2015 年 38 巻 7 号 p. 1012-1019
2015 年 38 巻 7 号 p. 1012-1019
Sphingosine-1-phosphate type-1 receptor (S1P1) agonists have the potential to inhibit the egress of lymphocytes, and have been demonstrated to provide protective effects on some acute inflammatory diseases. However, the value of S1P1 agonists on acute pancreatitis (AP) remains unclear. The aim of this study was to explore the effect of SEW2871, a S1P1-selective agonist, on caerulein-induced AP in mice. AP was induced by giving eight intraperitoneal injections of caerulein (50 µg/kg/h) at hourly intervals. SEW2871 was administered by gavage, at a dose of 20 mg/kg, at 0 h and 12 h after the first intraperitoneal injection of caerulein. The mice were sacrificed at 24 h. Severity of AP, serum amylase and lipase activity, levels of serum cytokines, pancreatic myeloperoxidase (MPO) activity, CD45+CD4+ T lymphocytes in blood, CD4+ T cell infiltration in the pancreas, and proinflammatory cytokine production were assessed. Furthermore, the expression of signal transducer and activator of transcription (STAT) 3 and phospho-STAT3 (p-STAT3) in the pancreas was also evaluated. The results revealed that the administration of SEW2871 ameliorated the severity of AP, by a reduction of serum pancreatic enzyme activity and levels of cytokines, decreased pancreatic MPO activity, depletion of CD4+CD45+ T lymphocytes in the blood and a reduction of CD4+ T cell infiltration in the pancreas. Furthermore, the expression of proinflammatory cytokines mRNA and p-STAT3 were also suppressed by SEW2871 treatment. These results suggest that SEW2871 treatment attenuates the severity of caerulein-induced AP in mice, which may provide a new therapeutic approach for AP therapy.
Acute pancreatitis (AP), a common digestive disease, is believed to have intracellular activation of digestive enzymes and pancreatic acinar cell injury as its initial pathophysiologic course, followed by a pancreatic inflammation, characterized by local infiltration and activation of immune cells, including neutrophils, lymphocytes, monocytes/macrophages, etc., which produce loads of proinflammatory mediators.1–3) Excessive inflammatory cytokines results in systemic inflammatory response syndrome (SIRS) and/or organ failure,4,5) as evidenced by the fact that levels of circulating inflammatory cytokines are correlated with the severity of AP.6) Immune cells are thus a potential drug target for alleviating the course of AP.
Sphingosine-1-phosphate (S1P), a physiological signaling molecule, can influence regulation of cellular proliferation, migration, differentiation, endothelial integrity, heart rate and the control of immune cell trafficking through five G-protein-coupled receptors.7) The type-1 S1P receptors (S1P1) are mainly expressed in immune cells, determine their trafficking and mediate immune response.8,9) Therefore, it is not surprising that S1P affects the immune system and has been implicated in inflammatory diseases, such as multiple sclerosis, renal ischemia-reperfusion injury, and influenza virus infection.10–14) Recently, it has been reported that FTY720, a non-selective S1P receptor agonist, can attenuate pancreatic inflammation and fibrosis in the chronic pancreatitis in rats,15) ameliorate the severity of taurocholate-induced severe acute pancreatitis (SAP),16) and decrease pulmonary inflammation and injury in a rat model of acute lung injury caused by acute necrotizing pancreatitis.17) However, non-selective S1P receptor agonists showed an obvious disadvantage of leading to sinus bradycardia as a complication of the activation of type-3 S1P receptors (S1P3), which may result in poor usability in the early phase of AP.18) Therefore, the selective S1P1 agonists seem to be promising therapeutic drugs for the treatment of AP.
SEW2871, a selective S1P1 agonist, was originally identified by high-throughput screening of commercial chemical libraries with a Fluorescence Imaging Plate Reader (FLIPR) calcium flux assay in the year of 2004.11,18) Recent research has revealed that SEW2871 can ameliorate renal ischemia–reperfusion injury by inhibiting lymphocytes egress and reducing the early CD4+ T cells infiltration in kidney.13) Evidence shows that the T lymphocytes, particularly CD4+ T cells, play a pivotal role in the development of tissue injury during AP.19) Hence, we performed the present study to evaluate the therapeutic effects of SEW2871 on caerulein-induced acute pancreatitis in mice for the first time.
