2015 年 38 巻 8 号 p. 1240-1244
2015 年 38 巻 8 号 p. 1240-1244
Adenovirus (Ad) vectors are widely used in gene therapy and in vitro/in vivo gene transfer. However, Ad-mediated gene transfer in epithelial cells shows low efficiency, because Ad fiber cannot bind to the primary receptor, the coxsackievirus and adenovirus receptor (CAR), present in tight junctions. Caco-2 monolayer cells cultured on Transwell-chamber plates for approximately 2 weeks are widely used for drug membrane permeation studies, but Ad-mediated gene transfer is difficult in Caco-2 monolayer cells. First, we examined the efficiency of gene transfer into Caco-2 monolayer cells. Luciferase production in cultured Caco-2 cells transduced with Ad vectors was 20-fold lower on day 12 than on day 1. In contrast, the expression of CAR protein in Caco-2 cells gradually increased along with the duration of culture. For efficient gene transfer into Caco-2 monolayer cells, the binding ability of Ad vectors with CAR was found to be important. Capric acid (C10), a medium-chain fatty acid is a tight-junction modulator used as a pharmaceutical agent. We found that a novel gene transfer method using transduction with Ad vectors in the presence of C10 led more efficiently to LacZ expression in Caco-2 monolayer cells than Ad vectors alone. The results of the present study indicate that C10 could be very useful for Ad-mediated gene transfer in human colonic Caco-2 epithelial cells.
Genetic engineering techniques, such as short interfering RNA (siRNA) technology and the improvements of gene-transfer technology, are powerful tools for elucidating the molecular mechanisms of drug permeation and drug metabolism. Caco-2 cells, which are derived from human colonic epithelial cells, are widely used for membrane permeation studies of drugs in epithelial cells. Caco-2 cells are also used in membrane barrier studies because they form cell monolayer sheets by forming tight junctions. However, Caco-2 cells shows low gene-transfer efficiency, and the formation of cell monolayer sheets further decreases gene-transfer efficiency.1)
Recombinant adenovirus (Ad) vectors are potentially useful for gene transfer to a wide variety of cells and tissues both in vitro and in vivo. The initial step of Ad infection involves at least two sequential steps. The first is the attachment of the virus to the cell surface by binding of the knob domain of the fiber to coxsackievirus and adenovirus receptor (CAR).2) Following attachment, interaction between the RGD motif of penton bases with the secondary host-cell receptors, αvβ3- and αvβ5-integrin, facilitates the internalization of the vector via receptor-mediated endocytosis.3,4) One disadvantage is that Ad vectors result in inefficient gene transfer to cells lacking the primary Ad receptor, CAR.5–7) In polarized epithelial cells, CAR is closely associated with tight junctions, where it contributes to the barrier to the paracellular flow of solutes and macromolecules.8) A two-cell gap in each tight junction has a width of <1 nm, a gap throuth which virus particles cannot pass. CAR in tight junctions limits virus infection across epithelial surfaces.
In the present study, we propose an enhanced adenovirus gene transfer method in Caco-2 cells using C10, a tight junction modulator. The results suggest that C10 could be of very useful for Ad-mediated gene transfer experiments using Caco-2 monolayer cells.
Caco-2, a human colonic cell line, was maintained in Dulbecco’s modified Eagle’s medium (DMEM) medium containing 10% fetal bovine serum in an atmosphere of 5% CO2. Cells from passages 45 through 55 were used for experiments.
Measurement of Transepithelial Electrical ResistanceConfluent monolayers of Caco-2 cells were grown in Transwell-chambers. The formation of tight junction (TJ) barriers in Caco-2 monolayers was monitored by the measurement of transepithelial electrical resistance (TER) using a Millicell-ERS epithelial volt-ohmmeter (Millipore Co.). After 7–14 d of culture, the TER values reached a plateau. The TJ-developing cells were then used for Caco-2 monolayer cells. Caco-2 monolayer cells were treated with C10 on the apical side of the chamber and TER values were measured. The TER values were normalized according to the area of the Caco-2 monolayer cells. The background TER value of a blank Transwell chamber was subtracted from the TER value of the cell monolayers.
Ad Vector PreparationWe used wild-type Ad vector, Ad-L2 and Ad-LacZ, and the fiber-modified Ad vectors, AdΔF-L2, AdΔP-L2 and AdΔS-L2, are ablating of CAR, integrin and heparin sulfate proteoglycan (HSPG) binding, respectively (Table 1). Vectors were prepared as described previously and purified by CsCl2 step gradient ultracentrifugation followed by CsCl2 linear gradient ultracentrifugation.9–11) Particle (VP) titer of vectors were calculated according to the report by Maizel et al.12)
| Ad vector | CAR (Fiber knob) | Integrin (Penton base) | Heparan sulfate (Fiber shaft) |
|---|---|---|---|
| Conventional Ad | |||
| Ad | Intact | Intact | Intact |
| Mutant Ad | |||
| AdΔF | Mutation | Intact | Intact |
| AdΔP | Intact | Mutation | Intact |
| AdΔS | Intact | Intact | Mutation |
·Fiber knob mutation sequence: -TEG- -NAV- (intact sequence: -TEGTAYTNAV-)·Penton base mutation sequence: -MND-TS-RAE- (intact sequence: -MND-HAIRGDTFAT-RAE-)·Ad type 5 fiber shaft mutation sequence: -GAGA- (intact sequence: -KKTK-)
Caco-2 cells were seeded in a 24-wells plate or Transwell chambers. After 1, 3, 6 and 12 d, they were transduced with Ad vectors (3000 VP/cell) for 1.5 h. After a 48 h culture period, luciferase production in the cells was measured using a luciferase assay system (PicaGene LT2.0, TOYO INK, Japan). Undifferentiated Caco-2 cells and differentiated Caco-2 cells were cultured in Transwell chambers for 1 and 12 d, respectively. We repeated all transfection experiments and obtained similar results.
