2015 年 38 巻 8 号 p. 1220-1226
2015 年 38 巻 8 号 p. 1220-1226
Cytidine monophosphate (CMP) N-acetylneuraminic acid (Neu5Ac) hydroxylase (CMAH) is an essential enzyme for N-glycolylneuraminic acid (Neu5Gc) synthesis. In humans, Neu5Gc cannot be synthesized because of a deletion in the CMAH gene. Since Neu5Gc research has not been actively performed in comparison with Neu5Ac research, little is known about the function of Neu5Gc. Possible reasons are that CMAH for controlling Neu5Gc synthesis is not understood well at the molecular level, that commercial Neu5Gc is expensive, and that addition of exogenous Neu5Gc to glycoconjugates is not a general method because of the difficulty in obtaining CMAH. One solution to these problems is to achieve large-scale production of CMAH with enzymatic activity. To produce and purify CMAH as simply as possible, we generated simian CMAH as a secretory protein with a histidine tag using a baculovirus protein expression system. After culture of baculovirus-infected cells in serum-free medium, secretory simian CMAH (approximately 180 µg) was highly purified from the supernatant (150 mL) of cell culture. HPLC analysis showed conversion of CMP-Neu5Ac to CMP-Neu5Gc by the secretory CMAH. We succeeded in producing secretory CMAH with enzymatic activity that is easy to purify. In addition, peptide-N-glycosidase F treatment of CMAH indicated that secretory CMAH was a glycoprotein with N-glycan. It will also contribute to research on Neu5Gc function by easy-to-use methods for controlling Neu5Gc synthesis, for exogenous addition of Neu5Gc to glycoconjugates and by application to industrial Neu5Gc synthesis.
N-Glycolylneuraminic acid (Neu5Gc) is synthesized by cytidine monophosphate (CMP) N-acetylneuraminic acid (Neu5Ac) hydroxylase (CMAH; EC 1.14.18.2), Neu5Gc is expressed in most mammals but not in humans. In the cytosol of a cell, CMAH converts CMP-Neu5Ac to CMP-Neu5Gc by hydroxylation of the N-acetyl group in the presence of cofactors including Fe2+ and ferro (Fe2+)-cytochrome b5, which is produced from ferri (Fe3+)-cytochrome b5 by cytochrome b5 reductase with coenzyme reduced nicotinamide adenine dinucleotide (NADH)1) (Fig. 1). Human CMAH lacks enzymatic activity, because of elimination of a putative iron binding site2) or a frame shift mutation3) resulting from a 92-base-pair deletion in exon 6 of the human CMAH genome. However, Neu5Gc is incorporated into human tissues and the human body from exogenous resources such as red meat and biotherapeutic glycoprotein.4,5) Some human cancers such as melanoma and colon cancer express Neu5Gc, possibly because these cancers actively incorporate Neu5Gc.6,7)

Underlined atoms in Neu5Gc are subjected to hydroxylation.
Neu5Gc glycan functions as a negative regulator for activation of B cells.8) In the human body, anti-Neu5Gc antibodies are produced by Neu5Gc intake and xenotransplantation. Anti-Neu5Gc antibodies enhance the potential barrier to xenoplantation from species such as pigs, whose organs are rich in Neu5Gc,4) carcinoma progression through antibody-mediated inflammation for a Neu5Gc-containing tumor,9) and clearance of Neu5Gc-containing biotherapeutic glycoproteins such as a monoclonal antibody produced from animal cell lines.5) Subtilase cytotoxin, a bacterial toxin secreted by Shiga toxigenic Escherichia coli, binds to Neu5Gc.10) Some strains of influenza A virus also bind to Neu5Gc.11–14) It is thought that Neu5Gc plays a role in the immune system and functions as a microbial (toxin) receptor. However, the biological role of Neu5Gc has not been investigated in detail, compared to the many studies on Neu5Ac. Some possible reasons are that CMAH for controlling Neu5Gc synthesis is not molecular level, that commercial Neu5Gc is expensive, and that addition of exogenous Neu5Gc to glycoconjugates is not a general method because of the difficulty in obtaining CMAH. One solution to these problems is to achieve large-scale production of CMAH with enzymatic activity.
