2014 Volume 37 Issue 8 Pages 1269-1273
2014 Volume 37 Issue 8 Pages 1269-1273
In this study we investigated whether κ-opioid receptor stimulation by dynorphin A (1–13), a potent fragment of endogenous peptide, attenuated repeated stress-induced behavioral impairments in mice. In order to reduce the motivation to escape, mice were preexposed to inescapable electric footshock (day 0), and then dynorphin A (1–13) was administered to mice prior to the stress from the next day for 4 d (days 1–4). Dynorphin A (1–13) (1500 pmol/5 µL intracerebroventricular (i.c.v.)) attenuated the repeated stress-induced escape failures from the shock, and this improvement was inhibited by the pretreatment of nor-binaltorphimine (4.9 nmol/kg subcutaneously (s.c.)), a κ-opioid receptor antagonist. In the neurochemical experiments, we detected an increase in 5-hydroxyindoleacetic acid (5-HIAA) content, but not in serotonin (5-HT) content, and an increase in the 5-HIAA/5-HT ratio was observed in the amygdala of the group with footshock compared with the group without shock. Additionally, the changes in 5-HIAA content and the ratio were reversed by dynorphin A (1–13). However, there were no differences in 5-HT or 5-HIAA content or their ratios in the hippocampus among the three groups. These results suggest that dynorphin might alleviate the stress-induced behavioral impairments accompanied by regulation of the 5-HTergic system in the brain.
The number of patients presenting with stress-induced behavioral abnormality is increasing in Japan as well as other developed countries, and thus it is very important to find a cure. Stress-induced behavioral abnormality in rodent is widely employed as a model of mood disorders.1) In the paradigm adopted in this study, i.e., the learned helplessness task, exposure to an uncontrollable aversive stimulus leads to a decreased ability to escape from subsequent aversive situations. Pre-exposure to inescapable electric footshock stress leads to a high escape failure rate in subsequent learning trials. This model is being used increasingly to investigate the neurobiology of affective illness and to explore candidates for medicines that relieve stress,2) such as antidepressants which activate serotonergic system. In addition, many studies have revealed that the amygdala/hippocampus as well as the frontal cortex play a critical role in controlling such repeated aversive stress directly and/or indirectly.3,4) Therefore, we focus on the serotonergic system in the amygdala/hippocampus.
Opioid systems in the brain are known to control mood function and pain.5) Opioid peptides and their receptors are found at high concentrations in regions of the limbic system. However, their involvement in the pathogenesis of mood disorders, particularly depressive illness, remains an enigma. Accumulating evidence from animal research has revealed that μ-, δ-, and κ-opioid receptors distinctly control mood-related processes. Delta-opioid receptor agonists show promise as antidepressants, whereas μ-opioid receptor agonists are characterized by their inherent abuse liability and thus, their risk-benefit ratio as antidepressants remains difficult to evaluate.5) Although most researchers have focused on the roles of the endogenous κ-opioid system in neuropsychiatric disorders,6) the details are still hazy. Previously, our group has been shown that synthetic κ-opioid receptor agonist, U-50,488H attenuated the fear memory impairments by acute electric footshock in mice.7,8) In this study, we investigated whether κ-opioid receptor stimulation by dynorphin A (1–13) attenuate repeated stress-induced behavioral deficits in mice. Additionally, neurochemical changes in this model have been examined to clarify the significance of κ-opioid system in the brain.
Male ddY mice (Nihon SLC Co., Ltd., Shizuoka, Japan), 9 weeks old, were used. The animals were housed in a controlled environment (24±1°C temperature, 55±5% humidity) and given food and water ad libitum. Room lights were off between 8:00 and 20:00. All experiments were conducted following the Guidelines for Animal Experiments of Meijo University (Approved number: Yaku-Jitsu-25 and 12) and the Guiding Principles for the Care and Use of Laboratory Animals approved by the Japanese Pharmacological Society (2007).
DrugsDynorphin A (1–13) (15, 150, 1500 pmol/5 µL, intracerebroventricular (i.c.v.), Sigma-Aldrich Co., U.S.A.) was dissolved in isotonic saline solution, and was administered 10 min before each training trial. For the antagonistic experiment, nor-binaltorphimine (nor-BNI; 4.9 nmol/kg, subcutaneously (s.c.), Sigma-Aldrich), which is dissolved in isotonic saline solution, was treated 25 min before each training.
