2016 年 39 巻 10 号 p. 1588-1595
2016 年 39 巻 10 号 p. 1588-1595
The aim of this study was to develop and to investigate a film of compound Calculus Bovis Sativus (CBS) and ornidazole film. A uniform mucoadhesive film was herein successfully obtained by a film-forming solusion containing insoluable drug. This film, as a valid adjunct for the treatment of oral mucosal ulcer, consisted of two main drugs (CBS, ornidazole) and three polymers (hydroxypropyl methyl cellulose, chitosan, poly(vinyl alcohol) (PVA)). The film was prepared with the film-forming suspension, using casting-solvent evaporation technique. The drug content, release behavior, swelling index and mucoadhesive properties of the film were detected. Then the effects of the prepared film on a glacial acetic acid-induced oral mucosal ulceration model of rabbits were evaluated. Moreover, the in vivo release of bilirubin and ornidazole in saliva were also detected in the oral mucosae of healthy volunteers. The films showed favorable in vitro drug release behaviors and swelling properties. Mucosal wounds in the animals were significantly relieved. With the films well tolerated, the salivary concentrations of ornidazole were maintained above the minimum inhibitory concentration against CBS for about 2 h. The compound CBS and ornidazole film functioned better than the film only containing CBS and ornidazole did. Therefore, it is a potentially efficient drug delivery system for the treatment of oral ulcers.
As a common oral mucosal disease, oral ulcer is characterized by recurrent, painful ulcerations and burning sensation, affecting approximately 5–20% of general population.1) In addition, oral ulcer is associated with a variety of underlying systemic diseases, including chronic inflammatory bowel diseases, gluten enteropathy, agranulocytosis, and cyclical neutropenia.2) However, the etiology of oral mucosal ulcerative lesions remains elusive. Various possible causative agents, such as local, systemic, immunologic, genetic, allergic, nutritional ones and microorganisms, have been developed.3) With complex and unclear causes, oral ulcer is often hard to cure. Thus, this study tried to develop a new effective compound and a suitable dosage form.
Buccal mucoadhesive film has been used as an effective dosage form for local oral ulcer owing to smooth homogeneous surface morphology, elegant appearance, high elasticity and acceptable mucoadhesive strength.4) Compared with mouth wash liquid, gel, ointment and tablet, the film attaches tighter to the mucosa, so it can protect the wound area from pathogenic microorganisms and improve the local effective concentration and residence time of the target drug.5,6)
Calculus Bovis (CB), naturally derived from the dry gallstones or bile duct calculi of bovine, is capable of diminishing inflammation, resisting spasm, relieving fever, normalizing function of the gallbladder and sedation. Because of the scarcity of natural resource and high price, Calculus Bovis Sativus (CBS; also called in vitro Cultivated CB) was developed as a substitute. CBS has similar pharmacological effects to those of CB.7) In traditional Chinese medicine, CB was used as a main ingredient to treat oral ulcers, exerting its pharmacological effects by resisting inflammation.8) In addition, the microenvironment of oral cavity is moist and rich of bacteria, which may lead to bacterial infection of the ulcer wound, thus delaying wound healing.9) Ornidazole (OD), a nitroimidazole drug, is commonly used to treat the infections of maxillofacial or periodontal anaerobic bacteria which are the common bacterial colonization of oral ulcers. Both of them have been used independently to treat oral ulcer in clinical practice. Meanwhile, the compound formulation of CBS and nitroimidazoles (Artificial CB, Metronidazole Tablet or capsule) has been widely used to treat oral diseases in China for decades. Hence, co-administering OD with CBS may have a synergistic effect on oral ulcers. In order to develop a novel buccal administration preparation, CBS and OD were herein used as the two main drugs to fabricate a compound CBS and OD film in this study.
