Abstract
Wogonin, a natural flavonoid, is one of the bioactive compounds of the medicinal herb Eucommia ulmoides OLIV. widely used in southeastern Asia for treating hypertension. However, the molecular mechanisms for the therapeutic benefits remain largely unclear. The present study investigated the vasodilatory effect of wogonin and its possible mechanisms. The flavonoid (0.1–100 µM) caused concentration-dependent relaxations in endothelium-intact aortic rings precontracted with norepinephrine (NE, 1 µM) or potassium chloride (KCl, 60 mM). Preincubation with wogonin (10, 100 µM) for 20 min significantly inhibited the contractile responses to NE (0.1, 1, 10 µM) or KCl (7.5, 15, 30, 60 mM). Relaxant responses to wogonin were not inhibited by NG-nitro-L-arginine methylester (100 µM) or endothelial denudation. In a Ca2+-free Krebs’ solution, wogonin not only blocked Ca2+ influx-dependent vasoconstriction by either NE (1 µM) or KCl (100 mM), but also inhibited NE (1 µM)-induced tonic contraction, which is dependent on intracellular Ca2+ release. Wogonin also suppressed the elevation of [Ca2+]i induced by KCl (60 mM) after exhausting the calcium store in sarcoplasmic and endoplasmic reticula with thapsigargin (1 µM) or by ATP (100 µM) in primary vascular smooth muscle cells. These findings suggest that wogonin-induced responses are mainly due to the inhibition of both intracellular Ca2+ release and extracellular Ca2+ influx.
Eucommia ulmoides OLIV. bark, as a tonic herb, has been widely used in traditional herbal prescriptions in China, especially for treating hypertension. Experiments in vivo and in vitro have shown that different compounds contained in Eucommia ulmoides OLIV. bark exerted anti-hypertensive activities. Geniposidic acid and genipin could reduce the blood pressures of hypertensive rats. Caffeic acid could induce the nitric-oxide (NO) synthase in endotheliocyte. Besides, liriodendrin, (+)-syringaresinol and (+)-pinoresinol di-O-β-D-glucopyranoside could inhibit the cAMP activity.1) These compounds might contribute to the anti-hypertensive effect collectively. However, the anti-hypertensive effect of Eucommia ulmoides OLIV. bark remains largely unclear.
Pharmacological studies have revealed that Eucommia ulmoides OLIV. bark extract induce endothelium and NO-cyclic guanosine monophosphate (cGMP) dependent relaxation in the rat thoracic aorta.2) Another report demonstrated that the endothelium-dependent vascular relaxation induced by the bark extract is mediated by NO and endothelium-derived hyperpolarizing factor in small vessels.3) However, the vasorelaxing components have been unclear. Recently, we found oroxylin A and wogonin isolated from Eucommia ulmoides OLIV. bark could significantly lower the perfusion pressure.4) In the previous study, we had reported that oroxylin A could relax rat thoracic aorta and it was endothelium and NO dependent.5) The present study was undertaken to investigate vasodilatory effect of wogonin and its mechanism.
Wogonin (Fig. 1) is a flavone and has a variety of cardiovascular protective effect. It could regulate migration, proliferation and apoptosis of vascular smooth muscle cells.6–8) Besides, wogonin could inhibit angiogenesis, suppress collagen deposition in cardiac fibroblasts and inhibit ischemic brain injury.9–12) There is no evidence for vascular relaxation effect of wogonin. We describe here that wogonin, unlike oroxylin A, is an endothelium- and NO-independent vasodilatory flavonoid. One report demonstrated that wogonin offered a wide margin of safety.13) It has therapeutic potential for the treatment of cardiovascular and cerebrovascular diseases. However, it was reported that little wogonin was detected in rat plasma after intragastric administration of wogonin (5 mg/kg).14) So the route of administration of wogonin might be intravenous administration based on the clinical administration method.13)
MATERIALS AND METHODS
ReagentsThe following drugs were used: NaCl, potassium chloride (KCl), MgSO4, KH2PO4, NaHCO3, CaCl2 and D-glucose (The North Medical Chemical Reagent Factory, Taijin, China); thapsigargin, ATP, norepinephrine (NE), acetylcholine (ACh), NG-nitro-L-arginine methylester (L-NAME), ethylenebis(oxyethylenenitrilo)tetraacetic acid (EGTA) and dimethyl sulfoxide (DMSO) (Sigma Chemical Co., St. Louis, MO, U.S.A.); Wogonin (Chinese Institute for Drug and Biological Product Control, Beijing, China). DMSO was used as a solvent for wogonin. Distilled water was used to dissolve all other drugs. All concentrations are described as the final concentration of the medium in the organ bath.
