2014 年 37 巻 9 号 p. 1475-1479
2014 年 37 巻 9 号 p. 1475-1479
The uptake mechanism of aristolochic acid I (AAI) was investigated using Caco-2 cells cultured on dishes and permeable membranes. The uptake of AAI from the apical membrane of Caco-2 cells cultured on a dish was rapid, and a decrease in the pH of the incubation medium significantly increased uptake. Incubation at low temperature (4°C) and treatment with sodium azide (a metabolic inhibitor) or carbonylcyanide p-trifluoromethoxyphenylhydrazone (a protonophore) significantly inhibited the AAI uptake. Coincubation with L-lactic acid or benzoic acid, typical substrates for the proton-linked monocarboxylic acid transporters (MCTs), significantly decreased the AAI uptake, as did coincubation with α-cyano-4-hydroxycinnamate (an inhibitor of MCTs). Dixon plotting revealed the competitive inhibition of benzoic acid on the AAI uptake. To confirm the AAI uptake via MCTs, the apical-to-basolateral transport of AAI was investigated using the Caco-2 cells cultured on the permeable membranes. The transport of AAI at pH 6.0 was markedly higher than that at pH 7.4, and was significantly decreased by coincubation with benzoic acid. These results suggest that the uptake of AAI from the apical membrane of Caco-2 cells is mediated mainly by MCTs along with benzoic acid.
Aristolochic acids (AAs) are a family of nephrotoxic, carcinogenic and mutagenic compounds commonly found in the Aristolochiaceas family of plants, which are commonly used in Chinese herbal medicine.1) AAs refer to a mixture of structurally related nitrophenanthrene carboxylic acids whose major constituents include 8-methoxy-6-nitro-phenanthro-[3,4-d]-1,3-dioxolo-5-carboxylic acid (aristolochic acid I; AAI) and its 8-demethoxylated form (aristolochic acid II; AAII).2,3) AAs are believed to be the causative agents in Balkan endemic nephropathy and Chinese herb nephropathy.4) Botanic products known or suspected to contain AAs are not permitted to be sold worldwide, although they are still in used in traditional medicines in Asian countries. Recently, Xue et al.4) reported that organic anion transporter (OAT) 1 and 3, which transport drugs from the blood into the tubular epithelium, are responsible for the transportation of AAI into renal tubular cells and the subsequent nephrotoxicity.
To date, 14 subtypes of monocarboxylic acid transporters (MCTs) have been identified, of which the first four (MCT1–MCT4) subtypes have been experimentally demonstrated to catalyze the proton-linked monocarboxylic acid transport.5) MCTs have an important role in the intestinal absorption of not only short-chain monocarboxylic acids, such as L-lactic acid, propionic acid and pyruvic acid, but also exogenous monocarboxylic acid compounds, such as benzoic acid, ferulic acid, nonsteroidal anti-inflammatory drugs and phenoxyacetic acid herbicides.6–11) The human colorectal adenocarcinomal cell line Caco-2 is a useful model with which to study the intestinal absorption of various compounds as the cells are morphologically and functionally similar to human small intestinal epithelial cells.12) Caco-2 cells express various MCT subtypes such as MCT1, MCT3, MCT4, MCT5 and MCT6.13) L-Lactic acid has been widely used as a typical substrate for MCT1, MCT2, MCT3 and MCT4 and α-cyano-4-hydroxycinnamate (CHC) has been used as a specific inhibitor of MCT1, MCT2 and MCT4,5,14,15) but Km and Ki values of MCT4 are higher than those of other subtypes.5) Benzoic acid has been reported as a typical substrate for MCT16)
Although the hepatic metabolism and toxicity of AAs have been studied in detail, little is known about the intestinal absorption mechanism of AAs. As AAI and AAII both possess a carboxylic group, the intestinal absorption of AAs is expected to proceed via MCTs. In the present study, we investigated whether the uptake of AAI from the apical membrane of Caco-2 cells is mediated via MCTs.
AAI, carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP), CHC, sulfobromophthalein, ferulic acid, p-coumaric acid, estrone-3-sulfate, γ-aminobutyric acid and 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS) were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). p-Aminohippuric acid (PAH), acetic acid, pyruvic acid, benzoic acid, salicylic acid, phthalic acid, L-lactic acid, citric acid, L-proline, gylcylsarcosine, choline chloride, sodium azide (NaN3), 2,4-dichlorophenoxyacetic acid (2,4-D) and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Fetal bovine serum (FBS) and nonessential amino acid (NEAA) were obtained from Life Technologies Co. (Carlsbad, CA, U.S.A.). All other chemicals used were of the highest purity commercially available.