CBS (pulverized by versatile pulverizer, smashed through 200 mesh sieve, insoluble in water) was gifted from Wuhan Jianmin Dapeng Pharmaceutical Co., Ltd. (Wuhan, P. R. China). OD (soluble in water) was gifted from Wuhan Lakeside Double-Crane Pharmaceutical Co., Ltd. (Wuhan, P. R. China). Hydroxypropyl methyl cellulose (HPMC) and carbomer were purchased from Colorcon China Co., Ltd. (Shanghai, P. R. China). Chitosan (CS) was acquired from Nantong Xingcheng Biological Industrial Limited Co., Ltd. (Nantong, P. R. China). Poly(vinyl alcohol) (PVA) was obtained from Kuraray China Co., Ltd. (Shanghai, P. R. China). Sodium carboxymethyl cellulose (CMC-Na) was purchased from Anhui Shanhe Pharmaceutical Excipirients Co., Ltd. (Anhui, P. R. China). Glycerin (Gly) and phosphate buffer saline (PBS) were obtained from Wuhan Google Biological Technology Co., Ltd. (Wuhan, P. R. China). The standards of bilirubin and OD were acquired from National Institutes for Food and Drug Control (Beijing, P. R. China).
MethodsFormulation of Compound CBS and OD FilmsCompound CBS and OD films were prepared by the solvent casting technique using different polymer ratios of CS, PVA and HPMC. Different concentrations of polymer solutions and drugs were mixed in specified ratios as shown in Table 1. CS was swelled in 1% (w/v) acetic acid, while PVA and HPMC were both soluble inflations in deionized water for 12 h. Then, HPMC solution was completely dissolved by being heated to over 90°C. After being cooled slightly, the solution of CS and PVA was mixed well with HPMC solution on a magnetic stirrer. For the above solutions, known quantities of CS, HPMC and PVA were added and mixed thoroughly. Gly, CBS and OD were mixed and stirred at high speed for 1 h to obtain a homogenous, clear suspension. Then the suspension was stirred at a low speed in order to remove air bubbles. Finally, the suspension was poured into a Petri dish (10 cm in diameter). Films were freeze-dried at −80°C for 24 h. Afterwards, the dried films were removed, checked for any imperfections or air bubbles and cut into 1 cm in diameter using a specially fabricated circular stainless steel cutter. The samples were packed in aluminum foil and stored in a glass container at room temperature.
| Formulation | CS (m/v) | HPMC (m/v) | PVA (m/v) | Gly (mL) | CBS (g) | OD (g) |
|---|---|---|---|---|---|---|
| F1 | 1.2% | 0.8% | 1% | 1.5 | 0.2 | 0.24 |
| F2 | 1.2% | 1.2% | 1% | 1.5 | 0.2 | 0.24 |
| F3 | 1.2% | 1.6% | 1% | 2 | 0.2 | 0.24 |
| F4 | 1.2% | 0.8% | 1.5% | 2 | 0.2 | 0.24 |
| F5 | 1.2% | 1.2% | 1.5% | 2.5 | 0.2 | 0.24 |
| F6 | 1.2% | 1.6% | 1.5% | 2.5 | 0.2 | 0.24 |
Five randomly selected films (1 cm2) from each formulation were weighed to calculate the mean thickness. Meanwhile, the thickness was determined using a bench thickness gauge at five different positions of the film. The data were expressed as the mean±standard deviation (S.D.).
Drug Content DeterminationCBS content was evaluated by bilirubin content in the compound CBS and OD film. Bilirubin and OD contents were determined as follows: Each film was cut impalpably into pieces, dissolved in moderate trichloromethane–methanol–phosphoric acid mixture (75 : 25 : 0.18, v/v/v) in a volumetric flask, and ultrasonicated for 15 min. The resultant solution was filtered through a 0.45 µm membrane filter. After suitable dilution, the samples were determined by high performance liquid chromatography at 315 and 450 nm, and reported as an average of three determinations. The liquid chromatography was performed as follows:
Bilirubin and OD were separated on a Hypersil ODS-2 column (4.6×250 mm, 5 µm). The mobile phase was acetonitrile–1% acetic acid with gradient elution. Detection wavelengths of bilirubin and OD were 450 and 315 nm, respectively. The column temperature was 30°C and the injection volume was 10 µL. The linear range of bilirubin was within 1.58–51 µg·mL−1 (y=0.0921x+0.0774, r=0.9992), and the linear range of OD was within 3.22–103 µg·mL−1 (y=0.0407x−0.043, r=0.9990). And the lower limit of line range means the detection limit.
