2014 年 37 巻 9 号 p. 1534-1542
2014 年 37 巻 9 号 p. 1534-1542
Lipid-mediated delivery of DNA into cells holds great promise both for gene therapy and basic research applications. The primary approach to improving transfection efficiency is the design and synthesis of novel cationic lipids. Alternatively, using the synergistic effect of different cationic mixtures can provide another approach to increasing transfection efficiency. This paper describes the synergistic effect of lipids with different polarheads, central core structures and hydrophobic tails. The enhancement of cellular transfection into HEK293 cells was observed by combining two lipids having aminoglycerol and di(hydroxylethyl)amino core structures at a 1 : 1 weight ratio. Additionally, the liposome formation of these lipids with the helper lipid, 1,2-dioleoyl-propyl-3-phosphatidylethanolamine (DOPE), at the weight ratio of 1 : 1 can provide higher transfection efficiency into HEK293, MCF-7 and HeLa cells than Lipofectamine™ 2000. Our finding indicated that cationic liposomes comprised of a mixture of lipids with different polarheads, central core structures and hydrophobic tails should be very promising in liposome-mediated gene delivery in vitro and in vivo.
Gene therapy has gained significant attention over past two decades as an alternative method to treat genetic disorders1,2) as well as cancers.3) One fundamental of gene therapy is the delivery system that can introduce and stabilize genetic material. Since free oligonucleotides and DNA are rapidly degraded by serum nucleases,4) many efforts are focused on developing the carriers to protect and deliver these materials into targeted cells. Two delivery systems namely viral and non-viral vectors are currently developed to solve the problems. Viral carriers have known to be one of the most effective gene delivery methods for in vivo application.5–8) Over one thousand gene therapy clinical trials have been completed or approved; two-thirds of which performed by viral vectors.8) However, the limitation of viral vectors concerning toxicity, immunogenicity, scale-up procedures and their relatively small capacity for therapeutic DNA has prompted the development non-viral vectors. Delivery systems based on non-viral vectors, for example cationic lipids, dendrimers or cationic polymers, have been the focus of much recent research.9) Non-viral systems have proved to be generally less toxic or immunogenic, more easily to produce and a greater stability.
Cationic liposomes are one of the most extensively studied of non-viral vectors because of their lesser immunogenic nature, ease of production and handle, and ability to deliver large pieces of DNA. Cationic liposomes, like other non-viral vectors bearing positive charge, interact with the negatively charged phosphate backbone of nucleic acids to form a compact structure and facilitate cellular uptake by endocytic routes.10–13) Since the first application of cationic lipid in DNA delivery,14) numerous cationic lipids have been synthesized and demonstrated the capability of delivering genetic materials into various cells.9) Some of cationic liposomes-mediated gene transfers have been used in gene therapy clinical trials.8) However, significant limitations of cationic liposomes are low transfection efficiency and much research activity has been focused on increase efficiency. The main approach to improving the transfection properties was to synthesize new kinds of cationic lipids. The alternative strategy, apart from employing the helper lipids such as 1,2-dioleoyl-propyl-3-phosphatidylethanolamine (DOPE) or cholesterol, to improve tansfection efficiency is the use of mixture cationic lipids.
Previous studies have shown that the use of a mixture of cationic lipids with the same head group but with different chain lengths dramatically enhances the transfection of human umbilical artery endothelial cells (HUAEC).15) The synergistic effect was not only limited to the homologous, but it also exhibited to cationic lipids with different polarhead and hydrophobic tails.16) Mixture of multi-components to form lipoplexes has also been reported.17) Asymmetric vesicles formulated with lipids having very different headgroups have been prepared. This vesicle is expected to be a delivery system with increase in biocompatibility and flexibility.18) Recently, we described the cationic lipids 119) and 220,21) (Fig. 1) which exhibited transfection efficiency higher than and comparable to the commercially available transfection reagents. These lipids contain different polarheads, central core structure, and hydrophobic tails; 1 comprise of the commonly used template, aminoglycerol, primary amino headgroup and dodecanoyl tails whereas 2 has di(hydroxyethyl)amino core structure, polyamine polarhead and myristoyl tails. Therefore, to investigate how transfection changes with the liposome comprised of cationic lipids with different polarheads, central core structures and hydrophobic tails, cationic liposome consisting of 1 and 2 was prepared and tested for transfection efficiency in mammalian cells. The size and zeta potential of the prepared liposome were also evaluated.

Solvents and reagents were purchased from commercial suppliers and used without further purification. IR spectra were recorded as neat on a Perkin-Elmer FT-IR spectrum 400 spectrometer attenuated total reflectance (ATR). 1H- and 13C-NMR spectra were recorded on a Bruker AVANCE400 spectrometer operating at 400 and 100 MHz, respectively. Chemical shifts are given in ppm relative to residual CHCl3/CDCl3 (δH 7.24/δC 77.00, central signal of the triplet related to carbon). Mass spectra were obtained using a Finnigan LC-Q mass spectrometer. High resolution mass spectra were performed on a Bruker micrOTOF II mass spectrometer. Unless indicated otherwise, column chromatography and TLC were carried out using Merck silica gel 60 (finer than 0.063 mm) and precoated silica gel 60 F254 plates, respectively. Spots on TLC were detected by spraying with ninhydrin reagent followed by heating.