Cell CultureCaco-2 cells at passage 45 were obtained from the RIKEN Cell Bank (Tsukuba, Japan), and the cells in this study were used between passage 50 and 76. As described previously, the cells were cultured on 35-mm six-well culture dishes coated with rat tail collagen type I (Becton Dickinson, Bedford, MA, U.S.A.),9,10) or permeable membranes (Cell Culture Insert, 0.9 cm2 growth area: Becton Dickinson)16) in DMEM containing FBS (10%), NEAA (1%), streptomycin (100 U/mL) and penicillin G (70 µg/mL) at 37°C in a humidified atmosphere of 5% CO2–95% air. The culture medium was replaced three times a week, and confluent Caco-2 cell monolayers cultured for 7–9 d on the dishes or for 21 or 22 d on the membrane were used in this study.
Uptake Study by Caco-2 CellsThe uptake experiment was performed as described previously.9–11) The incubation medium used for the uptake study was Hanks’ balanced salt solution (HBSS) containing 25 mM D-glucose and 10 mM 2-(N-morpholino)ethanesulfonate (MES) (pH 5.5, 6.0 or 6.5) or 10 mM N-hydroxyethylpiperazine-N′-2-ethanesulfonate (HEPES) (pH 7.0, 7.4 or 8.0). The culture medium was removed, and the cells were preincubated at 37°C or 4°C for 20 min in 1.5 mL of the incubation medium at pH 7.4. After preincubation, the medium was aspirated, and the cells were incubated with 1.5 mL of fresh incubation medium containing AAI for the designated times at the same temperature as the preincubation. Na+-free HBSS was prepared by replacing NaCl with choline chloride, and omitting NaH2PO4, to examine whether the uptake of AAI was Na+ dependent.11) In the metabolic inhibition experiments, the cells were treated with 10 mM NaN3 or 25 µM FCCP in the incubation medium at 37°C for 20 min,9–11) prior to incubation with AAI. To investigate the uptake of AAI via specific mechanisms, the cells were coincubated with 50 µM AAI and various compounds. AAI and the compounds used were dissolved in dimethyl sulfoxide or methanol, and added to the incubation medium at a final concentration of 1% or lower.
After incubation with AAI, the cell surface was quickly washed three times with ice-cold incubation medium. The cells were suspended in 1.0 mL of extraction solution (1 N H3PO4 : methanol=1 : 1) for 60 min at room temperature, and the cells were scraped off and collected using a cell scraper.9–11) The suspension was centrifuged at 13000×g for 10 min, and a 50-µL aliquot of the supernatant was injected onto the HPLC system.
Transport ExperimentsThe transport experiment was performed as described previously.16) The confluent Caco-2 cell monolayers on permeable membranes, with a transepithelial electrical resistance of more than 350 Ωcm2, were used for the transport experiment. The culture medium was removed, and the cell monolayers were washed twice with incubation medium (pH 7.4) warmed to 37°C. After washing, the cell monolayers were preincubated for 20 min at 37°C in the incubation medium at pH 7.4. After preincubation, the cell monolayers were incubated with 50 µM AAI for the designated time from the apical side of the monolayers at pH 6.0 or 7.4 at 37°C. The medium volume of the apical and basolateral sides was 0.5 and 1.5 mL, respectively. The transport of AAI from the apical side of the cell monolayers to the basolateral side was measured using an HPLC system.
Determination of AAI and ProteinAAI was determined using an HPLC system consisting of a Shimadzu LC-10A pump and SPD-10A UV detector (Kyoto, Japan) equipped with an Inertsil VP-ODS column (4 mm i.d.×250 mm; GL Sciences, Tokyo, Japan). The mobile phase and the wavelength were 50 mM Na2HPO4 buffer (pH 2.1) containing acetonitrile (3 : 2, v/v) and 400 nm, respectively. The calibration curve of AAI was linear over the concentration range of 1–20 nmol/mL and the coefficients of variation were lower than 4%.
The protein concentration was determined using a Bio-rad dye reagent (Richmond, CA, U.S.A.) with bovine serum albumin as the standard.
Statistical AnalysesThe data were analyzed by Scheffe’s multiple comparison test after analysis of variance using the Statcell 2 program, and differences with values of p<0.05 were considered to be significant. Data are shown as the mean±standard error (S.E.).