Western BlottingProtein samples were prepared by the incubation of Caco-2 cell pellets in the presence of 20-mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) (pH 7.5), 2-mM ethylene glycol bis (2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 10% glycerol, 1%-Triton X-100, 5-mM dithiothreitol, and 2-mM phenylmethylsulfonyl fluoride on ice for 30 min. Total protein (10 µg) in sample buffer with 4% β-mercaptoethanol was separated in a sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis (SDS-PAGE) gel, followed by electrotransfer to a nitrocellulose membrane. After blocking in 1% skim milk, the membranes were incubated with an anti-human CAR mouse antibody (1 : 1000) (clone RmcB; Cell Signaling, U.S.A.), followed by incubation in the presence of peroxidase-labeled anti-mouse immunoglobulin G (IgG) goat antibody (1 : 10000) (Cell Signaling). The membranes were developed using chemiluminescence (ECL Western blotting detection system), and signals were read using an LAS-1000 (FUJIFILM Co., Japan).
LacZ AssayCaco-2 cells were seeded in Transwell chambers. After a 7-d incubation period, the cells were transduced with Ad-LacZ (1000 VP/cell) in the presence of C10 (0.5 mg/mL) at the apical side of the Transwell-chamber. Twenty four hours later, Ad-LacZ was washed out. After an additional 24 h later the cells were washed with phosphate buffered saline (PBS), fixed with 0.5% glutaraldehyde, and stained with X-gal solution (1.3-mM MgCl2, 15-mM NaCl, 44-mM HEPES, 3-mM potassium ferricyanide, 3-mM potassium ferrocyanide, and 0.05% X-gal solution dissolved in dimethylformamide). LacZ production in the cells was measured using a reporter assay kit (TOYOBO Co., Japan).
Cytotoxicity AssayCaco-2 cells were seeded in Transwell chambers. After a 12-d incubation period, the cells were transduced with Ad-LacZ in the presence of C10. Twenty four hours later, the culture medium was collected at the apical and basal sides of the Transwell-chambers. The release of lactate dehydrogenase (LDH) from cells was analyzed using a cytoTox96 NonRadioactive Cytotoxicity Assay kit (Promega) according to the manufacturer’s protocol.
We evaluated gene-transfer activity in Caco-2 cells transduced with Ad-L2 on Transwell-chamber plates. Caco-2 cells in Transwell-chamber formed a stable tight junction barrier function at approximately 1 week, after which TER values were stable (data not shown). Ad-L2 mediated high levels of luciferase production in Caco-2 cells, but gene transfer efficiency gradually decreased at the days of culture increased (Fig. 1A). Luciferase production in Caco-2 cells transduced with Ad-L2 was 20-fold lower on day 12 than day 1. In contrast, the expression of CAR protein in Caco-2 cells gradually increased with days of culture (Fig. 1B). We found no correlation between CAR expression and Ad-mediated gene expression in Caco-2 monolayer cells. Next, to determine whether there was an increase in Ad-mediated gene expression and CAR binding, we studied the use of fiber-mutant Ad vectors. AdΔF-L2, AdΔP-L2 and AdΔS-L2 are ablating of CAR, integrin and HSP binding, respectively (Table 1). AdΔF-L2 result in approximately 1000-fold lower Caco-2 monolayer cell transduction than Ad-L2 (Fig. 2). On the other hand, AdΔP-L2 and AdΔS-L2 mediated similar levels of luciferase production in Ad-L2 (Fig. 2). The gene expression levels of the secondary Ad receptors, αvβ3 and αvβ5 integrins, in Caco-2 cells by reverse transcription polymerase chain reaction (RT-PCR) were similar on days 1 and 12 (data not shown). These data suggested that for improving the efficiency of gene expression in populations in Caco-2 monolayer cells, the binding of Ad particles and CAR to cell surfaces should be improved. Kesisoglou et al. have reported that immunofluorescence studies demonstrated increased CAR immunoreactivity in the cells at the migrating edge of Caco-2 monolayers as compared to areas of confluence. Furthermore, gene expression in populations in the migrating edge of Caco-2 monolayers is transduced more efficiently with Ad vectors than that areas of confluence.13) The expression of the cellular surface Ad receptor CAR is an important determinant for efficient Ad-mediated gene delivery to Caco-2 monolayer cells.