Animals and Ethics StatementMale Wistar rats weighing 250–300 g were used for the present study. Use of animals in this study was approved by the Tianjin University of Traditional Chinese Medicine Animal Care and Use Committee. The rats were group-housed in cages under controlled conditions of light, humidity and temperature. Water was provided ad libitum and standard particle feed were provided daily. All efforts were made to minimize the suffering of the animals and maximize their welfare.
Preparation of the Isolated AortaThe rats were sacrificed by decapitation. The thoracic aorta was rapidly removed and dissected from the rat. The aorta was cleaned of connective tissue and cut into 3–4 mm ring segments. Each ring was suspended in organ bath between two parallel stainless hooks. One hook was fixed and the other was connected to a force transducer. The organ bath filled with 10 mL of Krebs’ solution (composition, mM: NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, CaCl2 1.3, D-glucose 10) that was bubbled with 95% O2 and 5% CO2 to give a pH of approximately 7.4, and the temperature was maintained at 37°C. The Krebs’ solution was replaced with pre-warmed and oxygenated Krebs’ solution and the basal tension of the rings were raised by 0.5 g every 15 min until the rings were stretched to a basal tension of 2.0 g, which is optimal for inducing the maximum contraction of the aorta rings. Before starting the experiments, all preparations of the isolated aorta were equilibrated in the bathing medium for 90 min at least.
Measurement of Mechanical ResponsesAorta contractions and relaxations were displayed on an oscillograph. All aortic rings were contracted twice with 60 mM KCl to obtain a maximal response, and the aorta rings were repeatedly washed and equilibrated. Then the following experiments were done.15–21) 1) To investigate the vasodilatory effect of wogonin, NE (1 µM) or KCl (60 mM) was used to contract the aortic rings. Once a stable contraction was achieved, wogonin (0.1–100 µM) was added cumulatively. Besides, NE (0.01, 0.1, 1 µM) or KCl (7.5, 15, 30, 60 mM) was used to contract the aortic rings in the presence and absence of wogonin (10 µM or 100 µM) for 20 min. In vehicle control experiments, DMSO was added in the same volume as that used in the experiments with wogonin. 2) To investigate the involvement of endothelium in vasorelaxation to wogonin, the endothelium was removed and L-NAME (100 µM, an NO synthase inhibitor) was used. The endothelium was removed by lightly rubbing against the teeth of a pair of forceps. Success of the removal of endothelium was confirmed by a failure of relaxation in response to acetylcholine (ACh, 10 µM) in rings precontracted with NE (1 µM). In the presence of L-NAME, the aortic rings were exposed to L-NAME for 20 min prior to the application of NE (1 µM). Once a stable contraction was achieved, wogonin (0.1–100 µM) was added cumulatively. 3) To investigate the effect of wogonin on Initial Fast and Sustained Phases induced by NE, wogonin was pretreated or not pretreated for 20 min prior to NE addition. The initial contraction was first initiated with NE (1 µM) in Ca2+-free Krebs’ solution (containing 1 mM EGTA) and the sustained contraction was then induced by further addition of CaCl2 (10 mM). These experiments were performed in endothelium-denuded aorta. 4) To investigate the effects of wogonin on extracellular Ca2+ influx, concentration–response curves to CaCl2 (1, 3, 10, 30 mM) were obtained in the presence and absence of wogonin (10 µM or 100 µM) for 20 min. Aortic rings were first allowed to equilibrate at 2.0 g tension in a Ca2+ free Krebs’ solution, and then the rings were bathed in Ca2+-free high-K+ (100 mM) Krebs’ solution, which was prepared by replacing an equimolar concentration of NaCl with KCl. After 20 min incubation with wogonin, concentration–response curves to CaCl2 were constructed. In vehicle control experiments, DMSO was added in the same volume as that used in the experiments with wogonin. These experiments were also performed in endothelium-denuded aorta.