Caco-2 cells were incubated with 50 µM AAI at pH 6.0 for up to 10 min (Fig. 1). The initial uptake of AAI at 37°C was rapid followed by a gradual increase for up to 10 min. The uptake of AAI at 4°C linearly increased with incubation time, and the uptake at 10 min at 4°C was about 40% of that at 37°C. The uptake of AAI at 37°C in the presence of the metabolic inhibitor, NaN3, was about 60% of that in its absence.
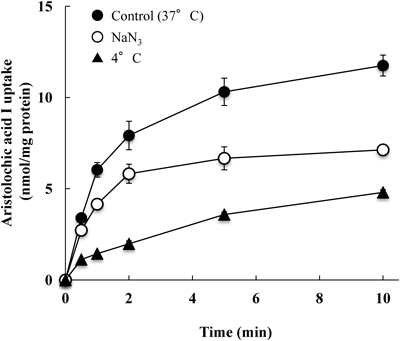
Caco-2 cells were incubated with 50 µM aristolochic acid I in a medium at pH 6.0 and at 37°C. Caco-2 cells were treated with 10 mM NaN3 at 37°C prior to incubation with aristolochic acid I at 37°C. Caco-2 cells were preincubated at 4°C and the incubated with aristolochic acid I at 37°C. Each value represents the mean±S.E. for 4–6 determinations.
The effect of extracellular pH on the uptake of AAI by Caco-2 cells was investigated (Fig. 2). The Caco-2 cells were incubated with 50 µM AAI for 1 min at pH 5.5, 6.0, 6.5, 7.0, 7.5 or 8.0. Decreases in pH markedly increased the uptake of AAI.

Caco-2 cells were incubated for 1 min with 50 µM aristolochic acid I in a medium at different pH levels. Each value represents the mean±S.E. for 4–6 determinations.
The effects of sodium ions and various compounds on the initial uptake of AAI by Caco-2 cells were investigated (Table 1).
| Compound | Concentration (mM) | Uptake (% of control) |
|---|---|---|
| Control | 100±3.4 | |
| Na-freea) | 96.7±3.0 | |
| FCCPb) | 0.025 | 43.7±0.9*# |
| DIDS | 1 | 107.2±2.3 |
| p-Aminohippuric acid | 10 | 91.1±1.4 |
| Acetic acid | 10 | 59.5±1.9* |
| Pyruvic acid | 10 | 70.3±2.2* |
| L-Lactic acid | 10 | 75.5±2.0* |
| Benzoic acid | 10 | 52.5±2.7* |
| Salicylic acid | 10 | 60.1±1.0* |
| Ferlic acid | 10 | 42.3±1.5*# |
| p-Coumaric acid | 10 | 50.4±1.5*# |
| 2,4-D | 5 | 66.3±3.1* |
| CHC | 10 | 55.7±2.4* |
| Phthalic acid | 10 | 95.0±3.4 |
| Citric acid | 10 | 91.2±3.1 |
| Estron-3-sulfate | 1 | 95.5±2.8 |
| Sulfobromophthalein | 1 | 93.6±4.1 |
| L-Proline | 10 | 101.4±3.1 |
| γ-Aminobutyric acid | 10 | 99.8±4.6 |
| Glycylsarcosine | 10 | 94.8±4.6 |
Caco-2 cells were incubated with 50 µM aristolochic acid I at 37°C for 1 min in the presence or absence of various compounds at pH 6.0. a) Caco-2 cells were incubated at 37°C and pH 6.0 for 1 min in a Na+-free medium containing 50 µM aristolochic acid I. b) Caco-2 cells were treated with 25 µM FCCP for 20 min, and then incubated with 50 µM aristolochic acid I at 37°C and pH 6.0 for 1 min. Each value represents the mean±S.E. for 4–6 determinations. *Significantly different from the control (p<0.05). #Significantly different from the value for L-lactic acid (p<0.05).
Na+-free conditions, achieved by the replacement of NaCl in the incubation medium with choline chloride, did not affect the uptake of AAI. However, pretreatment with FCCP (a protonophore) significantly inhibited the uptake of AAI by 56%.