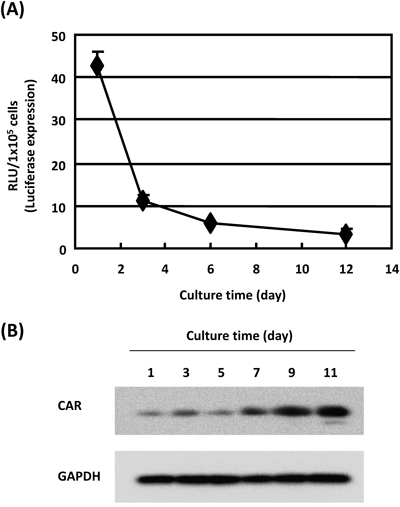
(A) Caco-2 cells were seeded in Transwell-chambers. After 1, 3, 6, and 12 d they were transduced with Ad vectors (3000 VP /cell) for 1.5 h. Luciferase production in the cells was measured using a luciferase assay system. (B) Caco-2 cells were seeded in Transwell-chambers. After 1, 3, 5, 7, 9, and 11 d, cells were harvested. We measured the expression level of CAR in Caco-2 cells at each time point by Western blotting analysis.

Caco-2 cells were seeded in Transwell-chambers. Undifferentiated Caco-2 cells and differentiated Caco-2 cells were cultured in Transwell-chambers for 1 and 12 d, respectively. After 1 or 12 d they were transduced with Ad vectors (3000 VP/cell) for 1.5 h. Luciferase production in the cells was measured using a luciferase assay system.
Next, to determine whether the opening of the tight junction induces an increase in Ad-mediated gene transduction in Caco-2 monolayer cells, these cells were transduced with Ad-L2 in the presence of C10, a tight junction modulator. Lindmark and coworkers have reported that C10 is open tight junctions by inducing redistribution of tight junction proteins, ZO-1 and occluding, increasing the mannitol permeability of the Caco-2 monolayer cells.14) Our date was also decreased the TER value by C10. After the C10 was washed out, TER recovered to its value before C10 treatment (Fig. 3). The amount of Ad-mediated LacZ expression was significantly increased in Caco-2 monolayer cells when the tight junction was opened by C10 that (Fig. 4A). Gene transfer by Ad-LacZ with C10 result in approximately 10-fold higher Caco-2 monolayer cell transduction than Ad-LacZ alone. More importantly, gene transfer by Ad-LacZ with C10 can widely express transgene in the Caco-2 monolayer cell (Fig. 4A). This finding is very important considering that a wide variety of cells could be transduced, making it very useful for drug permeation in drug metabolism studies.

Caco-2 cells were seeded into Transwell-chamber. When the TER was stable, Ad vector or C10 were added to the apical side at the indicated concentrations. TER values were monitored 24 h after the addition of Ad vector or C10. After an additional 24 h the cells were washed with PBS and TER values were monitored with an electrode. Data are representative of three independent experiments.

Caco-2 monolayer cells were cultured for 7 d. Next, the cells were infected with Ad-LacZ (1000 VP/cell) in the presence of 0.5 mg/mL C10 for 24 h. Twenty four hours later, the cells were washed with PBS. After an additional 24 h of culture, LacZ activity and X-gal staining was performed as described in Materials and Methods. (B) After 7 d of incubation, the cells were transduced with Ad-LacZ in the presence of C10. Twenty four hours later, the culture medium was collected at the apical and basal sides of the Transwell-chambers. The release of LDH from cells was evaluated using a cytoTox96 NonRadioactive-Cytotoxicity Assay kit. Data are presented as the results of three independent experiments.
Considering that little cytotoxicity was detected even following 24 h of application, gene transfer is possible without breaking the Caco-2 monolayer sheet (Fig. 4B). Bergelson and colleagues have also reported that ethylenediaminetetraacetic acid (EDTA) treatment on confluent Caco-2 cells increased infection by Ad vectors, because EDTA increased cell surface expression of CAR by opening tight junctions.8) On the other hand, Carolyn and co-workers have also reported that gene transfer by Ad vectors with C10 higher human airway epithelial cell transduction than Ad vectors with chelating reagent.15) These result suggested that this efficient Ad-mediated gene transfer method using C10 is also applicable to drug permeation studies using Caco-2 monolayer cells.
In the present study we developed an efficient Ad-mediated gene transfer method using the tight junction modulator, C10, result in significantly higher gene-transfer activity in Caco-2 monolayer cells. Furthermore, the tight junction barrier in the Caco-2 cell monolayer was restored by washing out C10 from the culture medium. We evaluated the effect of Ad vector and C10 on cytotoxicity in Caco-2 cells, finding that LDH activity was not increased by the addition of Ad vector and C10. This is the first report to describe the enhancement of gene transfer in Caco-2 monolayer cells by combination with adenovirus vector and C10. These results suggested that this novel method is useful for efficient transgene expression in Caco-2 monolayer cells.
This work was partly supported by a Grant-in-Aid for Scientific Research C (21590178) and Grant-in-Aid for Young Scientists B (19790135) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors declare no conflict of interest.