Synthesis of Cationic Lipid 1 (Chart 1)
Reagents and conditions: a) di-tert-butyl dicarbonate, CH2Cl2, 24 h; b) 4-nitrophenyl chloroformate, pyridine, CH2Cl2, 8 h; c) (±)-3-amino-1,2-propanediol, CH2Cl2 : MeOH (1 : 1), 12 h; d) dodecanoic acid, DCC, DMAP, CH2Cl2, 16 h; e) 20% TFA/CH2Cl2, 1 h.
(i) N-(tert-Butoxycarbonyl)-1,2-diaminoethane (4): A solution of di-tert-butyl dicarbonate (4.0 g, 18.6 mmol) in CH2Cl2 (200 mL) was added dropwise to a stirred solution of 1,2-diaminoethane (3) (5.3 g, 93.1 mmol) in CH2Cl2 (500 mL) and the mixture was stirred for 24 h. The solvent was evaporated and the residue was suspended in H2O (200 mL). The insoluble white solid was removed by filtration and the product was then extracted using CH2Cl2 (3×200 mL). The combined organic phase was dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified by column chromatography using CH2Cl2 and CH2Cl2–MeOH as eluting solvent with gradual increase in concentration of the more polar component to yield the title compound as a white solid (2.5 g, 84%). The spectroscopic data were in agreement with those found in the literature.22)
(ii) N-(1-(2,3-Dihydroxy)propyl)-N′-(2-(N-Boc)aminoethyl)carbamide (5): To a solution mixture of N-(tert-butoxycarbonyl)-1,2-diaminoethane (1.1 g, 6.8 mmol) and pyridine (0.8 mL, 10.3 mmol) in CH2Cl2 (10 mL) was added dropwise a solution of 4-nitrophenyl chloroformate (1.6 g, 8.2 mmol) in CH2Cl2 (10 mL) and the mixture was stirred for 8 h. The reaction was work up by removed the solvents under reduced pressure. The crude product was dissolved in CH2Cl2 (20 mL) and (±)-3-amino-1,2-propanediol (0.6 mL, 8.2 mmol) in MeOH (15 mL) was then added. The resulting mixture was stirred overnight at the room temperature. The solvent was evaporated and the residue was suspended in H2O (100 mL) and thoroughly extracted with EtOAc (3×50 mL). The combined organic phase was washed with H2O, dry over anhydrous Na2SO4 and the solvent was evaporated to dryness. The residue was purified by column chromatography using CH2Cl2 and CH2Cl2–MeOH as eluting solvent with gradual increase in concentration of more polar component to afford compound 5 (1.0 g, 53%) as colorless oil. IR: νmax 3348, 1678, 1590, 1157, 1045 cm−1; 1H-NMR (400 MHz, CDCl3+10 drops of CD3OD) δ: 1.36 (9H, s, C(CH3)3), 3.09 (2H, m, BocNHCH2), 3.15 (2H, m, NHCH2CH), 3.19 (2H, t, J=5.0 Hz, BocNHCH2CH2NHCO), 3.46 (2H, m, CH2OH), 3.60 (1H, m, CHOH); 13C-NMR (100 MHz, CDCl3+10 drops of CD3OD) δ: 28.1 (OC(CH3)3), 39.1 (NHCH2CH), 40.7 (BocNHCH2), 42.3 (BocNHCH2CH2NHCO), 63.4 (CH2OH), 71.3 (CHOH), 79.4 (OC(CH3)3), 156.9 (NHCONH), 160.2 (NHCOOtBu); high resolution-electrospray ionization-mass spectrum (HR-ESI-MS) (+ve) m/z: Calcd for C11H23N3NaO5 [M+Na]+ 300.1529, Found 300.1553.