Male ICR mice weighing 25–30 g (Experimental Animal Center of Yangzhou University, Jiangsu, China) were used in this study. All the animal experimental protocols were approved by the Animal Care and Use Committee of Nanjing University and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. The mice were housed on a 12 h light/dark cycle at a constant temperature of 22°C, with free access to water and standard rodent chow, and allowed to acclimatize for a week. The mice were fasted overnight on the day of the experiment but allowed access to water ad libitum.
AP Induction and SEW2871 AdministrationAP was induced by giving eight intraperitoneal injections of caerulein (Sigma, St. Louis, MO, U.S.A.), dissolved in normal saline, at a dose of 50 µg/kg/h at hourly intervals.20) SEW2871 (Cayman Chemical, Ann Arbor, MI, U.S.A.) was dissolved in 100% dimethyl sulfoxide (DMSO), and diluted with 50% Tween 20 for administration, and the final concentration of DMSO was 20%. As it has low solubility in aqueous solution and can keep a consistent lymphocytopenia up to 12 h at a dosage of 20 mg/kg as described previously,18) SEW2871 was administered by gavage at 0 and 12 h after the first intraperitoneal injections of caerulein (20 mg/kg, each). The mice (n=30) were randomly allocated into three groups (each group containing 10 mice): (1) the SEW2871 treated group: AP mice received SEW2871 by gavage twice (20 mg/kg, each); (2) the AP group: AP mice received equal volume of vehicle of SEW2871 (50% Tween 20 containing DMSO) by gavage; (3) the control group: common mice, without AP induction, received equal volume of vehicle of SEW2871 (50% Tween 20 containing DMSO) by gavage. At 24 h after the first caerulein injection, all the mice were sacrificed by excessive anaesthesia with pentobarbital sodium and heart exsanguination, and their blood samples and pancreatic tissues were harvested for experiments.
Histological ExaminationThe tail of the pancreas was fixed by 5% paraformaldehyde, and inflated under a pressure of 25 cm H2O with 0.5% of low melting agarose for histologic evaluation by hematoxylin and eosin (HE) staining. All the assessment was performed by an experienced pathologist blinded to the experiment design. The specimens were scored according to the criteria described previously.21)
Serum Pancreatic Enzymes Activity AssaySerum amylase activity and lipase activity were determined by commercially available kits (Biocalvin Company, Suzhou, China) according to the manufacturer’s instructions.
Serum Cytokines AssaySerum interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α) levels were analyzed by enzyme-linked immunosorbent assays (ELISA) with commercially available kits (Biocalvin Company) according to the manufacturer’s protocol, and 450 nm with a correction wavelength set at 570 nm on a spectrophotometer (Beckman Du 530 Life Science UV/Vis spectrophotometer; Beckman Coulter) was used.
Pancreatic Myeloperoxidase (MPO) Activity DeterminationMyeloperoxidase (MPO) activity, a marker for neutrophils infiltration,22) was measured in pancreatic tissues using an ELISA kit (Biocalvin Company) according to the manufacturer’s instructions, and data were expressed as units per gram wet weight of pancreas.
Flow Cytometric AnalysisBlood samples were processed on the day of collection for flow cytometry analysis. Erythrocytes were lysed with lysis buffer (Sigma) for 10 min at room temperature. Cell suspensions were washed twice in Roswell Park Memorial Institute (RPMI)-1640 (Sigma), and isolated cells were thoroughly suspended in each tube containing 500 µL RPMI-1640. For immunofluorescent staining, cells were counted and approximately one million cells transferred to each flow test tube. These cells were stained with phycoerythrin-conjugated anti-CD45 (30-F11; BD Biosciences), fluorescein isothiocyanate-conjugated anti-CD4 (RM4-5; BD Biosciences), or an appropriate negative control. The stained cells were then incubated at room temperature for 30 min in the dark. After that these cells were washed twice with 2 mL RPMI-1640 at room temperature and resuspended in 500 µL RPMI-1640. Finally, these cells were detected by flow cytometry (BD FACS Calibur), and data was analyzed by FlowJo software (version 7.6.1; Tree Star, Ashland, OR, U.S.A.).
