2015 年 38 巻 5 号 p. 663-668
2015 年 38 巻 5 号 p. 663-668
Small guanosine triphosphatases (GTPases) participate in a wide variety of cellular functions including proliferation, differentiation, adhesion, and intracellular transport. Conventionally, only the guanosine 5′-triphosphate (GTP)-bound small GTPase interacts with effector proteins, and the resulting downstream signals control specific cellular functions. Therefore, the GTP-bound form is regarded as active, and the focus has been on searching for proteins that bind the GTP form to look for their effectors. The Rab family small GTPase Rab27a is highly expressed in some secretory cells and is involved in the control of membrane traffic. The present study reviews recent progress in our understanding of the roles of Rab27a and its effectors in pancreatic beta-cells. In the basal state, GTP-bound Rab27a controls insulin secretion at pre-exocytic stages via its GTP-dependent effectors. We previously identified novel guanosine 5′-diphosphate (GDP)-bound Rab27-interacting proteins. Interestingly, GDP-bound Rab27a controls endocytosis of the secretory membrane via its interaction with these proteins. We also demonstrated that the insulin secretagogue glucose converts Rab27a from its GTP- to GDP-bound forms. Thus, GTP- and GDP-bound Rab27a regulate pre-exocytic and endocytic stages in membrane traffic, respectively. Since the physiological importance of GDP-bound GTPases has been largely overlooked, we consider that the investigation of GDP-dependent effectors for other GTPases is necessary for further understanding of cellular function.
Small guanosine triphosphatases (GTPases), comprising Ras, Rho, Rab, Ran, and Arf subfamilies, have guanosine 5′-triphosphate (GTP)- and guanosine 5′-diphosphate (GDP)-bound forms (Fig. 1). The conventional wisdom is that only the GTP-bound small GTPase interacts with effector proteins, and the resulting downstream signals control specific cellular functions.1,2) Therefore, the GTP-bound form is regarded as active, and proteins that bind the GTP form have been searched for to find their effectors. The activity of small GTPases is regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs).3,4) Small GTPases exist predominantly as GDP-bound forms under unstimulated conditions. Cell stimulation activates GEFs and the resulting conversion of the GDP- to the GTP-bound form transduces signals via their binding to effectors. GAPs terminate the signaling by promoting the intrinsic GTPase activity of small GTPases. Small GTPases participate in a wide variety of cellular functions including proliferation, differentiation, adhesion, and intracellular transport. Rab27a is a member of the Rab family that is highly expressed in pancreatic beta-cells and is involved in the control of membrane traffic.5–7) Ashen mice with a natural occurring mutation in Rab27a show glucose intolerance with decreased insulin secretion.8) Therefore, Rab27a has been proposed to regulate insulin secretion. Here, we review recent progress in our understanding of the roles of Rab27a and its effectors in the secretory process.

The activity of small GTPases is regulated by GEFs and GAPs. Small GTPases exist predominantly in their GDP-bound forms under unstimulated conditions. Cell stimulation activates GEFs, and the resulting conversion of the GDP- to the GTP-bound form transduces signals via their binding to effectors. GAPs terminate the signals by promoting the intrinsic GTPase activity of small GTPases.
Glucose, the most important secretagogue under physiological conditions, promotes insulin secretion via a regulatory pathway in pancreatic beta-cells (Fig. 2). Insulin synthesized in the beta-cells is eventually released by exocytosis via a series of stages. Synthesized insulin is packed into secretory vesicles (called insulin granules) and is transported near the plasma membrane. Exophilin8/Slac2-c/MyRIP is a GTP-dependent Rab27a effector and is involved in the regulation of this stage. In melanocytes, Exophilin8/Slac2-c/MyRIP acts as a linker between GTP-bound Rab27a and the motor protein Myosin Va, and transports melanosomes along F-actin.9) The same function is expected in pancreatic beta-cells, yet this model is still a matter of debate.10–15) Indeed, some reports suggest that there are several conditions under which Exophilin8/Slac2-c/MyRIP does not interact with Myosin Va.15) It is possible that Exophilin8/Slac2-c/MyRIP may regulate translocation of insulin granules via a distinct mechanism.11) Granuphilin/Slp4a is another GTP-dependent Rab27a effector and forms a complex with both Myosin Va and GTP-bound Rab27a.10) Therefore, there may be some subsets of insulin granules in pancreatic beta-cells.