(iii) N-(1-(2,3-Didodecanoyloxy)propyl)-N′-(2-(N-Boc)aminoethyl)carbamide (6): Dodecanoic acid (1.8 g, 9.0 mmol), dicyclohexylcarbodiimide (DCC) (1.9 g, 9.0 mmol) and 4-dimethylaminopyridine (DMAP) were dissolved in dry CH2Cl2 (20 mL). The reaction mixture was stirred at room temperature for 30 min, a solution of compound 5 (1.0 g, 3.6 mmol) in dry CH2Cl2 (10 mL) was then added and the resulting mixture was stirred overnight at the same temperature. The precipitated dicyclohexyl urea (DCU) was removed by filtration and the filtrate was evaporated under reduced pressure to give crude product. The product 6 (1.9 g, 82%) was obtained as white solid after usual column chromatographic purification. IR: νmax 3400, 3284, 2919, 1736, 1652, 1576, 1163 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 0.85 (6H, br t, J=6.6 Hz, 2×CH3), 1.23 (32H, s, 16×CH2), 1.41 (9H, s, OC(CH3)3), 1.58 (4H, br s, 2×CH2), 2.29 (4H, m, 2×CH2), 3.21 (2H, br s, CH2NHBoc), 3.26 (2H, br s, CH2CH2NHCO), 3.37 (2H, br t, J=5.1 Hz, NHCH2CH), 4.14 (1H, dd, J=12.0, 5.8 Hz, NHCH2CHCHaHbO), 4.25 (1H, dd, J=12.0, 3.5 Hz, NHCH2CHCHaHbO), 4.76 (1H, br s, NH), 5.00 (2H, br s, 2×NH), 5.03 (1H, m, NHCH2CHCH2O); 13C-NMR (100 MHz, CDCl3) δ: 14.0 (CH3), 22.6, 24.84, 24.87, 28.3, 29.1, 29.26, 29.29, 29.4, 29.5, 31.8, 34.0, 34.2, 40.1 (CH2NHBoc), 40.5 (NHCH2CH), 40.7 (CH2CH2NHCO), 62.8 (NHCH2CHCH2O), 70.9(NHCH2CHCH2O), 79.4 (OC(CH3)3), 156.7 (NHCOOtBu), 158.4 (NHCONH), 173.4 (OCOCH2), 173.5 (OCOCH2); HR-ESI-MS (+ve) m/z: Calcd for C35H67N3NaO7 [M+Na]+ 664.4871, Found 664.4898.
(iv) N-(1-(2,3-Didodecanoyloxy)propyl)-N′-(2-aminoethyl)carbamide (1): To a solution of compound 6 (1.9 g, 2.96 mmol) was added 20% trifluoroacetic acid (TFA) in CH2Cl2 (20 mL, excess) and the mixture stirred for 1 h. The solvent was removed under stream of nitrogen gas and further dried under vacuum for 2 h to dryness to obtain lipid 1 as white sticky solid (1.8 g, 100% as TFA salt). IR: νmax 3392, 2922, 1727, 1673, 1568, 1176, 1136 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 0.81 (6H, t, J=6.1 Hz, 2×CH3), 1.19 (32H, br s, 16×CH2), 1.52 (4H, br m, 2×CH2), 2.25 (4H, br m, 2×CH2), 2.97 (2H, br s, CH2NH2), 3.20 (1H, m, NHCHaHbCH), 3.32 (1H, m, NHCHaHbCH), 3.36 (2H, m, CH2CH2NHCO), 4.02 (1H, dd, J=11.9, 6.3 Hz, NHCH2CHCHaHbO), 4.20 (1H, dd, J=11.9, 3.1 Hz, NHCH2CHCHaHbO), 5.06 (1H, br s, NHCH2CHCH2O); 13C-NMR (100 MHz, CDCl3) δ: 13.9 (CH3), 22.5, 24.75, 24.78, 29.01, 29.04, 29.1, 29.2, 29.4, 29.5, 31.8, 34.0, 34.1, 37.5 (CH2CH2NHCO), 40.0 (NHCH2CH), 40.4 (CH2NH2), 62.8 (NHCH2CHCH2O), 70.5 (NHCH2CHCH2O), 159.5 (NHCONH), 173.9 (OCOCH2); HR-ESI-MS (+ve) m/z: Calcd for C30H60N3O5 [M+H]+ 542.4527, Found 542.4534.
Synthesis of Cationic N1,N1-Dimyristoyloxyethyl-spermine (2) (Chart 2)
Reagents and conditions: a) spermine, DMF, 16 h; b) Dde-OH, CH2Cl2, 16 h; c) di-tert-butyl dicarbonate, pyridine, CH2Cl2, 16 h; d) 2% hydrazine/DMF, 1 h; e) 2-bromoethanol, DIPEA, DMF, 16 h; f) myristic acid, DIC, DMAP, CH2Cl2 : DMF (4 : 1), 16 h; g) 20% TFA/CH2Cl2, 2 h.