Implantation of the CannulaEach mouse was anesthetized with pentobarbital (50 mg/kg, intraperitoneally (i.p.)) and mounted on a stereotaxic frame. The skull was exposed and holes were drilled. Each mouse was implanted with a lateral 28-gauge guide cannula (Nipro safelet cass, NIC, Osaka, Japan) that terminated into the right ventricle according to the atlas of Franklin and Paxinos9): +0.6 mm AP, +1.7 mm ML, and 4.5 mm DV from the Bregma. The guide cannula was cemented in place with dental acrylic and the skin resutured around the base of the cannula. A stainless steel dummy cannula (30-gauge) that extended 1 mm from the tip of the guide cannula was inserted and fixed in place when the animal was not injected. Following surgery, the mouse was allowed to recover overnight.
Footshock-Induced Behavioral ImpairmentsApparatus: Electric footshock stress was delivered in a Plexiglas chamber (W15×L18×H18 cm) with a stainless-steel grid floor consisting of rods spaced 1 cm apart (ENV-307 A-CT, Med Associates, Inc., U.S.A.) and this apparatus was placed in a soundproof box. This apparatus had a switch lever that would enable the mouse to escape from the electric footshock stress through the grids.
Pretreatment of Inescapable Electric Footshock: Mice were treated with scrambled inescapable electric footshock (0.6 mA, 30 s duration, 30 s interval) for 30 min.
Conditioned Avoidance Response: In order to evaluate their escape ability, active conditioned avoidance response was observed 24 h after the inescapable electric footshock stress. Each animal was placed in the soundproof box and subjected to 30 active conditioned avoidances. One training period consisted of avoidance (3 s) plus escape (30 s) with inter-trial interval (30 s). During the first avoidance time (3 s), a small light and a buzzer (85 dB) were presented (conditioned stimulus) and then, the animal was exposed to the electric footshock stress (0.6 mA, 30 s duration) (unconditioned stimulus). To escape from the electric footshock stress, the mouse had to press the lever in the box. “Escape failure” was considered when the mouse failed to escape the shock. Active conditioned avoidances were performed for four consecutive days and the number of escape failures was recorded.
Novel Object Recognition Test: We prepared another group for evaluating cognitive function after repeated stress. In this test, a mouse was habituated to a black plastic cage (30 cm×30 cm floor×50 cm height) for 15 min on day 4. On day 5, in the training trial, two same-sample objects (white film cases, ϕ3×6 cm) were placed in the cage (5 cm from the center each), and the mouse was allowed to explore them freely for 5 min. The time spent exploring each object was recorded manually. In the retention trial, which was conducted immediately after the training trial, the mouse was removed once and then placed back in the same cage, and one of the objects used in the training trial was replaced with a novel object (black dry battery cell, ϕ3×6 cm) for the mouse to explore freely for 5 min. Exploratory preference (%), which is the ratio of time spent exploring any one of the two same-sample objects in the training trial, and cognitive index (%), which is the ratio of time spent exploring the novel object in the retention trial to the total time spent exploring both objects, was used as an index of recognition memory.10,11)
Locomotor Activity: We prepared mice for the locomotor activity measurement. After the last treatment of nor-BNI on the day 4, mice were placed in a transparent acrylic cage (D25×W45×H40 cm), and locomotion was measured for 60 min using digital counters with infrared sensors (Scanet SV-10, Melquest, Toyama, Japan).
Biochemical Changes in the BrainCorticosterone Levels in the Plasma: To check the stress level and the effects of dynorphin, the animals were sacrificed after the behavioral test on the day 4. The trunk blood was collected in heparin-coated tubes and centrifuged at 1500 rpm for 20 min at 4°C, and the plasma was stored at −30°C until further processing. Plasma corticosterone concentrations were measured using a rodent corticosterone enzyme-linked immunosorbent assay (ELISA) test kit (Endocrine Technologies, Inc., CA, U.S.A.).
Monoamine Levels in the Brain Regions: We measured the content of serotonin (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA). On day 4 after the observation of active conditioned avoidances, mice were sacrificed by focused microwave irradiation for 1.4 s at 5 kW. The brains were quickly removed and the amygdala and hippocampus were dissected out on an ice-cold glass plate as in previous reports.12,13) Each section was rapidly frozen and stored in a deep freezer at −80°C until assayed. The contents of 5-HT and 5-HIAA were determined with an HPLC system equipped with an electrochemical detector (HTEC-500, Eicom, Kyoto, Japan). Briefly, each frozen brain sample was weighed and homogenized with an ultrasonic processor in 350 µL of 0.2 M perchloric acid containing isoproterenol as an internal standard. The homogenates were placed on ice for 30 min and centrifuged at 20000×g for 15 min at 4°C. The supernatants were mixed with 1 M sodium acetate to adjust the pH to 3 and injected into the HPLC system equipped with a reversed-phase octadecyl silica (ODS) column (Eicompak SC-5ODS; 3×150 mm; Eicom) and the electrochemical detector. The column temperature was maintained at 25°C, and the detector potential was set at +750 mV. The mobile phase was 0.1 M citric acid and 0.1 M sodium acetate, pH 3.6, containing 17% methanol, 180 mg/L sodium-L-octanesulfonate, and 5 mg/L ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA), and the flow rate was set at 500 µL/min.