Immunohistochemistry AnalysisConsecutive 4 µm thick serial sections were cut and routinely deparaffinized. Endogenous peroxidase was blocked with 3% H2O2/methanol. Nonspecific antibody binding was blocked by incubating the sections in blocking buffer (10% normal goat serum in phosphate-buffered saline (PBS)) for 30 min. Primary antibodies against CD4+ T cells (1 : 100 dilution; R&D Systems, U.S.A.) were applied overnight at 4°C. After being washed three times in PBS for 5 min each, the sections were incubated with horseradish peroxidase-conjugated immunoglobulin G (IgG) (1 : 500; Santa Cruz Biotechnology) for 60 min. 3,3-Diaminobenzidine (DAB)/H2O2 solution was used to visualize CD4+ T cells. CD4+ T cells were then identified, counted and analyzed on 6 consecutive high-power fields (HPFs) at a magnification of 400× by two investigators blinded to the grouping. Results are expressed as number of positive cells per HPFs.
Quantitative Real-Time Polymerase Chain Reaction (PCR) AnalysisTotal RNA was extracted from pancreatic tissues with RNAiso Plus (TaKaRa Bio, Dalian, China) according to the manufacturer’s instructions. The concentration of total RNA were detected with a spectrophotometer (OD260/280 1.8–2.0). In order to avoid RNA degradation, 900 ng RNA was immediately reverse transcribed to cDNA with the PrimeScript RT reagent kit (TaKaRa Bio), and the rest was kept at −80°C. The primers were designed according to PubMed GenBank and synthesized by Invitrogen Life Technologies (Shanghai, China). The primer sequences used to amplify mRNAs were shown in Table 1. The quantitative real-time PCR analysis for the cytokines expression was performed as described previously, applying real-time SYBR Green PCR technology. All samples were analyzed in triplicate. β-Actin was used as an endogenous reference “housekeeping” gene, and relative expression was calculated using the 2−ΔΔCt method.
| Primer | Sequence (5′ to 3′) | |
|---|---|---|
| TNF-α | Forward | AAAATTCGAGTGACAAGCCTGTAG |
| Reverse | CCCTTGAAGAGAACCTGGGAGTAG | |
| IL-6 | Forward | GCTGGTGACAACCACGGCCT |
| Reverse | AGCCTCCGACTTGTGAAGTGGT | |
| IL-17a | Forward | GCTCCAGAAGGCCCTCAGA |
| Reverse | AGCTTTCCCTCCGCATTGA | |
| IL-22 | Forward | TCTTGGTACAGGGAGGAGC |
| Reverse | CCTATCAGATTGAGGGAAC | |
| β-Actin | Forward | AGTGTGACGTTGACATCCGTA |
| Reverse | GCCAGAGCAGTAATCTCCTTCT |
Pancreatic tissue samples were homogenised in radioimmunoprecipitation assay buffer containing a cocktail of protease and phosphatase inhibitors. Protein concentrations were detected by the Bradford method. Equal amounts of protein (50 µg) per lane were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked for 2 h in blocking buffer (Tris-buffered saline/0.1% Tween 20 (TBST) containing 5% skim milk) and then incubated overnight at 4°C with primary antibodies against signal transducer and activator of transcription (STAT) 3 (1 : 2000 dilution; Cell Signaling Technology, U.S.A.), tyrosine phosphorylated STAT3 (p-STAT3) (1 : 2000 dilution; Cell Signaling Technology) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1 : 1000 dilution; Bioworld Technology, U.S.A.) in blocking buffer. After being washed with TBST (3×10 min), the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated IgG (1 : 30000 dilution; Bioworld Technology) for 70 min at room temperature. Detection was performed by incubating the membranes with ECL Plus (AMRESCO) and exposed to X-ray films. Band intensities were quantified after background subtraction and used to calculate the changes in the relative amounts of the corresponding proteins. Quantification of the blots was achieved densitometrically using the UN-Scan-It 6.1 software (Silk Scientific Inc., Orem, UT, U.S.A.).