Swelling IndexSwelling index was determined as described previously with minor modification.5,10) Each film was weighed and the initial weight was denoted as W1. Then the film was placed in a Petri plate containing 4 mL of phosphate buffer (pH 6.8). At the time interval of 1–4 h, the film was removed carefully from the Petri plate and excess solvent was soaked by a filter paper. The weight of the swelled film was measured and denoted as W2. Swelling index was calculated by using the following formula:
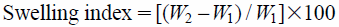 |
Surface pH was determined as described previously with minor modification.5,10) The surface pH of the prepared compound CBS and OD films was determined to evaluate the possible irritating effects on the mucosa. The films were allowed to swell by being kept in contact with 1 mL of pH 6.8 phosphate buffer for 2 h at room temperature, and pH was recorded by attaching an electrode to the surface of the film, allowing it to equilibrate for one minute.
In Vitro Mucoadhesive TimeIn vitro mucoadhesive time was determined as described previously with minor modification.6) The in vitro mucoadhesive time was detected (n=3) after the application of the films on freshly cut porcine buccal mucosa. The porcine buccal tissues were fixed on the internal side of a beaker with cyanoacrylate glue. Each film was divided in portions of 4 cm2 and then was cut into four parts. One side of each film was wetted with 50 µL of simulated saliva fluid and pasted to the porcine buccal tissue by applying a light force with the finger tip for 20 s. The beaker was filled with 800 mL of the simulated saliva fluid and kept at 37°C. After 2 min, the buccal cavity environment was simulated by stirring at 150 rpm, and film adhesion was monitored during 4 h.
In Vitro Mucoadhesive ForceTwo 3 cm-long segments of the membrane were prepared, spread and fixed on small glass bottles referred to as A and B, respectively (Fig. 1). Water was added slowly into a plastic bottle (referred to as C) for counter balancing. The total weight of C at this time point was W1. Then the film was soaked with 15 µL of normal saline and rubbed to B. Rapidly, A which was rubbed with sticky mucous membrane was covered onto B, and a 50 g weight set was applied for 5 min. After both sides of the mucous membrane fully bonded, the weight set was removed and water was added into C at a speed of 2 drops per second until drug film and the membrane separated completely. The total weight of C at this time point was W2. Each prescription was tested three times and the data were expressed as the mean±S.D. Mucoadhesive force was calculated by using the following formula:
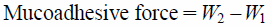 |
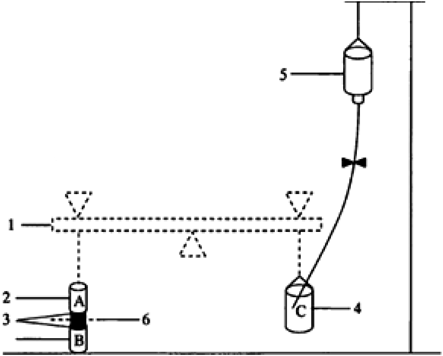
1, counter balance; 2, (A, B) glass carrier; 3, membrane; 4, (C) plastic palte; 5, infusion bottle; 6, film.
In vitro drug release was determined as described previously with minor modification.11) In vitro OD and bilirubin release studies were performed on the compound CBS and OD films using modified Franz diffusion cells (Shanghai Yuyan Instruments Co., Ltd., P. R. China). The receptor compartment was filled with release medium (5.6 mL of pH 7.4 phosphate buffer), held at a controlled temperature of 37.0±0.1°C, and stirred at 600 rpm. A 0.2 µm cellulose acetate membrane, which had been hydrated in pH 6 phosphate buffer overnight, was mounted on a diffusion cell. The compound CBS and OD films were cut to disc with a surface area of 1 cm2 and accurately weighed before being placed on the membrane. The cells were then fixed and tightly fastened with a clamp. At predetermined intervals, the samples (500 µL) were collected and replaced with fresh medium. The samples were determined by the same method used in drug content determination, and expressed as an average of three determinations.