A soluble secretory protein is generally convenient for purification of the protein and its use in various experiments. In this study, we succeeded in producing and purifying secretory CMAH by using a baculovirus-protein expression system. The secretory CMAH retained enzymatic activity for converting CMP-Neu5Ac to CMP-Neu5Gc. This secretory CMAH will contribute to progress in CMAH mutation studies. Furthermore, since a large amount of CMP-Neu5Gc can be synthesized by secretory CMAH, exogenous addition of Neu5Gc to terminal galactose in glyco-chains on cell and tissue surfaces will be possible by using sialyltransferases, thereby helping to advance Neu5Gc research.
mRNA of African green monkey kidney COS7 cells was extracted with TRIzol reagent (Invitrogen Corp., Carlsbad, CA, U.S.A.) and was converted to cDNA by using a TaKaRa RNA PCR kit (AMV) ver. 3.0 (TaKaRa Bio Inc., Shiga, Japan). The simian CMAH gene was amplified by the nested polymerase chain reaction (PCR) method with first PCR primers 5′-GGC TTA TGG GAG CAA ATG AGC CAC AG-3′ and 5′-AAT TCT TCT TGA AAT TTT CAT GTG GTT TTG CAT TCC-3′ and second PCR primers 5′-GGA ATT CCC ATG GGC AGC ACT GAA CAA ACA ACT G-3′ and 5′-CGG AAT TCC GTC ATG TGG TTT TGC ATT CCT GCG-3′ containing an EcoRI site using pfuUltra high-fidelity DNA polymerase (Agilent Technologies Inc., Palo Alto, CA, U.S.A.). The PCR product of the CMAH gene was digested with EcoRI enzyme and then inserted into a multicloning site between the two EcoRI sites of the pGEM-T easy vector (Promega, Madison, WI, U.S.A.). The CMAH gene was amplified by PCR using primers 5′-CGG CGG GAA TTC GAT GGG CAG CAC TGA AC-3′ and 5′-CGG CGG CTC GAG TCA TGT GGT TTT GCA TTC CTG-3′, which possessed EcoRI and XhoI restriction enzyme sites, respectively, at the 5′ end. The PCR fragment was inserted between the EcoRI site and XhoI site of the pBACgus-3 vector (Merck Millipore, Darmstadt, Germany) in order to add an N-terminal secretion signal peptide, histidine tag and linker sequence to the CMAH gene. The CMAH gene containing an N-terminal secretion signal peptide, histidine tag and linker sequence was amplified by PCR using primers 5′-CGG CGG GGT CTC GAA TTA TGC CCA TGT TAA GCG CTA TTG-3′ and 5′-TAT TGG TCT CCT CGA GTT AGT GGT GGT GGT GGT GG-3′, which possessed EcoRI and XhoI cleavage forms after BsaI digestion, respectively, at the 5′ end. The PCR fragment was inserted between the EcoRI site and XhoI site of the pFastBac 1 shuttle vector (Invitrogen Corp.).