The active carbonate resin 7 (562 mg, 1 eq) was added an excess solution of spermine (610 mg, 3.0 mmole) in DMF (10 mL) and the resulting suspension was then shaken overnight. The resin was filtered and washed successively with CH2Cl2, N,N-dimethylformamide (DMF), MeOH, DMF and CH2Cl2 (3×10 mL for each solvent) and dried under vacuum for 2 h to give the resin 8. To this resin was added an excess of 2-acetyldimedone (Dde-OH) in CH2Cl2 (10 mL) and the suspension was shaken overnight. The resin was successfully washed with solvents. The result resin was then treated with di-tert-butyl dicarbonate (Boc2O) (6 eq) and pyridine (20 eq) in CH2Cl2 (10 mL) and the suspension was shaken overnight. The resin was filtered and washed successively with suitable solvents and the resulting resin was further reacted with 2% hydrazine (N2H4) in DMF (10 mL) for two cycles of 30 min to give the resin 9. To the resin 9 was added an excess of 2-bromoethanol and N,N-diisopropylethylamine (DIPEA) (20 eq) in DMF (10 mL) and the suspension was shaken overnight. The resins were filtered and washed successively with CH2Cl2, DMF, MeOH, DMF and CH2Cl2 (3×10 mL for each solvent) to give diol resin 10. To the diol resin 10 was added a solution of myristic acid (4 eq), N,N-diisopropylcarbodiimide (DIC) (4 eq) and 4-dimethylaminopyridine (DMAP) in CH2Cl2/DMF (4 : 1). The suspensions were shaken overnight and washed with CH2Cl2, MeOH, DMF, MeOH and CH2Cl2 (3×10 mL for each solvent). The resulting resin was treated with a solution of 20% TFA in CH2Cl2 (10 mL) for 2 h. The filtrate was then collected and evaporated under a stream of nitrogen to give the crude lipid 2. The crude product was purified by Sephadex LH20 using MeOH as eluting solvent to give the lipid 2 (278 mg, 53%). The yield of the product was calculated based on the original loading of Merrifield resin (1.34 mmol/g). IR: νmax 2918, 2850, 1723, 1665, 1196, 1176, 1128 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 0.83 (6H, br s, 2×CH3), 1.21 (40H, br s, 20×CH2), 1.56 (4H, br s, 2×OCOCH2CH2), 1.75 (4H, br s, NHCH2CH2CH2CH2NH), 1.81 (2H, br s, NHCH2CH2CH2N), 2.04 (2H, br s, NH2CH2CH2CH2NH), 2.26 (4H, m, 2×OCOCH2), 2.65 (2H, br s, NHCH2CH2CH2N), 2.76 (4H, br s, N(CH2CH2O)2), 2.97 (8H, br s, NH2CH2CH2CH2NH, NHCH2CH2CH2CH2NH and NHCH2CH2CH2N), 3.04 (2H, br s, NH2CH2CH2CH2NH), 4.10 (4H, m, 2×NCH2CH2O); 13C-NMR (100 MHz, CDCl3) δ: 13.4 (CH3), 22.2, 22.3, 22.5, 23.2, 23.4, 24.4, 28.7, 28.8, 28.9, 29.0, 29.2, 31.4, 33.8, 36.1, 44.1, 46.0, 46.5, 46.6, 51.4, 52.2 N(CH2CH2O)2, 61.5 2×NCH2CH2O, 173.9 and 173.9 (OCOCH2); HR-ESI-MS (+ve) m/z: Calcd for C38H79N4O4 [M+H]+ 711.6721, Found 711.6717.
Liposome Preparation(i) Preparation of Cationic Liposomes: Stock solution of lipid 1 (5 µg/µL) and 2 (5 µg/µL) were prepared in absolute ethanol. Lipids 1 and 2 were mixed at various weight ratios (4 : 1, 3 : 1, 2 : 1, 1 : 1, 0.5 : 1 and 0.25 : 1) to produce solutions of mixture lipids in an Eppendorf tubes. The organic solvent was evaporated under a stream of nitrogen and further dried under high vacuum (>2 h). The resulting thin film was hydrated with phosphate buffered saline (PBS, pH 7.4) at room temperature for 1 h to give the final liposome concentration of 1 µg/µL. The mixture was vortexed for 1 min and sonicated for 20 min in a bath-type sonicator, producing small unilamellar vesicles.23,24) The liposomes were stored at 4°C for 24 h prior to use.
(ii) Preparation of Cationic Liposomes with and without DOPE: Stock solution of lipid 1 (5 µg/µL), lipid 2 (5 µg/µL) and DOPE (5 µg/µL) were made in absolute ethanol. To prepare cationic liposomes with DOPE, stock solutions of lipid and DOPE were mixed at weight ratio of 1 : 1 in an Eppendorf tubes. The processes to prepare cationic liposome are the same manner as described above. The liposome without DOPE was prepared as the same manner for DOPE-contained liposome excepted DOPE was not included.
DNA Binding AffinityDNA binding affinities of liposomes were measured at liposome/DNA ratios (w/w) of 5, 10, 15, 20, 25 and 30, by gel electrophoresis. The liposome/DNA complexes were prepared by adding the liposome solution to the DNA solution (the amount of DNA was fixed at 0.1 µg). The mixture was gently mixed by pipetting up and down for 2–3 times and the mixture was held at room temperature for 30 min. Each complex was added gel loading buffer (13.3% w/v sucrose in water) to get the final volume of 10 µL. The complexes solution was inverted to mix and each sample (10 µL) was loaded onto a 1% agarose gel (0.5×TBE buffer). The gel was run at 200 V, 400 mA for 2 h. DNA bands were viewed under UV light by ethidium bromide staining.