In Vitro PermeabilityIn vitro permeability was determined as described previously with minor modification.11,12) In vitro permeation studies were also performed on the compound CBS and OD films using modified Franz diffusion cells. Isotonic phosphate buffer (pH 7.4, 5.6 mL) at 37.0±0.1°C was used as a receptor and the receptor compartment was stirred at 100 rpm. Porcine esophageal mucosa, which was obtained from a local slaughterhouse, was used as a permeation membrane. The porcine esophageal tube was opened longitudinally and rinsed with pH 7.4 isotonic phosphate buffer. The mucosa was removed from the underlying muscular layer and immersed in isotonic saline at 60°C for 1 min. The epithelium was then peeled from the connective tissue and frozen at −20°C prior to use. The frozen mucosal membranes were brought to room temperature by immersion in pH 6.8 isotonic phosphate buffer for 15 min. The membrane was placed between the donor and receptor compartments, with the connective tissue surface facing down. Phosphate buffer (pH 6, 20 µL) was spread over the mucosal membrane to simulate the saliva. The films were then placed on the hydrated membrane, and the cells were subsequently fixed and tightly fastened with a clamp. Bilirubin and OD were analyzed according to the same methods used in drug content determination.
Efficacy of Rabbit Mucosal Ulcer ModelThirty six male New Zealand rabbits weighing 2.5–3.0 kg were obtained from the Center of Experimental Animal of Hubei Province (Wuhan, P. R. China). All animals were kept under the same laboratory conditions at 25±2°C, and given free access to standard laboratory chow and tap water. The protocol was approved by the local Animal Experimental Ethical Committee and all the experiments were performed in accordance with the guideline of the Council on Animal Care of Academia Sinica. The rabbits were given 50% glacial acetic acid on harelip for 60 s to establish the oral ulcer animal model,13,14) and randomly divided into control, compound (simultaneously containing CBS and OD), CBS and OD groups (n=9) that were attached compulsively with blank film, compound CBS and OD film, film only containing CBS or film only containing OD respectively for 0.5 h. The film areas were all 1 cm2. All animals were administered once each day.
Clinical healing of each oral ulcer was evaluated by subtracting the most recent reading from the initial ulcer measurement at each observation interval. Thus, clinical healing was defined using decrease in the ulcer area: clinical healing was better with increasing difference between the initial measurement and that on the observation day.
In each group, three animals were sacrificed separately on days 4, 8 and 12 for histological evaluation. Thirty six rabbits were included (nine animals in each test group for each observation period). The treatment started 24 h after ulceration (day 1). The doses of CBS and OD were 0.875 and 3 mg/kg, respectively, which were applied to the ulcer using finger to press. The applications were performed once daily.
Clinical photographs of the ulcers were recorded with a digital camera. In each photograph, a filter paper disc of 3 mm diameter was included to calibrate the ulcer area measurement. For the ulcer area was ellipse-shaped, a maximum diameter was also determined by calipers. All measurements were performed by the same researcher in a blinded fashion three times on the same day and a mean ulcer area was calculated.
For histological examination, nine animals from each group were sacrificed with an excess dose of pentobarbital on days 4, 8 and 12. The ulcers were excised by an experienced oral surgeon and fixed in 4% paraformaldehyde. In the laboratory, they were embedded in paraffin blocks and 4 mm-thick tissue sections were prepared.
Determination of Superoxidedismutase (SOD) Activity and Malondialdehyde (MDA) Content of Harelip TissueWhen the rabbits were killed on days 4, 8 and 12, a part of harelip tissue was cut and frozen at −80°C as a standby application. After being thawed, the frozen tissue was mixed with deionized water in a ratio of 4 : 1 (w/v) and homogenized for 3 min. Then the homogenate was centrifuged at 3000 rpm for 10 min to take the supernatant fluid. Then the SOD activity and MDA content of harelip tissue were determined respectively according to instructions.
Preliminary Evaluation of Buccal FilmsThis test was approved by the Ethical Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and performed by using film formulation F5 (three replicates) based on the results of the above experiments. The films were placed (with the drug-loading side) on the buccal mucosa (cheek) of three volunteers (healthy males, 20–35 years old), which swelled and then dissolved very slowly. At the end of the test, only some fragments were observed in the oral cavity. At each interval of the experiment, samples of saliva (about 0.5 mL) were collected, and bilirubin and OD concentrations were immediately determined by the same method used in the release study.
Statistical AnalysisIn all statistical analyses, p was set as 0.05 and SPSS 10.0 for Windows program (SPSS Inc., Chicago, IL, U.S.A.) was used for data analysis. Mean±S.D. was calculated through EXCEL.