Data Analysis: Values are expressed as mean number of escape failures±S.E. during each session. Results were statistically evaluated by the one-way ANOVA followed by the Dunn’s comparisons test or Student’s t-test. Significant level of difference was p<0.05.
Non-stressed animals displayed fewer escape failures than stressed animals throughout the experiments. It is revealed that dynorphin A (1–13) (1500 pmol) markedly reduced the number of escape failures on day 4 (F4, 35=3.12, p=0.037; Dunn’s test, p<0.01), whereas there are no significant differences among dynorphin A (1–13)-treated groups from the 1st to 3rd day (F4, 35=0.190, p=0.827 on day 1; F4, 35=0.64, p=0.617 on day 2; F4, 35=0.304, p=0.741 on day 3, respectively). This attenuation of escape failures by dynorphin A (1–13) (1500 pmol) was blocked by the pretreatment of nor-BNI (4.9 nmol/kg), a κ-opioid receptor antagonist.

Each column represents the mean±S.E.M. N=8 for each group. * p<0.01 vs. Stress/Dyn(0)-group, # p<0.01 vs. Stress/Dyn(1500)-group (Dunn’s multiple comparisons test).
As shown in the Fig. 2, there were no significant differences on the locomotor activity among 4 groups (F3, 28=0.361, p=0.711).

Each column represents the mean±S.E.M. N=8 for each group.
Three stressed groups showed higher concentration compared to non-stressed group. Additionally, dynorphin did not affect the corticosterone levels (non-stressed group: 5.3±1.3, stressed group: 11.2±0.7, stressed+Dyn 12.6±1.3, stressed+Dyn(1500)+norBNI 10.7±0.8 ng/mL (F3, 16=9.32, p<0.001; Dunn’s test, p<0.05 vs. non-stressed group).
Monoamine Levels in the BrainStatistical analysis revealed the high content of 5-HIAA (F3, 28=5.23, p=0.021; Dunn’s test, p<0.05, Fig. 3A) and 5-HIAA/5-HT ratio (F3, 28=6.04, p=0.032; Dunn’s test, p<0.05, Fig. 3B) in the amygdala of mice stressed compared to non-stressed group, whereas the 5-HT content was not changed (F3, 28=1.07, p=0.65). On the other hand, there were no significant differences in the 5-HT and 5-HIAA contents, and their ratio in the hippocampus among the groups (data not shown).
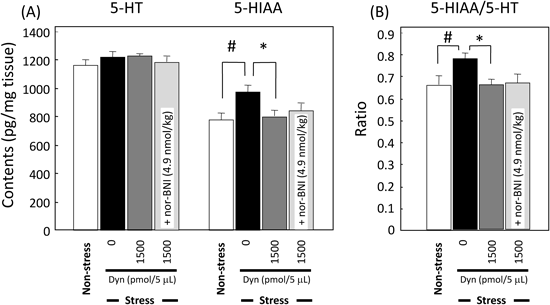
(A) 5-HT and 5-HIAA contents, (B) the ratio of 5-HIAA/5-HT. Mice were sacrificed immediately after the final trial of active conditioned avoidance task. Each column represents the mean±S.E.M. N=8 for each group. # p<0.01 vs. non-stress, * p<0.01 vs. Stress/Dyn(0) (Dunn’s multiple comparisons test).
Each group approaches one object for 15.2–20.3 s, there were no differences in exploratory preference among the groups in the training trial (Student’s t-test: p=0.69; Fig. 4A). In the retention trial, both groups exhibited greater preference for the novel object than the familiar object, and we could not find any differences on the recognition between the groups (Student’s t-test: p=0.53; Fig. 4B).
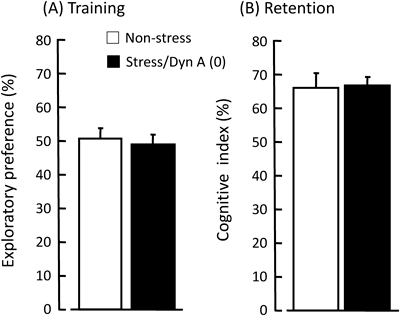
Each column represents the mean±S.E.M. N=5 for each group.
Here, we demonstrated that (i) repeated electric footshock induced escape failures in the active avoidance test, and (ii) κ-opioid receptor stimulation by dynorphin A (1–13) attenuated the those behavioral impairment accompanied with 5-HTergic system in mice.