Measurement of Ca2+ Influx and Intracellular Ca2+ ReleasePrimary vascular smooth muscle cells (VSMCs) were prepared from the thoracic aorta of 2–3-month old male Wistar rats via the tissue explants method, as described previously.22) The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) at 37°C in humidified atmosphere of 95% air and 5% CO2. More than 98% of the cells were positive for smooth muscle-specific α-actin, and exhibited the typical “hill and valley” growth pattern. Confluent cell at passages 3 to 7 were used for experiments. VSMCs were plated in 96-well black-walled clear-base plates at a density of 2×104 cells per well in DMEM with 10% FBS for 24 h. After pretreatment with various concentrations of wogonin in the presence or absence of extracellular Ca2+, the cells were incubated with Calcium 6 reagent (Molecular Devices, Sunnyvale, CA, U.S.A.) for 2 h. Then the VSMCs were checked on a FlexStation III (Molecular Devices), to monitor fluorescence (ex=485 nm, em=525 nm) before and after treatment with KCl (60 mM) or ATP (100 µM). Intracellular Ca2+ mobilization was measured as relative fluorescence units (RFU) and expressed as percentage of RFU at 0 s.
StatisticsStatistical analyses were performed using SPSS 11.5. Relaxant responses are given as percentage relaxation relative to precontraction levels to NE or KCl. Data were shown as mean±standard error of the mean (S.E.M.) from n number of experiments. Statistical significance was estimated by independent samples t-test between two groups. A p-value of less than 0.05 was regarded to be significant.
RESULTS
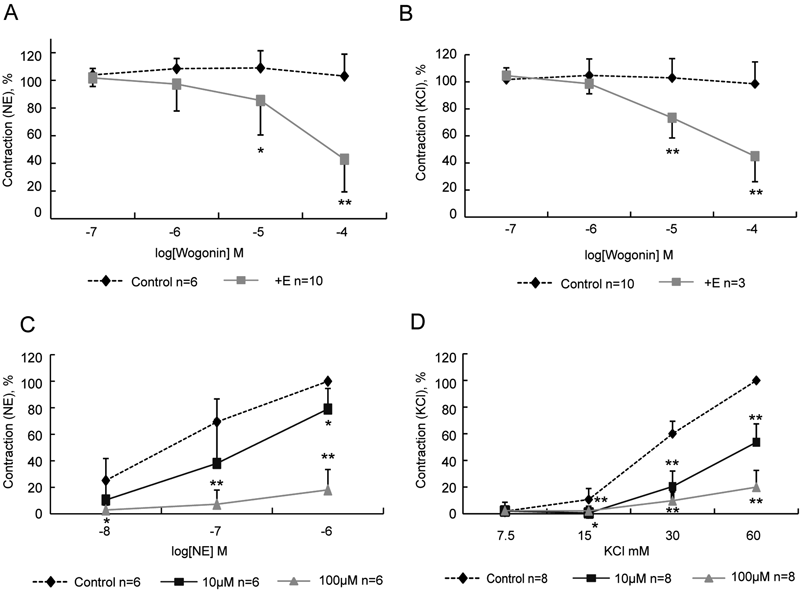
Effects of Wogonin on Rat Aortic RingsTo investigate the vasodilatory effect of wogonin, NE (1 µM) or KCl (60 mM) was used to contract the endothelium-intact aortic rings. Wogonin (0.1–100 µM) induced vasorelaxation in endothelium-intact aortic rings precontracted with NE (1 µM, n=10, Fig. 2A) or KCl (60 mM, n=3, Fig. 2B). Besides, NE (0.01, 0.1, 1 µM) or KCl (7.5, 15, 30, 60 mM) was used to contract the aortic rings in the presence and absence of wogonin (10 or 100 µM) for 20 min. NE (0.1, 1, 10 µM, n=6) or KCl (7.5, 15, 30, 60 mM, n=8) induced concentration-dependent contractions of rat aortic rings in Krebs’ solution. Pretreatment with 10 mM and 100 µM wogonin reduced the potency of contractile responses to NE (n=6, Fig. 2C) or KCl (n=8, Fig. 2D).