A recombinant baculovirus containing the CMAH gene (Bac-CMAH) was generated by using the Bac-to-Bac system (Invitrogen Corp.) according to the instruction manual. Briefly, Bacmid containing the CMAH gene was obtained from transformation of pFastBac 1 containing the CMAH gene to E. coli DH10Bac strain (Invitrogen Corp.). Lepidopteran insect Spodoptera frugiperda Sf9 cells (5×105 cells/mL) were seeded to a 6-well plate in 2 mL of Sf-900 III serum-free medium (SFM) (Invitrogen Corp.) (2 mL/well) containing 5% fetal bovine serum (FBS) at 28°C for 30 min. After washing the cells with SFM, Bacmid containing the CMAH gene was transfected into Sf9 cells using Cellfectin Reagent (Invitrogen Corp.). After culture at 28°C for 5 h, the culture supernatant was replaced with fresh 5% FBS SFM. P1 virus stock (2 mL) was obtained as a centrifuged supernatant (6000×g, 4°C, 5 min) cultured at 28°C for 72 h. Furthermore, a suspension culture of Sf9 cells (2×106 cells/mL, 25 mL) was inoculated with P1 virus stock (0.5 mL) and was cultured at 28°C for 96 h with shaking at 100 rpm. P2 virus stock was obtained as a 0.45 µm-filtrated supernatant after centrifugation (6000×g, 4°C, 10 min).
Titration of Recombinant BaculovirusSf9 cells (5×105 cells/mL) were seeded to a 6-well plate in 5% FBS SFM (2 mL/well) at 28°C for 30 min. The cells were inoculated with 10-fold dilutions of the P2 virus stock (1 mL/well) at 28°C for 60 min and then gently overlaid with 2 mL/well of 2% BacPlaque Agarose (Novagen, Madison, WI, U.S.A.) mixed with 2× Grace’s Insect Cell Culture Medium (Invitrogen Corp.). After 20 min at room temperature, the cells were overlaid with 2 mL/well of 5% FBS SFM and cultured at 28°C for 5–6 d. Plaques were stained with 1 mL/well of 0.025% Neutral red-phosphate buffered saline (PBS; pH 7.2, 131 mM NaCl, 14 mM Na2HPO4, 1.5 mM KH2PO4, and 2.7 mM KCl) at 28°C for 2 h and then counted.
Staining of Simian CMAH Protein in Bac-CMAH-Infected Sf9 CellsTo confirm the expression of simian CMAH protein in Bac-CMAH-infected Sf9 cells, cells (5×105 cells/mL) in a 6-well plate were inoculated with 50 µL of P2 virus stock and cultured at 28°C for 50 h. The cells were fixed with methanol and stained with mouse anti-polyhistidine monoclonal antibody (Sigma-Aldrich Corp., St. Louis, MO, U.S.A.) and horseradish peroxidase (HRP)-labeled goat anti-mouse immunoglobulin G (IgG)+M antibody (Jackson Immuno Research, West Grove, PA, U.S.A.). CMAH-expressing cells were stained as previously described.15)
Purification of Secretory Simian CMAHTo produce a large amount of secretory simian CMAH, a suspension culture of Sf9 cells (2×106 cells/mL) was inoculated at 1 multiplicity of infection (MOI) plaque forming unit/cell of Bac-CMAH and cultured in 150 mL of SFM containing 2 mM GlutaMAX-I (Invitrogen Corp.) at 28°C for 60 h with shaking at 100 rpm. After centrifugation (6000×g, 4°C, 10 min), the supernatant in SFM (approximately 150 mL) was concentrated to approximately 50 mL and then replaced by flowing 300 mL of 20 mM phosphate buffer–100 mM NaCl (pH 6.6) and subsequently 300 mL of 20 mM Tris–HCl buffer–100 mM NaCl (pH 7.9) using the ultrafiltration module MASTERFLEX L/S (Cole-Parmer International, East Bunker Court Vernon Hills, IL, U.S.A.) equipped with an ultrafiltration membrane, VIVAFLOW 200 (Sartorius Stedim Japan, Tokyo, Japan). To remove insoluble proteins from the concentrated solution, 7 volumes of the concentrated solution were mixed with 1 volume of eight-concentrated binding buffer (160 mM Tris–HCl, 4 M NaCl, 40 mM imidazole, pH 7.9) for His-Bind Resins (Novagen). The concentrated solution was ultracentrifuged at 111000×g for 1 h at 4°C using a CP65β machine with a P28S rotor (HITACHI KOKI Corp., Ltd., Tokyo, Japan). The supernatant was filtrated by a 0.45 µm membrane.
