 231.11,12) The PTN induced proliferation for these cell lines is carried out through PTN activating ALK which phosphorylates downstream intracellular substrates, SHC, phospholipase C-γ (PLC-γ) and insulin receptor substrate-1 (IRS-1) that further activates PI3K and Erk1/2.11,12) Similar as PTN, MK promotes proliferation of ALK expressed neuroblastoma SHSY-5Y, glioblastoma U87MG, epithelial WI-38 and HUVEC by activation of both PI3K/AKT and MAPK pathways.13) In addition to receptor ALK, MK and PTN also activate extracellular signal-regulated kinase (ERK1/2) and PI3K/AKT pathways through receptor RPTPζ on osteoblast-like cells88) and through both ανβ3 and RPTPζ on HUVEC.54) In the presence of MK, the MAPK and PI3K/AKT pathways participate in injured tissues recovery process, like the recovery of ischemic myocardial injury,23–27) cartilage trauma89–91) and amphetamine-induced neurotoxicity.92) MAPK and PI3K/AKT pathways also take part in the proliferation and self-renewal of stem cells, like embryonic stem cells (ES)20) and hematopoietic stem cell (HSC) after stimulation by PTN.21,22)
Janus Tyrosine Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) PathwayIn human G401 cells, MK but not PTN activates JAK/STAT pathway through a protein or a protein complex whose molecular weight is about 200 kDa.69) With MK stimulation, janus tyrosine kinases 1 (JAK1) and 2 (JAK2) associate with the 200 kDa protein and be activated through tyrosine phosphorylation.69) Then the JAK activates transcript factor STAT1α which will go in to nucleus for gene transcription.69)
Notch Signal PathwayThe notch signal pathway, which functions through cell to cell attachment, is very important for cell organization and communication. Beyond the important normal biological functions, for example embryogenesis, its malfunction associated with many pathological process, such as epithelial-mesenchymal transition (EMT) and carcinogenesis. MK was found driving the EMT process in human keratinocytes and pancreatic ductal adenocarcinoma (PDAC) through the notch signal pathway.66,67) After MK treatment, the epithelial-like cell lose its epithelial adhesive markers while increase the expression of the mesenchymal markers, and the cell became disorganized and more moveable.66,67) For the human keratinocyte cell line HaCaT, MK binds to the notch2 leading the cleavage of the intracellular domain (ICD) of notch2 (NCID) which is the active form of notch2. The NCID goes into the nucleus and induces the expression of HES1 which then recruiting JAK2 and STAT3 together for EMT associated gene transcription.66) While on the MK treated PDAC, the downstream signal of HES1 associated with the EMT process is NF-κB/RelA.67) Midkine-notch2-HES1 signal axis also plays a critical role in neuroblastoma tumorigenesis.93) Similarly, notch-HES signal was found to be active in the PTN promoted hematopoietic stem cell expansion and regeneration, while the downstream signal of HES here is PI3K/AKT.21)
Nuclear Targeting PathwayThe mitogenic and anti-apoptotic function of MK are also realized though its internalization, cell nuclear targeting and the subsequent transcription promotion.94) LRP and the membrane localized nucleolin play an important role in the internalization of MK.62,71) LRP and nucleolin are co-expressed on the cell membrane95) and the binding of MK to nucleolin can induce the assembly of the LRP related lipid raft71) that has been reported as playing an important role in endocytosis and signal transduction as platform on the membrane.96,97) After the MK is internalized, nucleolin and another cytoplasm protein, laminin binding protein precursor (LBP), mediate the nucleus translocation of MK.98) The nucleolus translocated MK localizes in the granular component (GC), dense fibrillar component (DFC) and the border between the DFC and fibrillar center (FC) of the nucleolus, and promotes the rRNA transcription, ribosome biogenesis, and cell proliferation of HepG2 cells.99) The nuclear translocation of MK can be regulated by proteasome and lysosome through the degradation of the internalized MK, where the proteasome inhibitor promotes the accumulation of MK in the nucleus.100)
Cell skeletal remodeling associated pathways activated by MK and PTN are mainly involved in the cell migration and differentiation of normal cells and metastasis of malignant tumors, while proliferation, survival and anti-apoptosis associated pathways are mainly involved proliferation and survival of normal and malignant tumor cells. It is noteworthy that these two pathways do not work independently; they can interfere with each other through crosstalk, like the cell skeletal remodeling associated FAK also activate the MAPK and PI3K/AKT pathways85) while the proliferation, survival and anti-apoptosis associated ALK activates β-adducin and Fyn through the median molecular PKC α/β likewise.78,80)
MK and PTN are two important cytokines which have multiple overlapped functions and what correspondingly are their diverse membrane receptors and complicated intracellular signal pathways. At present, anti-cancer drugs targeting MK and PTN and their receptors or intracellular pathways are being developed101–104); Targeting MK and PTN signaling pathways for curing drug addiction and neurodegenerative disorders also be discussed by Herradon group.105) Furthermore, more and more research support that MK and PTN not only have potential therapy for ischemic injury of heart and brain,23–28) Alzheimer’s disease,29) hematopoiesis of bone marrow,21,22) bone and muscle injury,31) and even human immunodeficiency virus (HIV) infection69) but also play an important role in tissue regeneration for adult body.14) So, in the future, clarification of the specific receptor and intracellular pathways of MK and PTN on different kinds of diseases should be basic for MK and PTN related new drug development.
We gratefully acknowledge the assistance of Shawn J. Tan and Mark Hartman for language revision. This work was supported by National Natural Science Foundation of China (81001388/H3004) and Medicine-engineering collaboration foundation of Shanghai Jiao Tong University (YG2010MS87).