Effects of L-NAME and Endothelial Denudation on Vasorelaxation to WogoninBecause wogonin induced vasorelaxation in endothelium-intact aortic rings precontracted with NE (1 µM) or KCl (60 mM), we investigated the involvement of endothelium in vasorelaxation to wogonin. Wogonin (0.1–100 µM) induced vasorelaxation in endothelium-intact aortic rings precontracted with NE (1 µM, n=10). Relaxant responses to wogonin were not inhibited by L-NAME (100 µM, n=8) and endothelial denudation (n=12) (Fig. 3).
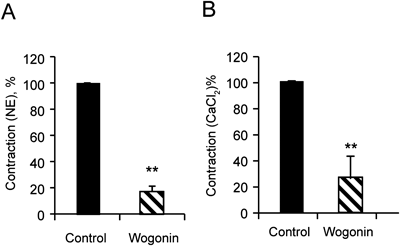
Effects of Wogonin on Initial Fast and Sustained Phases Induced by NEThe contractile response of aortic ring to NE can be separated into initial and sustained phases. Because pretreatment with 10 and 100 µM wogonin could reduce the potency of contractile responses to NE (0.1, 1, 10 µM) and the vasodilatory effect of wogonin is not dependent on endothelial cells, we investigated the effects of wogonin on Initial Fast and Sustained Phases induced by NE in endothelium-denuded aortic rings. The initial contraction was first initiated with NE (1 µM) in Ca2+-free Krebs’ solution (containing 1 mM EGTA) and the sustained contraction was induced by further addition of 10 mM CaCl2. Wogonin (100 µM) pretreated 20 min prior to NE addition markedly inhibited small tonic contractions elicited by NE (n=5, Fig. 4A) and the sustained contraction induced by further addition of 10 mM CaCl2 (n=5, Fig. 4B)..
Effects of Wogonin on CaCl2 Induced Concentration-Dependent Contractions in Ca2+-Free High-K+ Krebs’ Solution in Rat Aortic RingsThe present study demonstrated that wogonin could inhibit KCl-induced vasoconstriction. Because high K+ induced contraction by increasing extracellular Ca2+ influx via voltage-gated Ca2+ channels, we investigated whether wogonin could inhibit extracellular Ca2+ influx in Ca2+-free high-K+ Krebs’ solution. CaCl2 (1, 3, 10, 30 mM) induced concentration-dependent contractions of endothelium-denuded aortic rings in calcium-free buffer depolarized by 100 mM KCl. Pretreatment with wogonin (10, 100 µM) significantly reduced the potency of contractile responses to CaCl2 (n=7, Fig. 5).
Effects of Wogonin on Ca2+ Influx and Intracellular Ca2+ Release in Vascular Smooth Muscle CellsIn endothelium-intact aortic rings, wogonin not only blocked Ca2+ influx-dependent vasoconstriction by either NE or KCl, but also inhibited NE-induced tonic contraction, which depends on intracellular Ca2+ release. In order to further study the mechanisms, we investigated whether wogonin affect Ca2+ influx and intracellular Ca2+ release in primary VSMCs of rat. In the presence of extracellular Ca2+, VSMCs were treated with 1 µM thapsigargin to exhaust calcium store in sarcoplasmic and endoplasmic reticula. The increase in VSMCs [Ca2+]i evoked by 60 mM KCl was significantly suppressed by pretreatment of wogonin (10, 100 µM) (n=6, Fig. 6A). While in the calcium-free cell culture medium (containing 0.5 mM EGTA), wogonin exerted inhibitory effects on intracellular-calcium dependent [Ca2+]i increase induced by 100 µM ATP in a dose-dependent manner (n=6, Fig. 6B). These results indicated that both extracelluar-calcium dependent Ca2+ influx and intracellular Ca2+ release could be inhibited by wogonin treatment.