Particle Sizes and Zeta PotentialsThe particle size and surface charge of the cationic liposomes or cationic liposomes/DNA complexes were determined by photon correlation spectroscopy using a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, U.K.). The cationic liposomes or cationic liposomes/DNA complexes at varying weight ratios were diluted with distilled water that had been filtered through a 0.22 µm membrane filter to obtain the volume required for each measurement. All samples were measured in triplicate at room temperature.
Transfection ProcedureHuman embryonic kidney (HEK293) and cervical epithelial adenocarcinoma (HeLa) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) medium supplemented with 10% fetal calf serum (FCS), penicillin (100 units/mL), streptomycin (100 mg/mL) and L-glutamine (4 mM) at 37°C, 5% CO2. Human breast adenocarcinoma (MCF-7) was also growth as mentioned above except the medium containing 1% of insulin. For transfection, the cells were seeded up to 1×104 cells/well in a 96-well plate, to give 50−70% confluence to be used on the next day.
The growth medium was removed and the cells were washed with PBS and replaced with 100 µL of fresh serum-free DMEM medium. DNA (pCMV-encoding β-galactosidase)/cationic liposome complexes (lipoplexes) were prepared as follows. An appropriate volume of each cationic liposome (1 µg/µL) was added to the plasmid DNA (1 µL, 0.1 µg/µL) and the complex was incubated at room temperature for 30 min before being diluted with phosphate-buffered saline to make a final DNA concentration of 0.1 µg/10 mL. The lipoplexes (10 µL) were then added to the cells and left to incubate at 37°C, 5% CO2 for 4 h. The cells were then washed with PBS and fresh growth medium was added and further incubated for 48 h. For Lipofectamine™ 2000 transfection, the method was carried out according to the manufacturer’s instruction.
After transfection, the cells were washed once with Dulbecco’s phosphate buffered saline (D-PBS) containing 0.1 g/L calcium and magnesium and then fixed with 100 µL fixative (2% formaldehyde, 0.05% glutaraldehyde in D-PBS) for 5 min at room temperature. The cells were washed and 100 µL of substrate/stain solution (1 mg/mL X-gal in stain solution; 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2) incubated at 37°C for 2 h. The cells were washed with D-PBS and the blue cells were then counted under inverted microscope. The number of transfected cells in each well of 96-well plate (0.32 cm2) was calculated to be a number of positive cells per cm2.
Combinatorial synthesis provides a library of bioactive compounds to discover the lead. Lipid 1, which was one of the lead components found from a 60-compound library,19) exhibited higher transfection efficiency than the commercially available transfection agent, Effecene™. To obtain this compound in high purity and quantity, the lipid 1 was synthesized by a conventional method (Chart 1). The synthesis was started by the preparation of N-(tert-butoxycarbonyl)-1,2-diaminoethane (4) by high dilution method.25) The reaction of 4 with 4-nitrophenyl chloroformate followed by treatment with (±)-3-amino-1,2-propanediol afforded diol 5 in 53% yield over three steps. The diol 5 was then esterified with dodecanoic acid using DCC as coupling agent to provide compound 6 in 82% yield. Removal of the Boc-protecting group by the standard method yielded the lipid 1 in quantitative yield.
The lipid 2 which consisted of spermine polarhead was synthesized by solid phase chemistry (Chart 2). The protected spermine having one free primary amino group was prepared using the modified literature method.26) Firstly, the active carbonate Wang resin 727) was reacted with an excess of spermine to give the resin 8. The primary amino group was selectively protected over the presence of secondary amine with 2-acetyldimedone (Dde-OH).28) This reaction is highly selective most likely due to the stabilization provided by a strong intramolecular H-bond. The remaining secondary amines were then protected with tert-butyloxycarbonyl (Boc) groups and the Dde protecting group was selectively removed under mild condition with 2% hydrazine hydrate in DMF to obtain the resin 9. The liberated amine was converted to the desired di(hydroxyethyl)amino by treating the resin 9 with excess 2-bromoethanol in the presence of DIPEA to generate the diol resin 10. The assembly of the lipid was accomplished by reacting the alcohols with myristic acid using DIC as coupling agent in the presence of DMAP as a catalyst. The final product was cleaved from the resin by treatment with 20% TFA in CH2Cl2. The filtrate was collected and concentrated in vacuo; the resulting lipid was further purified by Sephadex LH20 column chromatography. The structures of the synthesized cationic lipids were confirmed by spectroscopic means (IR, NMR and HR-MS).
Characterization of the Cationic Liposomes/DNA ComplexesThe lipoplex formation between the cationic liposome preparing from single lipid and mixture lipids was analyzed by varying the lipid/DNA weight ratio using agarose gel retardation assays (Fig. 2). The results from the agarose gel electrophoresis illustrated that cationic liposomes were able to form lipoplexes. Excess DNA was barely detectable using lipid-to-DNA ratio of 5 in all of the cationic assemblies. The results indicated that liposome in the presence of lipids 1 and 2 did not change the DNA binding property to form the lipoplexes.