Compound CBS and OD films were successfully prepared by the solvent casting technique. Physical characteristics of the films are summarized in Table 2. All the films were tan and flexible, with the thicknesses ranging from 306 to 473 µm. The film masses ranged from 31.68 to 46.20 mg, and the drug content uniformities ranged from 0.868 to 1.041 mg/cm2. As suggested by the low S.D. in thickness, weights and drug contents, there were no significant differences between batches. The microenvironment pH of different batches was nearly 6.5.
| Formulation | Thickness (µm) | Bilirubin content (mg/cm2) | OD content (mg/cm2) | Mass uniformity (mg) | Surface pH |
|---|---|---|---|---|---|
| F1 | 306.2±23.5 | 0.868±0.115 | 3.04±0.45 | 33.48±2.34 | 6.4±0.2 |
| F2 | 336.0±11.0 | 0.897±0.142 | 2.96±0.32 | 31.68±1.82 | 6.5±0.2 |
| F3 | 336.2±11.5 | 0.937±0.153 | 3.17±0.36 | 38.14±1.82 | 7.1±0.1 |
| F4 | 462.2±45.2 | 1.041±0.254 | 3.24±0.59 | 46.20±6.36 | 6.9±0.3 |
| F5 | 426.2±39.2 | 0.972±0.211 | 3.16±0.44 | 46.16±2.27 | 6.2±0.1 |
| F6 | 473.8±17.0 | 0.884±0.106 | 3.02±0.34 | 44.66±1.82 | 6.3±0.1 |
Swelling index was determined by immersing pre-weighed patch of 1×1 cm2 in 4 mL water. The strips were taken out carefully at 0.5, 1 and 2 h intervals, blotted with filter paper, and weighed accurately. The average swelling index of all patches is shown in Table 3.
| Time (h) | Swelling index (%) | |||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |
| 0.5 | 19.36±0.67 | 20.48±0.55 | 22.38±0.42 | 24.94±0.65 | 24.64±0.61 | 25.44±0.64 |
| 1 | 30.16±0.54 | 32.37±0.46 | 32.72±0.37 | 35.96±0.46 | 33.71±0.54 | 31.87±0.37 |
| 2 | 45.28±0.42 | 43.33±0.74 | 46.57±0.58 | 46.98±0.38 | 44.61±0.48 | 45.65±0.52 |
The in vivo mucoadhesive force (g) and mucoadhesive time (h) for different formulations were found as per procedure given in methods, and the results are displayed in Table 4. All films had mucoadhesive times ranging between 2.93±0.35 and 4.84±0.11 h, and mucoadhesive force ranging between 294±12.3 and 436±20.2 g. All the films showed good mucoadhesive properties. With higher concentration of hydroxypropylmethyl cellulose (HPMC) 15 KM which is known to have outstanding mucoadhesive property, the films showed higher in vitro mucoadhesive force and longer in vitro mucoadhesive time. Formulation F5 with 3.9% total polymer concentration and F6 with 4.3% total polymer concentration had the best mucoadhesive properties. In addition, plasticizer at a higher concentration may promote the mucoadhesive property.
| Formulation | In vitro mucoadhesive force (g) | In vitro mucoadhesive time (h) |
|---|---|---|
| F1 | 294±12.3 | 2.93±0.35 |
| F2 | 305±15.7 | 2.97±0.15 |
| F3 | 347±16.1 | 3.23±0.27 |
| F4 | 393±18.9 | 3.51±0.14 |
| F5 | 436±20.2 | 4.84±0.11 |
| F6 | 412±18.6 | 4.53±0.24 |
The in vitro drug release profile of OD from compound CB films in PBS (pH 6.8) is shown in Fig. 2. No bilirubin was detected. About 60% of OD was released within 90 min, and then the cumulative percentage increased gradually. The drug release increased with rising concentration of polymer HPMC 15 KM, which may be ascribed to the hydrophilicity of HPMC 15 KM. It was characterized with a strong water absorption capacity, thus accelerating the dissolution, diffusion and release of easily soluble drug OD.
