2016 Volume 39 Issue 8 Pages 1293-1299
2016 Volume 39 Issue 8 Pages 1293-1299
To develop an effective oral delivery system for plasmid DNA (pDNA) using cationic liposomes, it is necessary to clarify the characteristics of uptake and transport of cationic liposome/pDNA complexes into the intestinal epithelium. In particular, evaluation of the involvement of an unstirred water layer (UWL), which is a considerable permeability barrier, in cationic liposome transport is very important. Here, we investigated the effects of a UWL on the transfection efficiency of cationic liposome/pDNA complexes into a Caco-2 cell monolayer. When Caco-2 cells were transfected with cationic liposome/pDNA complexes in shaking cultures to reduce the thickness of the UWL, gene expression was significantly higher in Caco-2 cells compared with static cultures. We also found that this enhancement of gene expression by shaking was not attributable to activation of transcription factors such as activator protein-1 and nuclear factor-kappaB (NF-κB). In addition, the increase in gene expression by mechanical agitation was observed at all charge ratios (1.5, 2.3, 3.1, 4.5) of cationic liposome/pDNA complexes. Transport experiments using Transwells demonstrated that mechanical agitation increased the uptake of cationic liposome/pDNA complexes by Caco-2 cells, whereas transport of the complexes across a Caco-2 cell monolayer did not occurr. Moreover, the augmentation of the gene expression of cationic liposome/pDNA complexes by shaking was observed in Madin–Darby canine kidney cells. These results indicate that a UWL greatly affects the uptake and transfection efficiency of cationic liposome/pDNA complexes into an epithelial monolayer in vitro.
Oral plasmid DNA (pDNA) delivery using non-viral vectors, including cationic liposomes, is expected to become an attractive gene therapy because of the following beneficial features compared with parenteral administration: 1) a large mucosal surface area, 2) ease of administration, 3) improvement of safety, 4) increase in patient compliance, and 5) reduction of cost.1–3) The most advanced research on the applications of oral pDNA delivery systems is oral DNA vaccination.1,4,5) Oral DNA vaccination enables elicitation of both antigen-specific mucosal immunoglobulin A (IgA) and systemic IgG. In addition, this method stimulates cell-mediated responses including CD4- and CD8-positive T-lymphocytes. Therefore, this type of vaccination is well accepted as a method that is superior to parenteral-injected vaccination. Oral DNA vaccination using cationic liposomes achieves effective immunization reactions in vitro and in vivo.1,4,5) Thus, oral gene therapy using cationic liposomes, especially oral DNA vaccination, would provide potential benefits for the treatment of a variety of gastrointestinal diseases and infections. However, the development of an oral pDNA delivery method is more difficult than that of a parenteral administration method because it is necessary to consider the absorptive process of cationic liposomes/pDNA complexes.
To develop an efficient oral delivery system for pDNA using cationic liposomes, it is essential to predict intestinal absorption of cationic liposomes. To estimate intestinal drug absorption, in vitro permeability experiments are widely used.6–8) In particular, Caco-2 cells are one of the most frequently used cell types as a model of the intestinal epithelium because Caco-2 cells form a monolayer that exhibits apical brush border microvilli, tight intercellular junctions, and a polarized distribution of brush border enzymes.6–8) Therefore, a Caco-2 cell monolayer is useful to evaluate the characteristics of oral absorption and transfection of cationic liposomes/pDNA complexes.
On the other hand, several studies have shown that the results of drug permeability obtained from in vitro experiments using a Caco-2 cell monolayer differ from those obtained from in vivo experiments or clinical studies.9,10) These differences are considered to be attributed to the presence of an unstirred water layer (UWL) on the surface of epithelial cells.11–13) A UWL is a considerable permeability barrier that greatly affects in vitro, but not in vivo, drug absorption experiments.13) Therefore, it is very important to elucidate how a UWL affects the uptake and transport of cationic liposomes/pDNA complexes into epithelial cells in experiments performed in vitro. The characteristics or mechanisms of nanoparticle transport in epithelial cells have been reported,14–18) whereas few reports have addressed the effect of UWLs.
Here, to examine whether cellular uptake and transport of pDNA via cationic liposomes into a Caco-2 cell monolayer are affected by the presence of a UWL, we evaluated the transfection efficiency of pDNA in Caco-2 cells via cationic liposomes under conditions of a reduction in the UWL thickness by mechanical agitation. In addition to Caco-2 cells, we investigated the effect of mechanical agitation on the uptake of cationic liposomes/pDNA complexes into Madin–Darby canine kidney (MDCK) cells.
Caco-2 human epithelial colorectal adenocarcinoma cells as well as MDCK-I and MDCK-II epithelial cells were obtained from DS Pharm Biomedical Co., Ltd. (Osaka, Japan). RAW264 mouse macrophage-like cells were obtained from Riken Bioresource Center (Osaka, Japan). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), nonessential amino acids, penicillin G (100 U/mL), and streptomycin (100 µg/mL) at 37°C with 5% CO2.