Statistical AnalysisSPSS 17.0 (SPSS Inc., Chicago, IL, U.S.A.) was used for the statistical analysis. Data were reported as means ±standard errors. One-way ANOVA was used for multiple comparisons, followed by Tukey’s post-hoc test, and the results were considered statistically significant if p-values were <0.05.
Firstly, the HE-stained pancreatic specimens from the three groups were observed and the severity of AP was scored. Compared to the mice in the control group (Fig. 1A), AP mice that received vehicle treatment showed more infiltrations of immune cells in the pancreas, severer pancreatic edema and acinar cell injury (Fig. 1B), and the mean histological scores were also significantly higher (p<0.001) (Fig. 1D). SEW2871 treatment obviously reduced pancreatic acinar cell injury and inflammatory cells infiltration (Fig. 1C), and lowered mean histological scores when compared with mice in the AP group (p<0.001) (Fig. 1D).

Representative HE-stained sections from three groups (400× magnification) are shown. A. The control group. B. The vehicle-treated AP group, which showed pancreatic acinar cells injury, edema formation and inflammatory cells infiltration in pancreas. Arrow indicates no obvious damage of acinar cells. C. The SEW2871-treated group, which showed markedly decreased infiltration of inflammatory cells and less acinar cells injury. Arrow indicates being broken of acinar cells. D. The histological scores of three groups. Values represent mean±S.E.M. (n=10). Mean values were significantly different from those of the vehicle-treated AP group: * p<0.001, # p<0.001.
Eight intraperitoneal injections of caerulein caused a marked increase in serum amylase activity and lipase activity in the AP group (p<0.001) when compared with the mice in the control group, and the application of SEW2871 reduced the elevation (Figs. 2A, B). And the induction of AP also resulted in a significant increase in serum IL-6 (p=0.001) and TNF-α (p=0.001), which indicated a systemic inflammatory course, and the application of SEW2871 also inhibited their elevation (Figs. 3A, B). Furthermore, the MPO activity in pancreas significantly increased after the induction of AP (p=0.003), and it was significantly decreased after the administration of SEW2871 compared with AP mice (p=0.031) (Fig. 4).

A. The serum amylase activity. B. The serum lipase activity. Values represent mean±S.E.M. (n=10). Mean values were significantly different from those of the vehicle-treated AP group: * p<0.001, # p<0.001.

A. Serum interleukin (IL)-6 levels. B. Serum tumor necrosis factor-alpha (TNF-α) levels. The induction of acute pancreatitis (AP) resulted in a significant increase in serum IL-6 (* p=0.001, compared with the control group). The application of SEW2871 markedly decreased the IL-6 levels (# p=0.005, compared with the vehicle-treated AP group). Serum TNF-α levels were significantly higher in AP mice compared with the control group (* p=0.001). The application of SEW2871 markedly attenuated this increase (# p=0.002, compared with the vehicle-treated AP group). Values represent mean±S.E.M. (n=10).

The measured MPO activity was significantly higher in acute pancreatitis (AP) mice compared with the control group (* p=0.003). The application of SEW2871 attenuated this increase (# p=0.031, compared with the vehicle-treated AP group). Values represent mean±S.E.M. (n=10).
Flow cytometric analysis was performed to investigate the effect of SEW2871 on the CD45+CD4+ T lymphocytes in peripheral blood. After the induction of AP, a significantly increased percentage of CD45+CD4+ T cells was found in the blood of vehicle-treated AP mice when compared with mice in the control group (p<0.001) (Fig. 5). And in the blood of SEW2871 treated mice, the percentage of CD45+CD4+ T cells was significantly lower than the vehicle-treated AP mice (p<0.001) (Fig. 5).
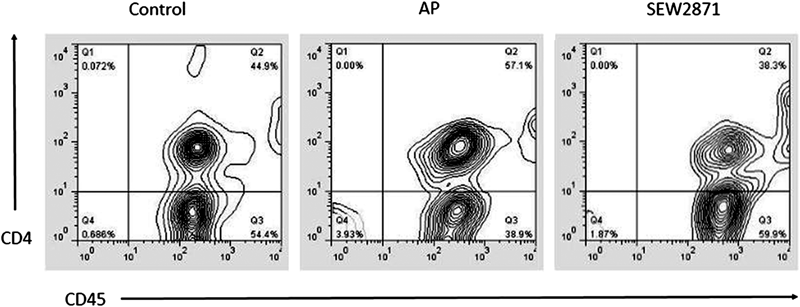
The numbers in the upper right quadrant represent the total percentage of CD45+CD4+ T cells in blood lymphocytes. Representative results are shown.