DISCUSSION
The present study investigated the vasodilatory effect of wogonin and its possible mechanisms. Wogonin (0.1–100 µM) caused concentration-dependent relaxations in endothelium-intact aortic rings precontracted with NE (1 µM) or KCl (60 mM). Preincubation with wogonin (10, 100 µM) for 20 min significantly inhibited the contractile responses to NE (0.1, 1, 10 µM) or KCl (7.5, 15, 30, 60 mM). Relaxant responses to wogonin were not inhibited by L-NAME (100 µM) and endothelial denudation. In a Ca2+-free Krebs’ solution, wogonin did not only block Ca2+ influx-dependent vasoconstriction by either NE (1 µM) or KCl (100 mM), but also inhibited NE (1 µM)-induced tonic contraction, which depends on intracellular Ca2+ release. Besides, wogonin suppressed the elevation of [Ca2+]i induced by KCl (60 mM) after exhausting calcium store in sarcoplasmic and endoplasmic reticula with thapsigargin (1 µM) or by ATP (100 µM) in VSMCs.
Vascular endothelium, which releases endothelium-dependent vasodilators, plays an important role in maintaining normal function of vascular tension. NO is a main endothelium-dependent vasodilator, formed by NO synthase using L-arginine as a substrate. NO penetrates vascular smooth muscle cells and activates soluble guanylyl cyclase which catalyzes guanosine triphosphate to transform into cGMP. cGMP-activated protein kinase G reduces sensitivity of contractile elements to Ca2+, inhibits the Ca2+ influx, and relaxes the blood vessel.23) In the present study, we found that wogonin induced vasorelaxation in endothelium-intact aortic rings precontracted with NE or KCl. Wogonin-induced vasodilation was neither affected by removal of endothelium nor by treatment with L-NAME in rat aortic rings precontracted with NE. These results suggest that the vasodilatory effect of wogonin is not mediated by the NO and endothelium pathway.
Intracellular Ca2+ controls vascular smooth muscle contraction by binding with calmodulin to form a complex of calcium and calmodulin. This complex activates myosin light chain kinase, phosphorylates myosin light chains and causes vascular smooth muscle contraction. The concentration of intracellular Ca2+ can be regulated by extracellular Ca2+ influx through both receptor-operated Ca2+ channels and voltage-dependent Ca2+ channels, as well as store-operated Ca2+ channels, or by intracellular Ca2+ release from Ca2+ stores in endoplasmic reticulum.24,25) NE can manipulate the concentration of intracellular Ca2+ by interference with either Ca2+ influx or intracellular Ca2+ release via receptor-operated Ca2+ channels. The fact that wogonin inhibited NE-induced vasoconstriction indicated that wogonin could affect receptor-operated Ca2+ channels. The contractile response of aortic ring to NE could be separated into initial and sustained phases. The initial phase was caused by release of Ca2+ from endoplasmic reticulum to the cytosol and the sustained phase was caused by influx of Ca2+ from extracellular fluid. The study then investigated the effect of wogonin on the release of Ca2+ in endoplasmic reticulum and extracellular Ca2+ influx in a Ca2+-free Krebs’ solution. It was found that wogonin (100 µM) markedly inhibited small tonic contractions elicited by NE and the sustained contraction induced by further addition of 10 mM CaCl2. These results clearly show that wogonin relaxes the isolated rat aorta by inhibiting both Ca2+ influx and Ca2+ release. Sixty millimoler KCl induced contraction by increasing extracellular Ca2+ influx via voltage-gated Ca2+ channels. The present study demonstrated that wogonin could inhibit KCl-induced vasoconstriction. In addition, it significantly inhibited CaCl2-induced vasoconstriction in a Ca2+-free high K+ Krebs’ solution. These facts indicated that wogonin could inhibit extracellular Ca2+ influx via voltage-gated Ca2+ channels. Moreover we find that wogonin could inhibit high K+ or ATP induced [Ca2+]i elevation in cultured VSMCs. It confirmed the vasodilatory effect of wogonin by inhibiting Ca2+ influx and intracellular Ca2+ release.
Flavonoids consumption directly reduced prevalence of cardiovascular diseases in human.26) Several flavonoids have been developed as vasodilator drugs, some with and others without an endothelium-dependent vasodilation effect.18,27) Our previous study reported that oroxylin A, a flavone isolated from the bark of Eucommia ulmoides OLIV. could relax rat thoracic aorta and the effect was endothelium and NO dependent.5) We describe here that wogonin, anothor flavone in Eucommia ulmoides OLIV. bark, is an endothelium- and NO-independent vasodilatory flavonoid. These results are important for validating the traditional use of Eucommia ulmoides OLIV. bark and developing novel antihypertensive agents. However, the potential signal pathway is still unclear. Our previous study found that wogonin exhibited phytoestrogen activities, which shows extensive cardiovascular bioactive.28) We need to do more work to study whether the potential signal pathway of wogonin-induced responses is associated with its phytoestrogen activities.