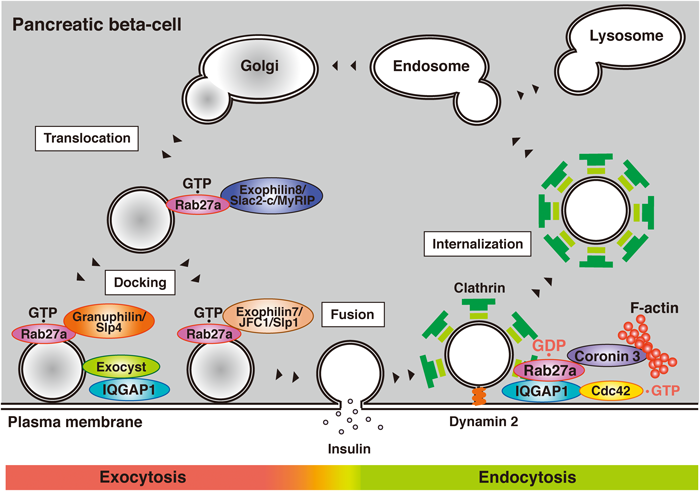
Insulin granules are transported near the plasma membrane and attach to its inner surface. GTP-bound Rab27a regulates this stage via its interaction with GTP-dependent effectors including Exophilin8/Slac2-c/MyRIP, Granuphilin/Slp4a, and Exophilin7/JFC1/Slp1. Glucose stimulation promotes the fusion of secretory membrane to the plasma membrane and insulin is released. Glucose stimulation also converts Rab27a from its GTP to its GDP-bound form. At the same time, glucose stimulation converts GDP-bound Cdc42 to its GTP-bound form. The activation of Cdc42 recruits both GDP-bound Rab27a and its effector coronin 3 to IQGAP1 at the cell periphery and regulates endocytosis at this membrane site.
Transported granules in the cytoplasm attach to the inner surface of the cell membrane (called docking) (Fig. 2). Granuphilin/Slp4a is involved in the regulation of this stage.16–18) Granuphilin/Slp4a interacts with the closed form of Syntaxin1a, a cell membrane associated soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein.17) In Granuphilin/Slp4a knock-out mice, the number of insulin granules docked to the plasma membrane is decreased.16) Moreover, insulin secretion from the mice is increased by glucose stimulation. These results indicate that Granuphilin/Slp4a inhibits the fusion of insulin granules via their tethering to the cytoplasmic surface of the plasma membrane. Exophilin7/JFC1/Slp1 is a GTP-dependent Rab27a effector that regulates another docking state of insulin secretion.19) Unlike Granuphilin/Slp4a, Exophilin7/JFC1/Slp1 does not bind Syntaxin1a. Moreover, the fusion event from undocked granules is impaired in Exophilin7/JFC1/Slp1 knock-out mice. These results indicate that insulin granules have multiple docking states and that these two effectors regulate different types of docking.
We searched for Rab27a-interacting proteins by affinity column chromatography and identified several,6,7) one of which is coronin 3.20) Coronin 3 is a ubiquitously expressed coronin that shares a central WD40 domain with other coronins.21,22) Surprisingly, coronin 3 selectively forms a complex with GDP-bound Rab27a but not with GTP-bound Rab27a.20) This is consistent with the structure of coronin 3 in that it does not contain a synaptotagmin-like homology domain (SHD), a potential GTP-bound Rab27a binding site.23) Coronin 3 also lacks a GDP dissociation inhibitor (GDI) consensus sequence. GDIs are regulators of small GTPases.24–26) In unstimulated cells, GDIs form a complex with GDP-bound small GTPases and induce their intracellular distribution from the plasma membrane to the cytosol. This suggests that coronin 3 and GDIs function in a different way. Coronins regulate membrane internalization in some types of cells.22) In the insulin-secreting beta-cell line MIN6, the silencing of coronin 3 inhibits the internalization of both endocytic and secretory membrane markers.20) The uptake is also inhibited by the expression of the dominant negative coronin 3, which inhibits the GDP-bound Rab27a-dependent function of native coronin 3 by forming a complex with GDP-bound Rab27a. This inhibition is rescued by co-expression of GDP-bound Rab27a, suggesting that the interaction between coronin 3 and GDP-bound Rab27a is necessary for endocytosis of secretory membrane in pancreatic beta-cells (Fig. 2). Moreover, we have shown that glucose stimulation converts Rab27a from the GTP- to the GDP-bound form, and that the peripheral GDP-bound Rab27a recruits coronin 3 from the cytosol to the plasma membrane20) (Fig. 3). These results indicate that glucose promotes both exocytosis and endocytosis in pancreatic beta-cells.