Immunohistochemistry analysis was performed to investigate the infiltration of CD4+ T cells in pancreas. As shown in Fig. 6A, few cells presented a positive morphology in the control group. The infiltration of CD4+ T cells in pancreas was significantly increased following the induction of AP (p<0.001) (Figs. 6B, D). And in pancreas of SEW2871 treated mice, the infiltration of CD4+ T cells was significantly lower than the vehicle-treated AP mice (p<0.001) (Figs. 6C, D).

Representative photomicrographs from three groups (400× magnification) are shown. A. The control group, which presented few positive cells in pancreas. B. The vehicle-treated AP group, which showed markedly increased positive cells. C. The SEW2871-treated group, which showed less CD4+ T cells infiltration into the pancreas compared with the vehicle-treated AP group. D. The number of CD4+ T cells per high-power field (HPF). Values represent mean±S.E.M. (n=10). Mean values were significantly different from those of the vehicle-treated AP group: * p<0.001, # p<0.001.
In order to explore whether ameliorated disease in SEW2871-treated mice was associated with changes in the production of cytokines, gene expression for inflammatory cytokines was quantified in pancreas by quantitative real-time PCR analysis. As presented in Fig. 7, the mRNA expression of IL-6, TNF-α, IL-17a and IL-22 significantly increased after the induction of AP (p<0.001), while the administration of SEW2871 lowered the elevation markedly (p<0.001).

Values represent mean±S.E.M. (n=10). Mean values were significantly different from those of the vehicle-treated AP group: * p<0.001, # p<0.001.
To further elucidate the mechanism of the protective effect of SEW2871, Western blotting of STAT3 and p-STAT3 expressions was performed in all the three groups. As shown in Fig. 8, the expression of p-STAT3 was significantly increased after the induction of AP, and could be significantly reduced by the SEW2871 treatment (p=0.039). While the levels of STAT3 were not affected.

A. STAT3 and p-STAT3 expressions detected by Western blot analysis. B. Relative expression of p-STAT3. The results were quantified using the UN-Scan-It 6.1 software (Silk Scientific Inc., Orem, UT, U.S.A.). Data represent mean±S.E.M. (n=10). Mean values were significantly different from those of the vehicle-treated AP group:* p<0.01, # p<0.01.
Tremendous progress has been achieved in the treatment of AP in the past several decades, but there are still two peak mortalities in this dynamic disease process, and the first one occurs in the early phase of AP (often within the first week of onset), due to SIRS and/or organ failure,23) which is generally considered as a result of excessive inflammatory disorder associated with a dysregulated immune response.24–26) Therefore, much of the ongoing research makes efforts to reduce organ damage and protect against SIRS by immunoregulation.16,27–29) The first published report on immunosuppression for AP was as early as 1980, in which cyclophosphamide was used for AP treatment in humans.30) Since then, various immunosuppressive agents have been studied in the treatment of AP, including glucocorticoids, cyclosporin, 5-fluorouracil, tacrolimus, rapamycin, FTY720, etc. However, most of these experiments have shown contradictory results, especially in the clinical trials. This may be partially explained by the complexity of the pathophysiology of AP and the multiple function of these immunosuppressive agents.