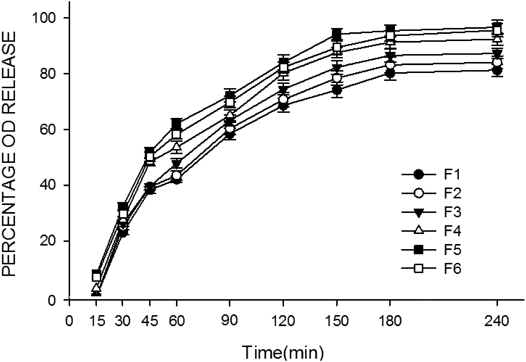
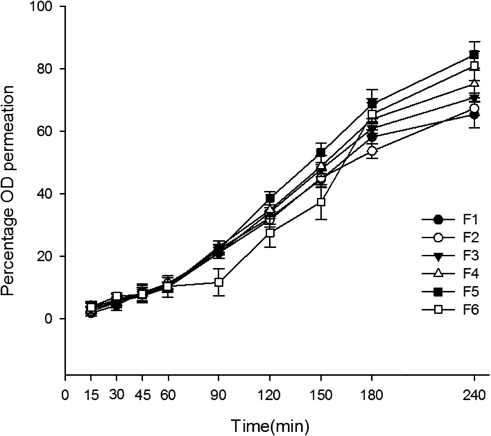
The in vitro permeation of OD from compound CBS and OD films in PBS (pH 7.4) is shown in Fig. 3. With good release property, all the prepared film formulations had cumulative OD releases ranging from 65.45 to 84.54%. Meanwhile, bilirubin was not detected due to extreme insolubility. In vitro drug permeation from formulation F5 decrease compared with that from formulation F6, which may be caused by higher concentration of HPMC 15 KM in F6. Higher concentration enhanced hydration and swelling of the film, which may form a hydrogel layer embracing drug molecule. Consequently the dissolution and diffusion of OD were delayed.
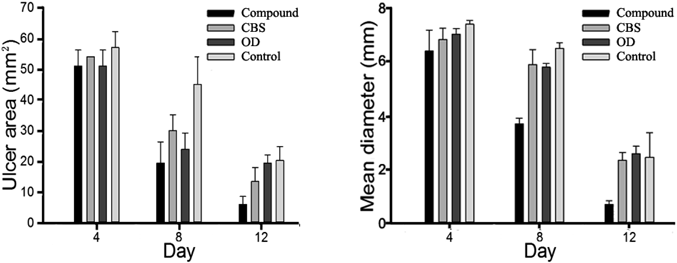
Formulation F5 was chosen in the following studies based on its favorable in vitro drug release profile and swelling ratio. The ulcer areas of the samples on days 4, 8 and 12 are presented in Fig. 4. During the period, the ulcer areas, which were largest on the first three days, decreased most obviously on day 9 and reached minimum on day 12. Comparisons between the area of compound group with those of CBS, OD and control groups by independent samples ANOVA gave p values of 0.067, 0.003 and 0.016, respectively. The compound film-treated animals had a significantly smaller average of all the mean ulcer areas measured on days 4, 8 and 12 than those of other groups. Simultaneously, a maximum diameter was observed every day during 12 d, with its variations shown in Fig. 4. Comparisons between the diameter of compound group with those of CBS, OD and control groups by independent samples ANOVA gave p values of 0.001, 0.001 and 0.004, respectively. The mean maximum diameter decreased gradually to 0.6 mm in the compound group, differing significantly from those of the other three groups.
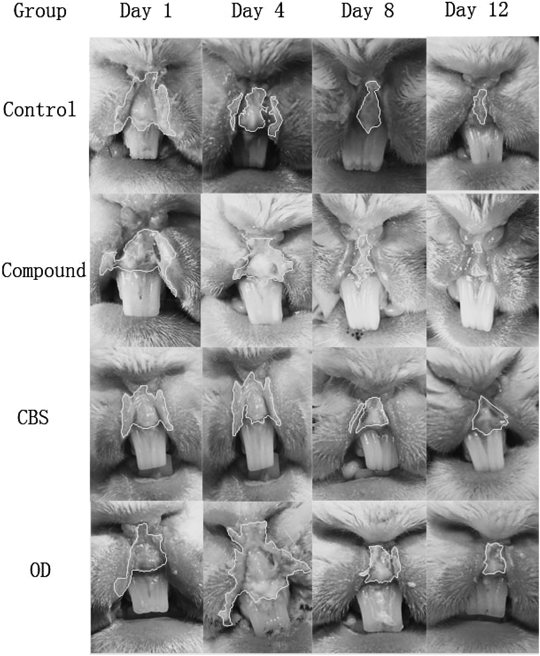
The images of pathological section and oral ulcer healing process on days 4, 8, and 12 are presented in Figs. 5 and 6. Ulcers of the compound group healed faster than those of the other three groups, and this group was completely cured at the end of this study.