In conclusion, it is clearly shown that wogonin induces endothelium-independent vasorelaxation. The vasodilatory effect of wogonin may be related to its ability to interfere with both extracellular Ca2+ influx and Ca2+ in endoplasmic reticulum release.
Acknowledgments
We acknowledge the financial support from the project supported by the National Natural Science Foundation of China (81173592, 81202800), Program for New Century Excellent Talents in University of Ministry of Education of China (NCET-13-0935), Program of International S&T Cooperation Project of China (2015DFA30430), “Major drug discovery” National Science and Technology Major Project of the Ministry of Science and Technology of China (2012ZX09101212, 2012ZX09304007), the Program for Changjiang Scholars and Innovative Research Team in University, PCSIRT (IRT1276).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) He X, Wang J, Li M, Hao D, Yang Y, Zhang C, He R, Tao R. Eucommia ulmoides Oliv.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol., 151, 78–92 (2014).
- 2) Kwan CY, Chen CX, Deyama T, Nishibe S. Endothelium-dependent vasorelaxant effects of the aqueous extracts of the Eucommia ulmoides Oliv. leaf and bark: implications on their antihypertensive action. Vascul. Pharmacol., 40, 229–235 (2003).
- 3) Kwan C-Y, Zhang W-B, Deyama T, Nishibe S. Endothelium-dependent vascular relaxation induced by Eucommia ulmoides Oliv. bark extract is mediated by NO and EDHF in small vessels. Naunyn Schmiedebergs Arch. Pharmacol., 369, 206–211 (2004).
- 4) Akinyi M, Gao XM, Li YH, Wang BY, Liu EW, Chai LJ, JawoBah A, Fan GW. Vascular relaxation induced by Eucommia ulmoides Oliv. and its compounds Oroxylin A and wogonin: implications on their cytoprotection action. Int. J. Clin. Exp. Med., 7, 3164–3180 (2014).
- 5) Wang H, Qu JT, Zhao X, Guo Y, Mao HP. Vasodilator effect of oroxylin A on thoracic aorta isolated from rats. Zhong Xi Yi Jie He Xue Bao, 10, 880–885 (2012).
- 6) Huang HC, Wang HR, Hsieh LM. Antiproliferative effect of baicalein, a flavonoid from a Chinese herb, on vascular smooth muscle cell. Eur. J. Pharmacol., 251, 91–93 (1994).
- 7) Lee SO, Jeong YJ, Yu MH, Lee JW, Hwangbo MH, Kim CH, Lee IS. Wogonin suppresses TNF-alpha-induced MMP-9 expression by blocking the NF-kappaB activation via MAPK signaling pathways in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun., 351, 118–125 (2006).
- 8) Liu YM, Wang X, Nawaz A, Kong ZH, Hong Y, Wang CH, Zhang JJ. Wogonin ameliorates lipotoxicity-induced apoptosis of cultured vascular smooth muscle cells via interfering with DAG-PKC pathway. Acta Pharmacol. Sin., 32, 1475–1482 (2011).
- 9) Lin CM, Chang H, Chen YH, Li SY, Wu IH, Chiu JH. Protective role of wogonin against lipopolysaccharide-induced angiogenesis via VEGFR-2, not VEGFR-1. Int. Immunopharmacol., 6, 1690–1698 (2006).
- 10) Lin CM, Chang H, Chen YH, Wu IH, Chiu JH. Wogonin inhibits IL-6-induced angiogenesis via down-regulation of VEGF and VEGFR-1, not VEGFR-2. Planta Med., 72, 1305–1310 (2006).
- 11) Kong EK, Huang Y, Sanderson JE, Chan KB, Yu S, Yu CM. Baicalein and Wogonin inhibit collagen deposition in SHR and WKY cardiac fibroblast cultures. BMB Rep., 43, 297–303 (2010).