Lane 1, DNA (pCMV-encoding β-galactosidase); lanes 2–7, liposomes /DNA complexes at weight ratios of 5, 10, 15, 20, 25 and 30 for a) liposomes–lipid 1, b) liposomes–lipid 2, and c) liposomes–lipids 1+2 at weight ratio of 1 : 1.
Further investigations of the particle size and zeta potentials of the liposomes and liposomes/DNA complexes were performed across the entire weight ratios of 2 to 40. As shown in Fig. 3a, the particle size of the liposome 1 (636±67 nm) was smaller than that of liposome 2 (997±120 nm). The particle sizes of these liposomes consisting of DOPE were smaller than those without DOPE. However, the mixture of lipids 1, 2 and DOPE formed larger liposome (1428±46 nm) than that of the mixture of lipids 1 and 2 alone (810±46 nm). The particle size of the formed lipoplexes with the cationic liposomes/DNA weight ratio varied from 2 to 40 was studied. It was found that most of the liposomes could bind and compact DNA into particles of cationic liposomes/DNA at the weight ratio of 5. The mean diameters of 1/DNA, 1-DOPE/DNA, 2-DOPE/DNA and 1,2/DNA lipoplexes were 500–600 nm, whereas the diameters of 2/DNA and 1,2-DOPE/DNA lipoplexes were 800–1000 nm. The zeta potential, which is a measure of the electrical field of cationic liposomes in an aqueous environment, is one of the important factors that controls their DNA binding ability. High zeta potential is preferred for higher DNA binding ability. From Fig. 3b, it was found that the liposomes of the lipids 1 and 2 have a zeta potential of 53.7±1.7 and 47.5±1.4 mV, respectively. The inclusion of DOPE as the helper lipid in the lipids 1 and 2 leads to an decrease in the zeta potential which is observed to be 39.2±0.8 and 43.3±0.8 mV, respectively. The zeta potential of the liposomes prepared by the mixture of lipids 1 and 2 was 47.1±1.6 mV. Addition of DOPE in the mixture of the lipids 1 and 2, the zeta potential (42.9±1.1 mV) was not much decreased. As shown in Fig. 3b, the zeta potential was slightly decreased along with the increase in cationic liposome/DNA weight ratio. Most of the cell membranes usually show negative charge, so it is expected that the high positively charged liposomes formulated by the mixture lipids 1 and 2 alone or with the combination of DOPE will significantly enhance the interaction between liposomes and cells and facilitate cellular uptake.

(◆) liposomes–lipid 1, (■) liposomes–lipid 1+DOPE (1 : 1), (▲) liposomes–lipid 2, (+) liposomes–lipid 2+DOPE (1 : 1), (*) liposomes–lipids 1+2 (1 : 1), and (●) liposomes–lipids 1+2+DOPE (0.5 : 0.5 : 1). Each value represents the mean±S.D. of three measurements.
Most of the cationic lipid carriers studied so far are liposomes composed of cationic lipid alone or a mixture of cationic lipid and a helper lipid, which was usually DOPE,29) DOPC30) or cholesterol.31) The alternative approach can be the use of combination of cationic lipids having the same headgroup but with tails of different chain lengths15) or cationic lipids with different polarhead and hydrophobic tails.16) To study the synergistic effect of cationic lipids with different polarheads, central core structures and hydrophobic tails, the lipids 1 and 2 were mixed at various weight ratios and tested for DNA delivery to human embryonic kidney cells (HEK293) using β-galactosidase as a reporter gene. The liposomes prepared from the lipids 1 and 2 were also evaluated for transfection efficiency. The transfection activity was reported as number of transfected cells per cm2. Figure 4 displays data generated from an assay employing plasmid DNA (0.1 µg/well) at liposomes/DNA weight ratio of 20 under serum-free condition. Liposomes comprises of the lipid 1 and 2 alone exhibited low transfection efficiency than the Lipofectamine™ 2000. As shown in Fig. 4, the weight ratio of 1 to 2 is important for higher transfection efficiency; the optimal ratio was 1 : 1. Combining the lipid 1 with 2 at weight ratio of 1 : 1 enhances about 10 and 3.5-fold the extent of transfection of HEK293 cells, compared with liposomes prepared from each of lipids 1 and 2, respectively. At this ratio, the transfection efficiency was about 2-fold higher than that of the commercially available transfection agent, Lipofectamine™ 2000. When the ratio of the lipids 1 to 2 decreases the transfection efficiency was also decreased. It has been shown that the synergistic effect of cationic liposomes on treansfection efficiency depended on the lipid composition. The minor change in cationic lipid component significantly effected on the transfection efficiency.17) Thus, our finding confirmed this observation. It has also been revealed that liposomes compose of very different lipid headgroups and/or aliphatic tails has been shown to produce asymmetric liposomes.18) This was expected to increase the biocompatibility and flexibility of liposomes as delivery system. The liposomes comprised of lipids 1 and 2 which have different polarheads, central core structures and hydrophobic tails may be constructed asymmetric liposomes, in particular at weight ratio of 1 : 1, and resulted in highly transfection efficiency. On the basis of the results (Fig. 4), the mixture of lipids 1 and 2 at weight ratio of 1 : 1 was chosen to further optimize transfection.