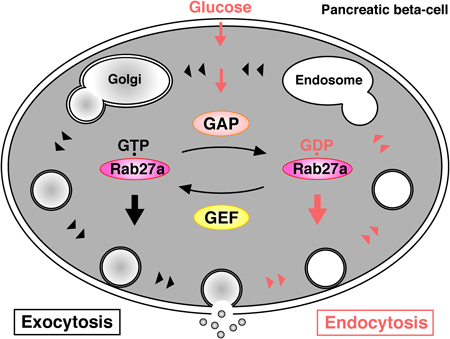
In the basal state, GTP-bound Rab27a controls insulin secretion at pre-exocytic stages via its GTP-dependent effectors. Glucose stimulation causes insulin exocytosis. Glucose stimulation also converts Rab27a from its GTP- to its GDP-bound form through the activation of Rab27a-GAPs. GDP-bound Rab27a controls endocytosis of the secretory membrane via its GDP-dependent effectors.
Phogrin, a transmembrane protein, closely co-localizes with insulin granules.27) Following insulin exocytosis, phogrin is internalized into small vesicles that recycle to an insulin-containing compartment for subsequent rounds of exocytosis.28) Phogrin is concentrated near the plasma membrane in cells expressing the dominant negative coronin 3, because endocytosis is inhibited.20) Interestingly, the level of phogrin exposed to the outer surface of the plasma membrane is decreased in these cells.29) As the total expression level of phogrin in the cells is not changed, these findings suggest that endocytosed phogrin is located underneath, but not inserted into, the plasma membrane. In general, endocytosis comprises multiple stages that include the assembly of endocytic machinery followed by invagination, scission, and targeting to the organelle (Fig. 2). The above-mentioned results suggest that coronin 3 forms a complex with GDP-bound Rab27a and regulates the retrograde transport of internalized secretory membrane, the stage after scission from the plasma membrane (Fig. 2).
Oligomerization of coronins and their interaction with F-actin modulate actin assembly.21,30–32) Coronin 3 also interacts with the actin-related proteins 2 and 3 (Arp2/3) complex through the C-terminal region and regulates the activity of Arp2/3 on actin nucleation.31) Both direct and indirect modulations of F-actin play a crucial role in the cellular function of coronin 3. In pancreatic beta-cells, the F-actin binding activity of coronin 3 is promoted by its interaction with GDP-bound Rab27a.33) Moreover, the direct actin modulation of coronin 3 is necessary for the endocytosis of secretory membrane. In contrast, GDP-bound Rab27a does not affect the interaction between coronin 3 and Arp2/3. Thus, coronin 3 is not a regulator of Rab27a such as GDIs or GEFs, but is rather a GDP-dependent effector, which functionally acts as an actin modulator thereby regulating the endocytosis of secretory membrane (Fig. 2).
3.2. IQ Motif Containing GTPase Activating Protein 1 (IQGAP1)IQGAP1, a GTP-dependent effector of Cdc42, regulates membrane cytoskeleton events through its interaction with F-actin, beta-catenin, E-cadherin, and CLIP170.34–40) We recently identified IQGAP1 as another GDP-dependent Rab27a effector.41) GDP-bound Rab27a interacts with IQGAP1 via its RasGAP related domain (GRD). This domain is a part of the GTP-bound Cdc42-binding region of IQGAP1.38–40) Interestingly, GDP-bound Rab27a forms a complex with IQGAP1 only when IQGAP1 interacts with GTP-bound Cdc42 (Fig. 2). Therefore, these proteins form a trimeric complex in pancreatic beta-cells. Since glucose shifts Cdc42 from the GDP to GTP-bound form,42) the formation of the complex is promoted by glucose stimulation. This glucose-induced complex formation recruits coronin 3 from the cytosol to the plasma membrane via its interaction with GDP-bound Rab27a.29) Moreover, the recruitment is essential for endocytosis of the secretory membrane. These results suggest that IQGAP1 determines the endocytic site via the recruitment of endocytic machinery, including GDP-bound Rab27a and coronin 3 (Fig. 2).