A His-bind column was made by stuffing 2 mL (1 mL of bed volume) of His-Bind Resin suspension into a Econo-Pack column (Bio-Rad Laboratries Inc., Hercules, CA, U.S.A.). After deairing of the column for 10 min, nickel ions were captured to resins by applying 5 mL of a charge buffer (50 mM NiSO4) to the his-bind column. The supernatant sample containing the secretory simian CMAH was applied to the his-bind column equilibrated by the addition of 4 mL of binding buffer (20 mM Tris–HCl, 500 mM NaCl, 5 mM imidazole, pH 7.9). A flowthrough was applied to the column again to efficiently adsorb the secretory simian CMAH to the resins. The secretory simian CMAH-adsorbed column was washed with 10 mL of the binding buffer and subsequently 6 mL of wash buffer (20 mM Tris–HCl, 500 mM NaCl, 40 mM imidazole, pH 7.9). The column was then washed with 5 mL of 20 mM Tris–HCl buffer–100 mM NaCl (pH 7.9) to decrease the salt concentration. The secretory simian CMAH adsorbed to the column was eluted to 12 fractions per 0.5 mL by adding 6 mL of elution buffer (20 mM Tris–HCl, 100 mM NaCl, 75–400 mM imidazole, pH 7.9). The fourth to tenth fractions (fractions 2–8 in Fig. 4) were used as purified secretory simian CMAH. The purified secretory CMAH was ultrafiltrated by centrifugation (6000×g, 4°C) using VIVASPIN20 (molecular weight cut off, 30 kDa) (Sartorius Stedim Japan, Tokyo, Japan) to concentrate the secretory simian CMAH and to decrease the concentration of imidazole.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and ImmunoblottingThe secretory simian CMAH elution fraction was 5-fold diluted with distilled water. Ten microliters of the diluted secretory CMAH or the cultured supernatant of Bac-CMAH-infected Sf9 cells was mixed with 10 µL of 2× concentrated sample buffer [100 mM Tris–HCl (pH 6.8), 4% SDS, 12% β-mercaptoethanol, 20% glycerol and bromophenol blue] and then boiled at 100°C for 5 min. The samples were electrophoresed at 25 mA/gel in 10% polyacrylamide gel. For coomassie brilliant blue G-250 (CBB) staining, the gel was fixed with fixation solution (10 mL methanol, 3.25 mL acetic acid and 36.75 mL distilled water) for 30 min and washed with distilled water. Bands in the gel were stained with GelCode Blue Stain Reagent (Thermo Fisher Scientific Inc., Waltham, MA, U.S.A.) overnight and subsequently decoloring solution (2.5 mL methanol, 3.5-mL acetic acid and 34 mL distilled water). For immunoblotting, proteins in the gel were blotted onto a polyvinylidene difluoride membrane at 15 V for 30 min. After blocking with 1% Block Ace (DS Pharma Biomedical Corp., Inc., Osaka, Japan) at 4°C overnight, the membrane was incubated with mouse anti-polyhistidine monoclonal antibody and subsequently HRP-labeled rabbit anti-mouse IgG antibody (Ab) (Jackson Immuno Research, West Grove, PA, U.S.A.) at room temperature for 2 h. The secretory simian CMAH band was stained as previously described.16)
Measurement of CMAH ActivityPurified secretory simian CMAH (1 or 5 µg) was mixed with 20 µM CMP-Neu5Ac, 1 mM NADH, 1 mM DTT, 0.5 mM FeSO4, and rat liver microsomal fraction (160 µg) in 100 µL of 10 mM Tris–HCl buffer (pH 7.5) containing 0.5% TritonX-100. The mixture was reacted at 37°C and stocked at −80°C until HPLC analysis of sialic acid species, Neu5Ac and Neu5Gc. Fluorometric determination of Neu5Ac and Neu5Gc was conducted by the modified HPLC method using 1,2-diamino-4,5-methylenedioxybenzene (DMB) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) as