The transfection efficiencies (cells/cm2) of the liposomes were compared to that of the commercially available reagent, the Lipofectamine™ 2000 (L2K). Each value represents the mean±S.D. of triplicate experiments.
The helper lipid, DOPE, has been known to increase the transfection efficiency of cationic liposomes to transfer and release DNA into the cytoplasm.32) To evaluate the effect of DOPE on gene delivery of the mixture of cationic lipids 1 and 2, this mixture lipids (1/2 at weight ratio of 1 : 1) was mixed with different amounts of DOPE to form cationic liposomes. In order to find out the most effective formulation, transfection with identical liposomes/DNA weight ratio of 20 was used. Their ability to deliver a plasmid encoding β-galactosidase into HEK293 cells was studied (Fig. 5). A synergistic effect on the transfection efficiency was clearly demonstrated by the combined use of mixture of lipids 1 and 2 with the helper lipid, DOPE. The highest transfection efficiency was achieved by using the combination of mixture of lipids 1 and 2/DOPE at the weight ratio of 1 : 1. This formulation dramatically increased the transfection efficiency for 11.5- and 2.3-fold, which was higher than those of liposomes comprised of lipid 1-DOPE and lipid 2-DOPE formulations, respectively. This optimal formulation exhibited 2.5-fold higher transfection efficiency than that of Lipofectamine™ 2000. Thus, the liposomes composed of mixture lipids/DOPE at weight ratio of 1 : 1 was chosen for further study.

The liposomes were formulated at various weight ratios of mixture lipids 1 +2 and DOPE. The transfection efficiencies (cells/cm2) of the liposomes were compared to that of the commercially available reagent, the Lipofectamine™ 2000 (L2K). Each value represents the mean±S.D. of triplicate experiments.
The transfection efficiency of the cationic liposome also depends on the cationic lipisomes/DNA ratio as previously reported by our group19,33,34) and others.35–37) To find out the optimal liposomes/DNA ratio, transfection experiments were performed against HEK293 cells by using mixture lipids/DOPE ratio of 1 : 1. As shown in Fig. 6, the liposomes formulations with mixture lipids/DOPE at 1 : 1 weight ratio showed maximum transfection efficiency at liposomes/DNA weight ratio of 5 and transfection profiles followed a bell-shaped graph. At this optimal liposomes/DNA weight ratio, liposome comprised of mixture lipids having different polarheads, central core structures and hydrophobic tails showed nearly 3-fold higher transfection efficiency than Lipofectamine™ 2000.

Transfection efficiency (cells/cm2) of the mixture lipids was compared to that of the commercial reagent, Lipofectamine™ 2000 (L2K). Each value represents the mean±S.D. of triplicate experiments.
One of the major drawbacks of cationic liposomes for their in vivo use is the inhibition of the transfection efficiency in the presence of serum. Most of cationic liposomes including many commercially available transfection reagents which exhibited high transfection activity in the absence of serum lost their efficiency when transfected in the presence of serum.34,38) In order to investigate the effect of serum on gene transfection efficiencies of mixture lipids, transfection experiments with our optimized lipid formulations were therefore performed in the presence of 10, 20, 30 and 40% serum. The results are shown in Fig. 7. The transfection efficiency of mixture lipids gradually decreased when the amount of serum increase. Interestingly, the transfection efficiency of mixture lipids at high serum condition (30–40%) showed comparable transfection efficiency to Lipofectamine™ 2000. It has been reported that size of the lipoplexes may be one of the factors contributing the serum resistance.39,40) Large lipoplexes (>700 nm) showed transfection efficiency in the presence or absence of serum, but small lipoplexes (<250 nm) exhibited transfection efficiency only in the absence of serum.39) Thus, high transfection efficiency of optimized lipoplexes formulation, total lipids/DNA ratio of 5, under high serum condition may be due to the large size of our lipoplexes aggregates (1428±46 nm, Fig. 3a).

Transfection efficiency (cells/cm2) of the mixture lipids was compared to that of the commercial reagent, Lipofectamine™ 2000 (L2K). Each value represents the mean±S.D. of triplicate experiments.