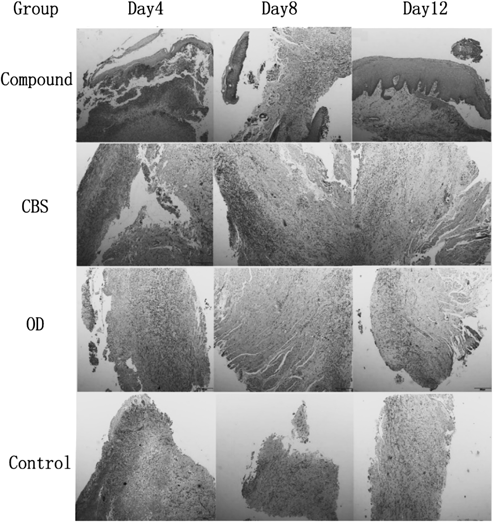
There is epithelial necrosis and abscission at the surface, and subepithelial inflammatory activity. And the degree of subepithelial inflammatory was obviously decreased.
As shown in Table 5, formulation F5 significantly enhanced the SOD activity and decreased the MDA content of oral tissues, being significantly different from those of control, CBS and OD groups.
| Day | SOD activity (U/mg prot) | MDA content (nmol/mg prot) | ||||||
|---|---|---|---|---|---|---|---|---|
| Compound | CBS | OD | Control | Compound | CBS | OD | Control | |
| 4 | 52.03±9.29 | 31.58±4.76 | 51.31±8.47 | 51.65±8.35 | 0.65±0.12 | 0.89±0.26 | 1.29±2.04 | 1.02±0.44 |
| 8 | 47.06±7.76 | 17.82±3.62 | 38.69±6.71 | 23.04±5.05 | 1.05±0.87 | 1.55±0.59 | 1.57±0.83 | 1.61±0.38 |
| 12 | 37.87±6.05 | 10.44±1.51 | 18.72±3.88 | 12.72±2.16 | 1.42±1.11 | 2.54±1.24 | 1.78±0.78 | 2.59±1.15 |
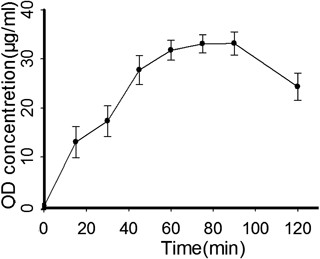
As a preliminary study, the salivary levels of OD diacetate obtained from the cheek mucosa of a healthy volunteer are shown in Fig. 7 (formulation F5). OD concentrations rose in a regular way, reaching the highest level (33.07 µg/mL) at 75 min and starting to decrease after 120 min. Notably, the OD level of film F5 was maintained higher than the minimum inhibitory concentration against Candida albicans (7.8 µg/mL) for about 2 h. Moreover, the films, which did not irritate or discomfort the cheek mucosa, showed a good comfortability and tolerability in the oral cavity.
In this study, the combination of CBS and OD was derived from Artificial CB and Metronidazole Tablet or capsule which has been commonly used in clinical practice. The traditional drug given by mouth has been proved to have a significant efficacy on oral ulcer.15) However, due to systemic administration, it inevitably leads to lower local effective concentration, and it has often been associated with concomitant gastrointestinal adverse reactions.16) Meanwhile, general treatment for oral ulcer is dominated by local treatment which not only eliminates the systemic side effects caused by systemic administration, but also ensures local effective concentration of the drug. Therefore, another substitute (CBS) and a new generation of nitromidazoles (OD) were chosen to prepare an oral mucoadhesive film. CBS was developed in vitro, based on the simulation of in vivo stone formation and biochemical process. It has been widely used in Southeast Asia, especially in China, Japan and Korea, to exhibit anti-spasmodic, fever-reducing, anti-inflammatory and gallbladder-repairing effects.17) Artificial CB is prepared in accordance with the main components of natural CB. Though CBS and artificial CB are both substitutes for natural CB, the composition, content, efficacy and pharmacological effects of CBS are almost identical to those of natural CB.18) Hence, CBS is better than artificial CB. In addition, compared to metronidazole, OD has a wider spectrum of activity, higher antimicrobial activity, stronger tissue permeability and lower side effects.19) Besides their respective advantages, they can also exert a synergistic effect owing to compatibility. Combining the advantage of oral mucoadhesive film, it will have a better efficacy.