previously described.11,17) Briefly, the reacted mixture (100 µL) was mixed with 50 mM H2SO4 (75 µL) and 2 µM N-propylneuraminic acid (25 µL) as an internal control. After incubation at 80°C for 12 h (hydrolysis of the glyco-chain), the mixture was filtrated by centrifugation using Amicon Ultra Centrifugal Filters (Ultracel 3K membrane) (Millipore, Billerica, MA, U.S.A.) at 15100×g for 90 min. The flow-through (60 µL) was mixed with DMB reagent (60 µL) composing of 7 mM DMB, 1 M β-mercaptoethanol and 18 mM sodium hydrosulfite (Sigma-Aldrich Corp.) in water. After incubation at 60°C for 2.5 h under protection from light (fluorescent derivatization of sialic acid), the mixture was cooled on ice (derivatization reaction stop). A 100-µL aliquot of the supernatant was injected into an HPLC system with a TSKgel ODS-100 V 5 µm (4.6×150 mm) column (TOSOH Inc., Tokyo, Japan) at 40°C with a flow rate of 1.2 mL/min of methanol–water=25 : 75 (v/v). Fluorescent intensity of sialic acid derivatives (excitation at 373 nm and emission at 448 nm) was measured by a fluorescent detector, FP-2020 Plus (JASCO Corp., Tokyo, Japan). For the establishment of calibration curves, standard mixtures of Neu5Ac and Neu5Gc (0.1, 1 and 10 µM) (Sigma-Aldrich Corp.) or water only were used. Each sample was compensated by the internal control.
Treatment of CMAH with Peptide-N-glycosidase F (PNGase)Purified secretory CMAH (1.925 µg) was denatured at 100°C for 3 min and treated with PNGase (TaKaRa Bio, Shiga, Japan) at 37°C for 17 h, according to the manufacturer’s instructions. Briefly, 2.5 µL of purified CMAH plus 1.5 µL of β-mercaptoethanol was heat-denatured. Then to the CMAH solution was added 5 µL of stabilizer solution (5% Nonidet P-40), 13 µL of distilled water, and 2 µL of PNGase (500 mU/mL). For mock treatment, distilled water was used instead of PNGase. After reaction of the mixture at 37°C for 17 h, 0.6 µg/well of proteins was applied to SDS-PAGE using 10% gel. Bands in the gel were stained with GelCode Blue Stain Reagent overnight and subsequently decoloring solution as described above. For immunoblotting, proteins in the gel were blotted onto a polyvinylidene difluoride membrane. After blocking with 1% Block Ace at 4°C overnight, the membrane was incubated with mouse anti-polyhistidine monoclonal antibody and subsequently HRP-labeled rabbit anti-mouse IgG Ab at room temperature for 2 h. The CMAH band was stained as previously described.16) A mixture of glycoproteins with N-glycan from a PNGase kit was used as a positive control. The mixture includes human α1-acid glycoprotein (five N-glycans), bovine fetuin (three N-glycans), hen ovalbumin (one N-glycan), human transferrin (two N-glycans), and human IgG (two N-glycans).
A soluble secretory protein is generally convenient for purification of the protein and its use in various experiments because it can be secreted into an SFM. We added a gp64 secretion signal peptide and a histidine tag to the N-terminal region of CMAH for easy purification of CMAH in a baculovirus protein expression system (Fig. 2). We generated a baculovirus possessing the CMAH gene with a secretion signal peptide and histidine tag, named CMAH-Bac. Sf9 cells were infected with CMAH-BAc for 50 h. The CMAH-Bac-infected cells were stained with an anti-polyhistidine monoclonal antibody, whereas the non-infected cells not, demonstrating expression of the CMAH with a histidine tag (Fig. 3).