Repeated Stress and Behavioral ImpairmentsIn the paradigm of this study, the motivation of pressing the levers for the escape from the footshock is an important key point to evaluate effects of reagents for medicines. Additionally, because there is a possibility that high dose of opioid peptide has analgesic effects, we checked the mouse behaviors in the apparatus, then found that all mice jumped and/or vocalized to the footshock. We also could not find any differences on the pain sensitivity among the 6 groups throughout this experiment (personal observation). When we measured the corticosterone level in the plasma as a stress marker, mice given inescapable footshock showed over two times as high concentration as that in non-stressed mice, indicating that such repeated footshock seem to give aversive stimuli to all groups.
It is demonstrated that brain areas rich in glucocorticoid receptors such as the basolateral amygdala (BLA) and prefrontal cortex,14) which controls emotional and working memory respectively, interacts with the hippocampus to modulate cognitive function.15) There are also reports that the exogenous treatment or stress-induced elevation of corticosterone impairs learning and memory.16,17) It is reported that acute/chronic stress disturbs brain function in human and rodents,18) so that we checked the cognitive function using the novel object recognition test. This task is considered to be dependent on cortex and hippocampus.19) As shown in the Fig. 4, there were no remarkable differences on the preference and cognitive behaviors between non-stressed and stressed group. Therefore, it seems that cognitive function is not affected by consequent stress in this study.
It is well known that stressors reliably increase hypothalamic-pituitary-adrenal (HPA) functioning and provoke increased central monoamine activity, especially 5-HTergic neuronal system.20,21) Additionally, acute/chronic stress changes the 5-HT levels and 5-HT turnover.22) As described the above, amygdale plays a critical role in the response to the stress, then, we checked the 5-HT and 5-HIAA contents in the amygdale. We could not find the differences on the 5-HT content in the amygdale, whereas its metabolite increased in the stressed group compared to non-stressed group. Because the brains were dissected after the behavioral test, it might not detect the transient changes of 5-HT synthesis, storage and release. There is a report that strong chronic stress, such as a social defeat stress upregulates the expression of monoamine oxidase-A (MAO-A) gene to accelerate the neurotransmitter degeneration.23) In this point of the increased metabolite, our result was consistent with the previous reports which the elevation of 5-HIAA was observed in the amygdala of mice in the very stressed situation.2,24) Taken together, repeated electric footshock impaired motivation-related lever press behavior accompanied with changing the 5-HTergic system in the amygdale of mice. Since we have a plan to determine the extracellular 5-HT level by brain microdialysis, the data will give the detailed mechanism which dynorphin might control 5-HTergic system in mice.
Roles of κ-Opioid Receptor Agonist on This ModelIt is reviewed that κ-opioid receptor ligand, dynorphins play very important roles on the brain function.6) Therefore, we examined effects of dynorphin A (1–13), an active fragment on this task, and found that it ameliorated only on the last day. A selective κ-opioid receptor antagonist also inhibited the improvement of escape failure by dynorphin A (1–13), suggesting that κ-opioid system may modulate the conditioned lever-press behavior in this task. On the other hand, dynorphin A (1–13) did not affect the corticosterone levels as well as locomotor activity, so that it does not appear that this peptide affects the HPA function directly. Whereas dynorphin A (1–13) recovered the 5-HIAA levels to the control (non-stressed group) level in the amygdale, nor-BNI did not affect the metabolite in this study. Nor-BNI is a long-lasting antagonist, so that we repeated it for 4 d at very low dose. Possibly, because the time-window of neurochemical changes by nor-BNI may be very small, it might be difficult for us to detect the effects in our study. In the different experiments, our group has reported that κ-opioid receptor agonists might improve the impairments of learning and memory via the cholinergic system.7) Therefore, the mechanism of attenuation by κ-opioid receptor agonist might be involved in the cholinergic system in the brain. Taken together, it is suggested that dynorphin A (1–13) may induce the expression and/or enhance the function of MAO-A, then ameliorate the behavioral changes in this model. However, the detail mechanisms underlying the interactions with κ-opioid receptor agonist and other neurotransmitters are needed to clarify in the next step.
Our results demonstrated that dynorphin alleviated the stress-induced behavioral impairments accompanied with regulation of 5-HTergic system in the brain. We hope that these data will contribute the development of a new therapy for treatment of acute and/or chronic stress disorder.
This work was supported by JSPS KAKENHI (Grant Numbers 24590304 and 22790233); the Research Project Supported by the Takeda Science Foundation; the Nakatomi Foundation; the Smoking Research Foundation Grant for Biomedical Research; the Sasakawa Scientific Research Grant from the Japan Science Society; and Basic Research Grants from the Japan Health and Research Institute and the Aichi Health Promotion Foundation. We are thankful to the late Professor Makoto Ukai and Mr. Yoshiaki Kawai for help in the experiment.