- 12) Chen CC, Hung TH, Wang YH, Lin CW, Wang PY, Lee CY, Chen SF. Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-kappaB signaling after experimental traumatic brain injury. PLoS ONE, 7, e30294 (2012).
- 13) Peng J, Qi Q, You Q, Hu R, Liu W, Feng F, Wang G, Guo Q. Subchronic toxicity and plasma pharmacokinetic studies on wogonin, a natural flavonoid, in Beagle dogs. J. Ethnopharmacol., 124, 257–262 (2009).
- 14) Du Y, Chen XY, Yang HY, Zhong DF. Determination of wogonin in rat plasma by liquid chromatography-tandem mass spectrometry. Yao Xue Xue Bao, 37, 362–366 (2002).
- 15) Kim B, Lee K, Chinannai KS, Ham I, Bu Y, Kim H, Choi HY. Endothelium-independent vasorelaxant effect of Ligusticum jeholense root and rhizoma on rat thoracic aorta. Molecules, 20, 10721–10733 (2015).
- 16) Chen H, Li S, Wang P, Yan S, Hu L, Pan X, Yang C, Leung GP. Endothelium-dependent and -independent relaxation of rat aorta induced by extract of Schizophyllum commune. Phytomedicine, 21, 1230–1236 (2014).
- 17) Tep-areenan P, Kendall DA, Randall MD. Mechanisms of vasorelaxation to 17beta-oestradiol in rat arteries. Eur. J. Pharmacol., 476, 139–149 (2003).
- 18) Tep-areenan P, Sawasdee P, Randall M. Possible mechanisms of vasorelaxation for 5,7-dimethoxyflavone from Kaempferia parviflora in the rat aorta. Phytother. Res., 24, 1520–1525 (2010).
- 19) Fu XC, Wang MW, Li SP, Zhang Y, Wang HL. Vasodilatation produced by orientin and its mechanism study. Biol. Pharm. Bull., 28, 37–41 (2005).
- 20) Xue YL, Shi HX, Murad F, Bian K. Vasodilatory effects of cinnamaldehyde and its mechanism of action in the rat aorta. Vasc. Health Risk Manag., 7, 273–280 (2011).
- 21) Mendes LJ, Capettini LS, Lobo LT, da Silva GA, Arruda MS, Lemos VS, Côrtes SF. Endothelial nitric oxide-dependent vasorelaxant effect of isotirumalin, a dihydroflavonol from Derris urucu, on the rat aorta. Biol. Pharm. Bull., 34, 1499–1500 (2011).
- 22) Wang H, Gao X, Zhang B. Tanshinone: an inhibitor of proliferation of vascular smooth muscle cells. J. Ethnopharmacol., 99, 93–98 (2005).
- 23) Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch., 459, 915–922 (2010).
- 24) Lai N, Lu W, Wang J. Ca(2+) and ion channels in hypoxia-mediated pulmonary hypertension. Int. J. Clin. Exp. Pathol., 8, 1081–1092 (2015).
- 25) Leblanc N, Forrest AS, Ayon RJ, Wiwchar M, Angermann JE, Pritchard HA, Singer CA, Valencik ML, Britton F, Greenwood IA. Molecular and functional significance of Ca(2+)-activated Cl(−) channels in pulmonary arterial smooth muscle. Pulm Circ., 5, 244–268 (2015).
- 26) Lu MF, Xiao ZT, Zhang HY. Where do health benefits of flavonoids come from? Insights from flavonoid targets and their evolutionary history. Biochem. Biophys. Res. Commun., 434, 701–704 (2013).
- 27) Wang HP, Lu JF, Zhang GL, Li XY, Peng HY, Lu Y, Zhao L, Ye ZG, Bruce IC, Xia Q, Qian LB. Endothelium-dependent and -independent vasorelaxant actions and mechanisms induced by total flavonoids of Elsholtzia splendens in rat aortas. Environ. Toxicol. Pharmacol., 38, 453–459 (2014).
- 28) Wang H, Li MC, Yang J, Yang D, Su YF, Fan GW, Zhu Y, Gao XM, Paoletti R. Estrogenic properties of six compounds derived from Eucommia ulmoides Oliv. and their differing biological activity through estrogen receptors α and β. Food Chem., 129, 408–416 (2011).