A: Structure of secretory CMAH. SS, gp64 secretion signal sequence; HT, 6×histidine tag. B: Gene and amino acid sequence of secretory simian CMAH.

Sf9 cells were infected with CMAH-Bac and cultured for 50 h. The cells were fixed with methanol and stained with mouse anti-polyhistidine monoclonal antibody. Non-infected cells were used as a control.
Sf9 cells were infected with CMAH-Bac at a MOI of 1 and cultured with SFM for 60 h with shaking at 100 rpm. The supernatant of the culture SFM was centrifuged to remove the cells, concentrated, and finally replaced with 20 mM Tris–HCl buffer–100 mM NaCl (pH 7.9) by ultrafiltration. The concentrated supernatant was diluted with an eight-concentrated binding buffer (160 mM Tris–HCl, 4 M NaCl, 40 mM imidazole, pH 7.9) for His-Bind Resins. To remove insoluble proteins, the diluted supernatant was ultracentrifuged and filtrated through a 0.45 µm membrane. The supernatant was applied to a His-Bind Resin column (1 mL of bed volume) in order to trap the secretory simian CMAH to a nickel column. After washing the column with binding buffer and wash buffer, the secretory simian CMAH adsorbed to the column was eluted to 12 fractions by gradient addition of elution buffer (20 mM Tris–HCl, 100 mM NaCl, pH 7.9) containing 75–400 mM imidazole. For SDS-PAGE analysis of elutes, approximately 65-kDa bands of secretory CMAH clearly appeared in fractions 2–8 as shown in Fig. 4 by both CBB staining (Fig. 4A) and immunoblotting using an anti-histidine tag antibody (Fig. 4B). We used a mixture of these fractions as purified secretory simian CMAH. Finally, approximately 180 µg of purified CMAH was obtained from 150 mL of SFM supernatant from Bac-CMAH-infected Sf9 cells.

A: CBB staining of purified secretory CMAH. B: Immunostaining of purified secretory CMAH using an anti-histidine tag antibody. Secretory simian CMAH was loaded to a nickel column, washed, and eluted with 500 µL of 100 mM (fractions 1 and 2), 150 mM (fractions 3 and 4), 200 mM (fractions 5 and 6), and 300 mM (fractions 7 and 8) imidazole. M, marker; S, supernatant from CMAH-Bac-infected Sf9 cells.
We examined purified CMAH enzymatic activity for converting the substrate CMP-Neu5Ac to CMP-Neu5Gc. Rat liver microsome was used to supply cytochrome b5 (incrementally cytochrome b5 reductase), which was required for CMAH activity (Fig. 1). A mixture of purified secretory simian CMAH and CMP-Neu5Ac was reacted in a reaction buffer containing Fe2+ and NADH at 37°C. After the reaction, contents of Neu5Ac and Neu5Gc were measured by HPLC analysis. When 5 µg of purified secretory simian CMAH was reacted for 15 min, Neu5Gc increased to 14.3 µM, whereas, Neu5Ac decreased from 19.4 µM to 7.9 µM (Fig. 5A). When 1 or 5 µg of purified secretory simian CMAH was reacted for 60 min, Neu5Gc increased and Neu5Ac decreased in a CMAH dose-dependent manner (Fig. 5B). These results demonstrate that the purified secretory CMAH possesses enzymatic activity to convert CMP-Neu5Ac to CMP-Neu5Gc.

A: Enzymatic activity of CMAH, conversion of Neu5Ac (open column) to Neu5Gc (closed column), was measured using the purified secretory simian CMAH (5 µg) for 0 and 15 min of enzyme reaction time. B: Conversion of Neu5Ac to Neu5Gc was measured using 0, 1, and 5 µg of the secretory simian CMAH for 60 min. Standard deviation was calculated from three independent experiments.