Coincubation with acetic acid, benzoic acid, ferulic acid, p-coumaric acid, salicylic acid or 2,4-D significantly decreased the uptake of AAI by about 35–60%. Coincubation with CHC (an inhibitor of some MCTs) significantly decreased the uptake of AAI by 44%, and that with pyruvic acid or L-lactic acid significantly but moderately decreased the uptake of AAI by 24 and 30%, respectively. In contrast, coincubation with phthalic acid (a dicarboxylic acid) or citric acid (a tricarboxylic acid) did not decrease the uptake of AAI.
Coincubation with p-aminohippuric acid (a typical substrate of organic anion transport systems; OATs), DIDS (an anion exchange inhibitor), or glycylsarcosine (a substrate of proton-coupled peptide transporter; PEPT1) did not decrease the uptake of AAI. Further, coincubation with estrone-3-sulfate (a substrate of the organic anion transporting polypeptide family; OATPs), sulfobromophthalein (a substrate of OATPs), L-proline (a substrate of proton-coupled amino acid transporter; PAT1) or γ-aminobutyric acid (a substrate of PAT1) did not decrease the uptake of AAI.
Competitive Inhibition of Benzoic Acid on AAI UptakeThe mode of the inhibitory effect of benzoic acid on AAI uptake by Caco-2 cells was analyzed using Dixon plotting (Fig. 3). Caco-2 cells were incubated for 1 min at 37°C and pH 6.0 with 25, 50 or 100 µM AAI in the absence or presence of benzoic acid (0, 1, 2 or 5 mM). The uptake data at 25, 50 or 100 µM AAI were well fitted to straight lines, and the intersection of the three straight lines was found at about −4.8 mM and 0.1 nmol/mg protein. These results suggest the competitive inhibition of benzoic acid on AAI uptake by Caco-2 cells with Ki and Vmax values of about 4.8 mM and 10 nmol/mg protein, respectively.

Uptake of aristolochic acid I for 1 min at pH 6.0 from the medium containing 25, 50 or 100 µM in the absence or presence of benzoic acid (0, 1, 2 or 5 mM) was measured. Each value represents the mean±S.E. for 4–6 determinations. The apparent Ki value determined by linear regression analysis was 4.8 mM.
In order to confirm the effect of the apical side pH on the uptake of AAI from apical membranes of Caco-2 cells, Caco-2 cell monolayers cultured on permeable membranes were incubated with 50 µM AAI from the apical side at pH 6.0 or 7.4 and at 37°C for up to 120 min. The apical-to-basolateral transport of AAI from the apical side at pH 6.0 and 7.4 increased with time (Fig. 4). However, the transport of AAI from the apical side at 120 min at pH 6.0 (7.83±0.23 nmol/cm2) was more than 3.5 times larger than that at pH 7.4 (2.21±0.09 nmol/cm2). Furthermore, coincubation with 5 mM benzoic acid (a typical substrate of MCTs) from the apical side for 120 min at pH 6.0 significantly decreased the apical-to-basolateral transport of AAI by 56% (data not shown in Fig. 4).

Caco-2 cell monolayers were incubated at 37°C with 50 µM aristolochic acid I added to the apical medium at pH 6.0 or 7.4. Each value represents the mean±S.E. for 3–6 determinations.
We investigated the intestinal absorption mechanism of AAI using Caco-2 cells. The uptake of AAI from the apical membrane was rapid, and both temperature and pH dependent (Figs. 1, 2). Pretreatment with a metabolic inhibitor (NaN3) or protonophore (FCCP) profoundly inhibited the uptake of AAI (Fig. 1, Table 1). Coincubation with CHC (a nonspecific inhibitor of MCT1, MCT2, MCT4) profoundly decreased the uptake of AAI, and coincubation with L-lactic acid (a typical substrate of MCT1–MCT4) or pyruvic acid (a substrate of some MCTs) moderately decreased AAI uptake (Table 1). Furthermore, coincubation with aromatic monocarboxylic acids (benzoic acid, salicylic acid, ferulic acid, p-coumaric acid and 2,4-D) profoundly inhibited the uptake of AAI, whereas coincubation with dicarboxylic acid (phthalic acid) or tricarboxylic acid (citric acid) did not have any effect (Table 1). These results suggest that the uptake of AAI from the apical membrane of Caco-2 cells is mediated mainly via proton-linked MCTs. The apical-to-basolateral transport of AAI from the apical side at pH 6.0 was markedly higher than that at pH 7.4 (Fig. 4), and coincubation with benzoic acid significantly decreased the transport of AAI. These results support the notion that AAI is uptaken from the apical membrane of Caco-2 cells via proton-linked MCTs.