In a previous single factor analysis, the film-forming property of different concentration of bioadhesive polymers was investigated, with 1–1.5% CS solution functioning optimally. Formulation consisting of CS and PVA 217 had an excellent film-forming property, but its mucoadhesive property was poor. Hence, CMC-Na, carbomer, HPMC 10 KM and HPMC 15 KM were added to prepare the mucoadhesive film, respectively. Both carbomer and CMC-Na produced white precipitates when mixed with CS. Meanwhile, the film added with HPMC 15 KM had a better mucoadhesive property than that of the film with HPMC 10 KM, so HPMC 15 KM was chosen to enhance the mucoadhesive property. The thickness of buccal epithelium in humans is approximately 500–800 µm,20) with its surface covered with a mucus layer thickened less than 1 µm.21) Patients may feel uncomfortable if a film with the thickness of over 500 µm is attached. In this study, with increasing concentration of total polymers, the films became thicker (Table 2). Further experiments were carried out to explore the concentration range of total polymers. When the concentration of total mucoadhesive polymers was controlled from 1.5 to 4.5%, the film thicknesses were less than 500 µm (Table 1). Thus, the concentration of film-forming solution (containing CS, PVA 217 and HPMC 15 KM) was chosen between 1.5 and 4.5%.
Since the solubility of CBS is poor, film-forming suspension has serious edge effect and self-aggregation during drying, which makes the middle part of the films thick and the edge thin. Many U.S. patents have endeavored to circumvent self-aggregation by adding gel formers or polyhydric alcohols respectively to increase the viscosity of film-forming solution prior to drying, or using conventional drying methods like a high-temperature air-bath with drying equipment.22,23) Although these drying methods can reduce self-aggregation by speeding up the drying process, aggregation still affects the uniformity of films. In order to solve this problem, a rapid freeze-drying method was employed to prevent random thermal motion of particles, through which we decreased the aggregation. After being freeze-dried, the film-forming solution was turned into a solid sticky mesh that could be evenly pressed into a uniform film by mechanical pressure equipment.
The major bioactive substances of CBS are bilirubin, cholic acid, deoxycholic acid, ursodeoxycholic acid, chenodeoxycholic acid and hyodeoxycholic acid.24) The Chinese Pharmacopoeia regulated that bilirubin was used to quantify CBS. In addition, it is reported that bilirubin, an indispensable ingredient in CBS, has many significant pharmacological effects including antioxidation.25) Since the active ingredient for curing oral ulcer in complicatedly constituted CBS is still unclear, the content of bilirubin was determined in this study to represent that of CBS. Besides, being extremely insoluble in PBS and artificial saliva, bilirubin was not detected in in vitro drug release or permeability studies. But it does not mean that the other ingredients of CBS cannot be released and absorbed.
In previous literatures, CBS elevated the SOD activity and decreases the MDA content in mouse’s brain, liver and heart,26,27) being consistent with the results of this study. The activity of SOD was enhanced and the MDA content was decreased in rabbit oral ulcers by compound CBS and OD film. Hence, oral ulcers may be treated by CBS through scavenging free radicals.
In summary, a compound CBS and OD film was successfully prepared by solvent casting technique, and characterized based upon good flexibility, suitable surface pH and mucoadhesive force, moderate swelling index and satisfactory mucoadhesive properties. Exerting a satisfactory therapeutic effect in vitro, the film can maintain an effective drug concentration in local oral mucosa. The compound CBS and OD film will be used to enhance the efficacy for treatment of oral ulcer, to reduce side effects. As a new drug delivery system, its efficacy on human will be further studied in our group. Simultaneously, the mechanism by which CBS treats oral ulcer will be another research focus.
The authors declare no conflict of interest.