IQGAP1 also plays an important role in exocytosis in pancreatic beta-cells (Fig. 2). IQGAP1 recruits vesicle-tethering exocysts and regulates the docking state of insulin granules.43) Since docking is not a prerequisite but is temporally inhibitory for glucose-induced membrane fusion,44) this complex may inhibit subsequent fusion events like Granuphilin/Slp4a. Interestingly, GTP-bound Cdc42 binds IQGAP1 and inhibits the interaction between IQGAP1 and exocysts.43) This may allow insulin release from pancreatic beta-cells by the promotion of fusion events between the insulin secretory membrane and the plasma membrane. We therefore consider that IQGAP1 plays a crucial role in the control of vesicle tethering and endocytosis by the recruitment of relevant regulators at each stage of the process (Fig. 2).
Membrane recycling is essential for the generation of specialized membranous organelles and for communication between these organelles. We consider that Rab27a-mediated membrane recycling is involved in the maintenance of the internal conditions that beta-cells need to support life (Fig. 4).
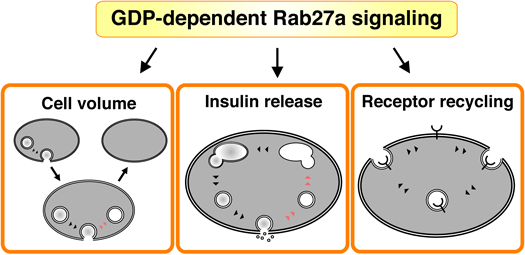
GDP-dependent Rab27a signaling regulates membrane recycling in pancreatic beta-cells. Membrane recycling is essential for the generation of specialized membranous organelles and for communication between these organelles.
Insulin is packed into secretory vesicles that are transported near the plasma membrane. Although glucose stimulation promotes the fusion of secretory membrane to the plasma membrane, beta-cell volume is kept constant before and after insulin secretion (Fig. 4). This raises the possibility that the fused membrane is taken up from the plasma membrane by endocytosis. Since Rab27a regulates both exocytosis and endocytosis,6,7) this small GTPase can work as a sensor protein by which beta-cells recognize their cellular volume.
4.2. Insulin ReleaseThe secretory membranes up-taken by endocytosis are transported to the Golgi apparatus through endosomes (Fig. 4). The membranes are known to be reused as secretory vesicles in which the newly synthesized insulin is packed.28) In other words, endocytic machinery, including GDP-bound Rab27a and its effectors, indirectly regulates insulin release. Indeed, insulin secretion is inhibited in beta-cells expressing the dominant negative coronin 3 (unpublished data). We consider that the endocytic machinery is a potential molecular therapeutic target for type 2 diabetes.
4.3. Receptor RecyclingThe activities of some receptors are regulated by their intracellular distribution. In particular, the appropriate number of receptors in the plasma membrane is modulated by endocytosis in various cells (Fig. 4). In pancreatic beta-cells, glucose recruits ATP sensitive potassium (KATP) channels via non-insulin-containing dense core granules.45) This receptor recycling is required for efficient glucose responsiveness of beta-cells and thereby glucose homeostasis. GDP-bound Rab27a may be involved in the recycling of some receptors via its GDP-dependent effectors.
Together with the pre-exocytic roles of Rab27a, glucose-dependent Rab27a conversion from the GTP- to GDP-bound form shifts the stage from exocytosis to endocytosis in pancreatic beta-cells (Fig. 3). Since the physiological importance of GDP-bound GTPases has been largely overlooked, we consider that the investigation of GDP-dependent effectors of other GTPases is necessary for further understanding of cellular function.
In general, the conversion to GDP-bound GTPases is regulated by their specific GAPs (Fig. 1). Rab27a has two GAPs, EPI64A and EPI64B.46) EPI64A presents and functions as the main Rab27a-GAP in melanocytes. In pancreatic acinar cells, EPI64B is involved in amylase release by modulating the Rab27a cycle.47) We recently searched for Rab27a-GAP-interacting proteins and identified some signaling molecules. We expect that the analysis of these molecules will reveal glucose-sensing mechanisms in pancreatic beta-cells.
We thank all members of our laboratory for helpful suggestions. This work was supported by the grant KAKENHI, Takeda Science Foundation, Suzuken Memorial Foundation, Novo Nordisk Pharma, Oita Broadcasting System Cultural Foundation and Research Fund at the Discretion of the President, Oita University.
The authors declare no conflict of interest.