Caco-2 cells possess several isoforms of MCTs, such as MCT1, MCT3, MCT4, MCT5 and MCT6,13) and MCT1, MCT3 and MCT4 catalyze the proton-linked monocarboxylic acid transport.5) We previously speculated that there are two types of MCTs in Caco-2 cells: one type consists of L-lactic acid-sensitive MCTs, and the other consists of L-lactic acid-insensitive (benzoic acid-sensitive) MCTs.9,10) As the uptake of AAI was inhibited profoundly and competitively by benzoic acid and moderately by L-lactic acid (Table 1, Fig. 3), AAI uptake appears to be mediated mainly via benzoic acid-sensitive MCTs. Recently, Kekuda et al.17) reported that MCT1 is the high affinity lactate transporter, MCT4 is the high affinity butyrate transporter, and several non-steroidal anti-inflammatory drugs and salicylate are transported by the butyrate transporter in the intestinal epithalial cell line IEC-18 treated with siRNAs for MCT1 and MCT4. Further studies using the MCT suppressed Caco-2 cells and/or the MCT over-expressed Caco-2 cells are necessary to clarify the mechanism of AAI uptake via MCTs.
We previously reported that the uptake of phenoxyacetic acids in MCPA, 2,4-D and 2,4,5-T9,10) and triclopyr11) by Caco-2 cells was mediated mainly via L-lactic acid-insensitive (benzoic acid-sensitive) MCTs and partly via L-lactic acid-sensitive MCTs. We previously reported the competitive inhibition of benzoic acid on the uptake of MCPA, 2,4-D, 2,4,5-T and triclopyr, with Ki values of 4.68, 3.42, 3.65, and 4.61 mM, using Lineweaver–Burk plots.9–11) In the present study, we analyzed the inhibitory effect of benzoic acid on the uptake of AAI using Dixon plotting (Fig. 3) as the solubility of AAI is markedly lower than that of phenoxyacetic acids and triclopyr. Dixon plots revealed that benzoic acid competitively inhibits the uptake of AAI with the Ki value of benzoic acid being 4.8 mM. This Ki value of benzoic acid is very similar to the values reported previously using Lineweaver–Burk plots. In addition, coincubation with 2,4-D significantly decreased the uptake of AAI (Table 1). These results are further evidence of the uptake of AAI by Caco-2 cells mainly via benzoic acid-sensitive MCTs.
The Na+-coupled monocarboxylic acid transporter (SMCT)-1 is localized on the luminal membrane in the intestine, and contributes to the intestinal absorption of monocarboxylic acid derivatives.18,19) However, the uptake of AAI was found to be Na+ independent (Table 1). Several Na+-independent, H+-dependent cotransporters, such as oligopeptide transporter (PEPT)-1, organic anion transporting polypeptide (OATP)-B and amino acid transporter (PAT)-1, localize in the brush border membranes of the human small intestine and Caco-2 cells.20–23) However, coincubation with glycylsarcosine (a typical substrate of PEPT), estro-3-sulfate (an inhibitor of OATPs), sulfobromophtalein (an inhibitor of OATPs), L-proline (a substrate of PAT1), or γ-aminobutyric acid (a substrate of PAT1) did not decrease the uptake of AAI (Table 1). The transport of AAI from the blood into the tubular epithelium is mediated by organic anion transporter (OAT) 1 and 3, which are expressed by basolateral membranes,4) andcoincubation with p-aminohippuric acid (a typical substrate of OATs) did not decrease the uptake of AAI. The possibility of AAI uptake from the apical membranes of Caco-2 cells via SMCT1, PEPT1, OATP-B, PAT1 and OATs can, therefore, be ruled out. CHC is an inhibitor of not only some MCTs but also anion exchanger.24) However, coincubation with DIDS (an anion exchange inhibitor) did not decrease the uptake of AAI (Table 1). The uptake of AAI from the apical membranes of Caco-2 cells via anion exchanger can be ruled out.
To our knowledge, the present study is the first report focused on the intestinal absorption of AAI and suggests that the absorption of AAI proceeds mainly via MCTs along with benzoic acid. Further study is necessary to investigate the intestinal absorption mechanism of AAII, which is an another major constituent found in the Aristolochiaceas family of plants.
This work was supported by Grant-in-Aid from Japan Society for the Promotion Science (C) (No. 24614012; to O.K.)