It is known that transfection agents have the ability to specifically deliver DNA into different cell types.19,33,34) To evaluate the transfection efficiency of these mixture lipids toward the different cell lines, human breast adenocarcinoma (MCF-7) and cervical epithelial adenocarcinoma (HeLa) cells, the experiments were performed by using optimum condition under serum-free conditions. The transfection efficiency of mixture lipids was compared with the commercially available transfection agent, Lipofectamine™ 2000, which was calculated as 100%. The results are illustrated in Fig. 8. The optimal liposome and lipoplexes formulations of mixture lipids that work well in HEK293 cells can be applied to MCF-7 and HeLa cells. The liposome comprised of mixture lipids 1 and 2/DOPE at 1 : 1 weight ratio exhibited 2-fold higher transfection efficiency to deliver DNA into HeLa cells than that of the Lipofectamine™ 2000. However, the ability of these lipids and Lipofectamine™ 2000 to transfer DNA into MCF-7 cells was comparable.

Transfection efficiency (cells/cm2) of the mixture lipids was compared to that of the commercial reagent, Lipofectamine™ 2000 (L2K). Each value represents the mean±S.D. of triplicate experiments.
Two mostly concerned criteria for gene delivery carriers are transfection efficiency and their cytotoxicity. To assess the relationship between cytotoxicity and gene expression efficiency, the toxicity of the liposomes/DNA complexes at weight ratios of 2 to 40 was determined by measuring changes in cell metabolic activity (MTT assay) and was shown as % cell viability as compared to the control cells in the presence of DNA. The results are shown in Fig. 9. It was found that the optimal lipoplexes formulation (liposomes/DNA weight ratio of 5) of the mixture lipids showed low toxicity (cell viability more than 80%) in MCF-7 and HeLa cells. The cell viability of these lipids against HEK293 cell was slightly lower than 80%. This result was in contrast to the high transfection efficiency of these lipids. The cell viability of Lipofectamine2000™ against MCF-7 and HeLa cell was higher and slightly lower than 80%, respectively. However, the cytotoxicity of the Lipofectamine™ 2000 was relatively high for HEK293 cell line and the cell viability was approximately 60%. Thus, a slight reduction in metabolic activity of these mixture lipids should not prevent it from further in vivo study as tranfection agent.
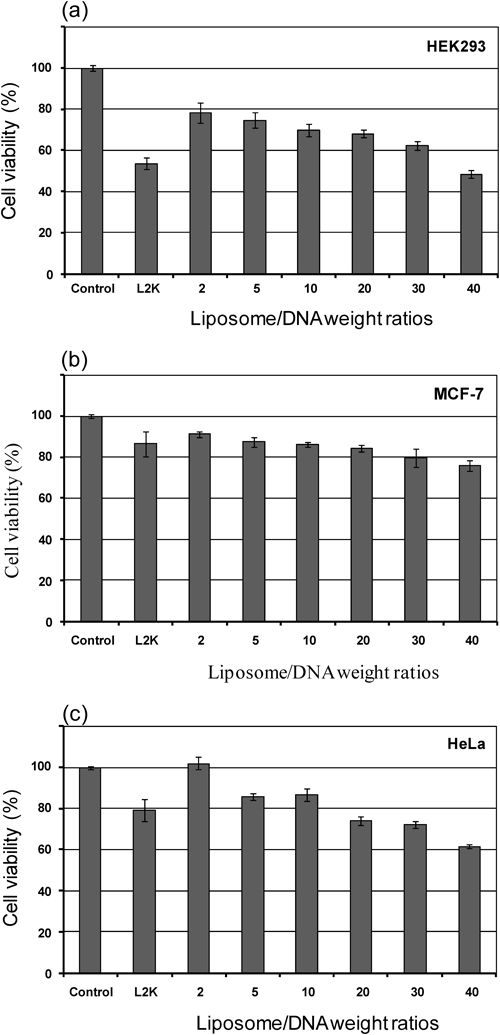
The commercially available agent, Lipofectamine™ 2000 (L2K) was also tested for comparison. Cell metabolic activity was determined by an MTT assay. Each value represents the mean±S.D. of triplicate experiments.
In conclusion we have demonstrated that the combination of lipids with different polarheads, central core structures and hydrophobic tails in the presence of helper lipid, DOPE, has allowed the discovery of highly efficient transfection agents with minimal cytotoxicity. These mixture lipids showed high efficiency to deliver DNA into HEK293, MCF-7 and HeLa cells than that of a commercially available transfection agent. We hope that the strategy illustrated here is useful for the development of cationic lipid-based gene delivery. Employing the synergistic effect of these lipids may be a promising approach for successful non-viral gene transfection.
This work was supported by Office of the Higher Education Commission (OHEC), Ministry of Education and The Thailand Research Fund (TRF) (RMU5480003). NN acknowledges the Royal Golden Jubilee Ph.D. program of TRF for studentship (Grant No. PHD/0217/2552). Supports from TRF (DBG5680006) and the Center of Excellence for Innovation in Chemistry (PERCH-CIC), OHEC, Ministry of Education are gratefully acknowledged.