Simian CMAH has two glycosylation sites based on the amino acid sequence, Asn-X-Ser and -Thr. It has not yet been shown whether CMAH is a glycoprotein or not. Secretory simian CMAH was treated with peptide-N-glycosidase F (PNGase) to digest N-glycans on the CMAH protein. PNGase treatment resulted in a decrease in the CMAH molecular weight (Fig. 6). CMAH was also confirmed by immunoblotting using an anti-histidine tag antibody. Two bands of CMAH suggested the existence of both secretory CMAH with and without N-glycan. The result indicated that CMAH was a glycoprotein with N-glycan.
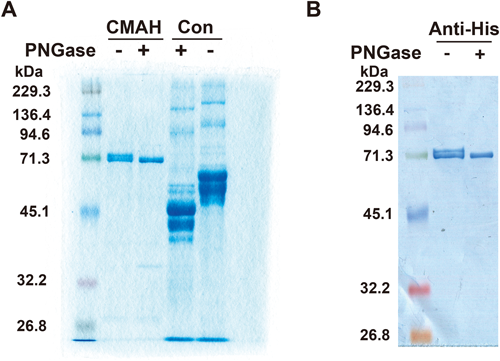
A: CBB staining of PNGase-treated CMAH. After SDS-PAGE of PNGase-treated or mock-treated CMAH, protein bands were stained with CBB. A mixture of glycoproteins with N-glycan was used as a positive control (Con). B: Immunostaining of PNGase-treated or mock-treated CMAH using an anti-histidine tag antibody.
In this study, we succeeded in producing a secretory simian CMAH with enzymatic activity that is easy to purify. This is the first report of the production and purification of secretory CMAH with enzymatic activity. In addition, the present study is also the first study showing that CMAH is a glycoprotein with N-glycan. The enzymatic properties of CMAH, such as enzymatic site and ion binding site, have not been investigated in detail. Our establishment of purified secretory simian CMAH will contribute to the progress of research on CMAH enzymatic properties from amino acid sequences. For example, the purified secretory simian CMAH will apply to functional analysis of CMAH by introducing mutations to the CMAH gene and make X-ray crystal structural analysis of CMAH easy. Elucidation of CMAH enzymatic properties at the molecular level will lead to a clear understanding of Neu5Gc metabolism and establishment of a strategy for controlling Neu5Gc expression in order to elucidate Neu5Gc function. Furthermore, the use of the purified secretory simian CMAH together with sialyltransferases will enable exogenous addition of Neu5Gc to terminal galactose of glycoconjugates on the surfaces of culture cells, red blood cells and artificial polymers. Neu5Gc-containing glycoconjugates are useful tools for screening of Neu5Gc-binding materials, such as influenza virus11–14) and bacterial toxin.10) Thus, the results of this study will help to advance studies on CMAH enzymatic function, Neu5Gc function and Neu5Gc-binding activity. A baculovirus protein expression system enables a secretory protein to be produced at an industrial level. Currently, commercial Neu5Gc is much more expensive than Neu5Ac. If the secretory CMAH is introduced for industrial Neu5Gc synthesis, commercial Neu5Gc will become easy to use, thus contributing to the progression of Neu5Gc research.
This work was supported in part by the Global COE Program from the Japan Society for the Promotion of Science and by JSPS KAKENHI Grant Number (Scientific Research C, 23590549; Challenging Exploratory Research, 26670064; Young Scientists A, 15H05644), Grants-in-Aid from Hokuto Foundation for Bioscience, Showa University Medical Foundation, the Uehara Memorial Foundation, Foundation for Promotion of Material Science and Technology of Japan, The Waksman Foundation of Japan, the Research Foundation for Pharmaceutical Sciences, Takeda Science Foundation, the Tokyo Biochemical Research Foundation, the Hamamatsu Scientific Research Foundation, the Sasakawa Scientific Research Grant from The Japan Science Society and Adaptable and Seamless Technology Transfer Program (A-step) through Target-driven R&D, JST.
The authors declare no conflict of interest.