Recently, the S1P axis, as a pivotal role in the control of immune cell trafficking, have attracted much attention, especially in the field of cancer and inflammatory diseases, including AP.7) Previous studies have indicated that SEW2871 had protective effects on renal ischemia-reperfusion injury.12,13) Here, we first investigated the effects of SEW2871 on AP. AP was induced by giving eight intraperitoneal injections of caerulein at a dose of 50 µg/kg/h at hourly intervals, which can lead to an AP model with about 35% pancreatic acinar cell necrosis reported by previous research.20) The severity of AP is evaluated by histological score of the pancreas, serum pancreatic enzymes activity, levels of serum IL-6 and TNF-α, and pancreatic MPO activity. Results showed that SEW2871 administration at 0 h and 12 h after the first intraperitoneal injections of caerulein reduced serum pancreatic enzymes activity and levels of serum IL-6 and TNF-α, lowered elevation of MPO activity in the pancreatic tissues, and histologic examination of the pancreas also revealed much less tissue damage. Thus, we can conclude that the administration of SEW2871 can attenuate the severity of caerulein-induced AP in mice. After caerulein-induced AP, the pancreatic acinar cell injury occurred, and SEW2871 treatment can only alleviate the severity of AP, and can’t reverse it entirely, so all these parameters observed were not inhibited to the control level by SEW2871 treatment.
SEW2871 treatment resulted in a remarkable improvement of all aspects of the severity of AP that we examined. A previous study showed that CD4+ T cells played a pivotal role in the development of tissue injury during AP.19) Thus, we firstly performed experiments to observe the change of CD45+CD4+ T cells in the peripheral blood and CD4+ T cells infiltration in pancreas. And then we measured the transcriptional levels of proinflammatory cytokines associated with CD4+ T cells in pancreatic tissues, including IL-6, TNF-α, IL-17a and IL-22. All of these results were in line with the findings of histological examination. With consideration of the effect of SEW2871 on inhibiting lymphocyte recirculation,18) the reduction of CD45+CD4+ T cells in blood and CD4+ T cells infiltration in pancreas might be due to the decreased homing of circulating lymphocytes, and the suppression of the proinflammatory cytokine production in pancreas thereby could be a result of the reduction of the activated T lymphocytes in pancreas.
As SEW2871 has the potential to inhibit the egress of lymphocytes, so it can cause immunosuppression. For the safety of SEW2871, previous research showed that SEW2871 at the dosage of 20 mg/kg can maintain the maximal suppression of circulating lymphocytes for 12 h, and the immunosuppression effect of SEW2871 could be controlled by adjusting its dosage.18) Therefore, we think that low-dose and short-range SEW2871 treatment is safe, and may provide a new therapeutic approach for AP therapy.
The STAT3 signaling pathway is well-known to be activated by the family of cytokine receptors and mediates a wide variety of biological effects including immune responses, cell differentiation and proliferation,31) and aberrant activation of the STAT3 signaling pathway has been reported in inflammatory diseases.32,33) Besides, as caerulein is a cholecystokinin (CCK) analogue, it binds to the CCK receptor to activate signaling in pancreatic acinar cells. The CCK2 receptor is a Gq protein coupled receptor that mediates the activation of STAT3 for cell proliferation.34) Therefore, our results showed that the expression of p-STAT3 was significantly increased after the induction of AP.
Previous study showed that activation of STAT3 signaling pathway resulted in edematous and inflammatory changes of pancreas in rats with experimental pancreatitis.35) In pancreatic acinar cells stimulated with TNF-α, the activation of STAT3 also mediated the development of pancreatic injury.36) And the inhibition of STAT3 signaling pathway reduced the severity of AP and improved the average survival time of rats with SAP,37) which suggested that the STAT3 signaling pathway could be a potential target for the treatment of AP. In this study, our results indicated that an important beneficial effect of SEW2871 was associated with a reduction of p-STAT3 expression in pancreas.
Besides, previous research showed that S1P1 could also positively regulate the proliferative/survival signaling pathways by inhibiting adenylate cyclase and/or activating phospholipase C in many cells,38,39) which might be other mechanisms underlying S1P1 activation for induction of protective effects and needed for further research.
In conclusion, our study demonstrated that the administration of SEW2871 alleviated the severity of caerulein-induced AP in mice. This was partly explained by depleting the peripheral CD4+ T cells, decreasing the release of serum cytokines, and thus reducing tissue damage. Furthermore, the proinflammatory cytokines mRNA and p-STAT3 expressions were also suppressed by SEW2871 treatment.
This study was supported by the National Natural Science Foundation of China (Grants 81170438 and 81200334) and the Jiangsu Provincial Special Program of Medical Science (BL2012006).
The authors declare no conflict of interest.