Construction of Cationic Liposome/pDNA ComplexesCationic liposomes were constructed according to the procedures reported by Kawakami and colleagues with several modifications.19) Briefly, 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) (Avanti Polar Lipids Inc., Alabaster, AL, U.S.A.) and cholesterol (Nacalai Tesque, Kyoto, Japan) were dissolved in chloroform at a molar ratio of 1 : 1. After the mixture was dried by evaporation and vacuum desiccated, the resultant lipid film was hydrated for 30 min at 70°C in a sterile 5% glucose solution under mechanical agitation. Cationic liposome/pDNA complexes were prepared by gently mixing with an equal volume of cationic liposomes and pGL4.50 [luc2/CMV/Hygro] Vector (Promega, Madison, WI, U.S.A.) at various charge ratios. The particle size and ζ-potential of the complexes were determined by a Zetasizer Nano ZS instrument (Malvern Instrument, Worcestershire, U.K.).
Preparation of Caco-2 and MDCK Cell MonolayersCaco-2 cells were seeded in 12-well culture plates at a density of 2×105 cells/3.8 cm2. For transport experiments, Caco-2 cells were seeded in a Transwell® with a 0.4-µm Pore Polycarbonate Membrane insert (Costar, Bedford, MA, U.S.A.) at a density of 5×104 cells/1.12 cm2. Cells reached confluency after 7 d and were cultured for a further 7 d. The culture medium was replaced every 48 h. MDCK-I and MDCK-II cells were also seeded in 12-well culture plates at the same density as Caco-2 cells. MDCK cells cultured for 4 d reached confluency.
Uptake Experiments of Cationic Liposome/pDNA ComplexesAfter preparation of Caco-2 and MDCK cell monolayers, the culture medium was replaced with 900 µL Opti-MEM I (Gibco BRL, Grand Island, NY, U.S.A.), and the cells were incubated for 30 min at 37°C with 5% CO2. Then, 100 µL Opti-MEM I containing naked pDNA or cationic liposome/pDNA complexes (1 µg pDNA/well) were added to the cells, followed by incubation for 30, 60 and 120 min with or without mechanical agitation using a water bath shaker (TAITEC, Saitama, Japan). After shaking, the medium was replaced with DMEM, and the cells were incubated for an additional 24 h at 37°C with 5% CO2. The cells were suspended in lysis buffer (0.05% Triton X-100, 2 mM ethylenediaminetetraacetic acid (EDTA), and 0.1 M Tris, pH 7.8) and centrifuged at 10000×g for 10 min at 4°C. The supernatant was mixed with luciferase assay buffer (Picagene, Tokyo Ink, Tokyo, Japan) and incubated for 30 min. Luciferase activity was then measured with an SH-8100 microplate reader (Corona Electric Co., Ltd., Ibaraki, Japan).
Quantitative RT-PCRAt 2 h after shaking, total RNA was isolated from Caco-2 cells using a GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich, St. Louis, MO, U.S.A.). Reverse transcription of mRNA was performed using ReverTra Ace® (Toyobo Co., Ltd., Osaka, Japan). The detection of cDNA (c-fos, c-jun, and glyceraldehyde-3-phosphate dehydrogenase (gapdh)) was conducted by real-time RT-PCR using SYBR® Premix Ex Taq (TaKaRa Bio Inc., Shiga, Japan) and a StepOne Real-Time PCR System (Applied Biosystems., Foster City, CA, U.S.A.). The primers for c-fos, c-jun, and gapdh were as follows: c-fos, 5′-CGT CTC CAG TGC CAA CTT CA-3′ (forward) and 5′-GGT CCG GAC TGG TCG AGA T-3′ (reverse); c-jun, 5′-CGG AGA GGA AGC GCA TGA-3′ (forward) and 5′-TTC CTT TTT CGG CAC TTG GA-3′ (reverse); gapdh, 5′-CCA TCA CCA TCT TCC AGG AG-3′ (forward) and 5′-CCT GCT TCA CCA CCT TCT TG-3′ (reverse).20)
Measurement of Intracellular Phosphorylated Nuclear Factor-kappaB (NF-κB) p65Caco-2 cells were collected and suspended in lysis buffer at 2 h after shaking. After centrifugation at 10000×g for 10 min at 4°C, the protein concentration in the supernatant was measured using a Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific K.K., Kanagawa, Japan). The amounts of phosphorylated NF-κB p65 in the supernatant were measured using a PathScan® Phospho-NF-κB p65 (Ser536) Sandwich ELISA Kit (Cell Signaling Technology Japan, K.K., Tokyo, Japan) according to the recommended procedures.
Transport Experiments with a Caco-2 Cell Monolayer Using TranswellsAt 24 h before the transport experiments were carried out, RAW264 cells were seeded in 12-well culture plates at a density of 4×105 cells/3.8 cm2. A Transwell insert was placed in the 12-well plate containing RAW264 cells. The culture media in apical and basolateral sides of the insert were replaced with 100 and 600 µL Opti-MEM I (Gibco BRL), respectively. After incubation of the cells for 30 min at 37°C with 5% CO2, 100 µL Opti-MEM I containing cationic liposome/pDNA complexes (5 µg pDNA/well) were added in the apical side. After 30 min of shaking, the medium was replaced with DMEM, and the cells were incubated for 24 h at 37°C with 5% CO2. Then, Caco-2 or RAW264 cells were suspended in lysis buffer and centrifuged at 10000×g for 10 min at 4°C. The supernatant was mixed with luciferase assay buffer and incubated for 30 min. Luciferase activity was then measured with the SH-8100 microplate reader.
Statistical AnalysisResults are presented as the mean±standard deviation (S.D.) of four experiments. ANOVA was used to test the statistical significance of differences between groups. Two group comparisons were performed with the Student’s t-test. Multiple comparisons between control groups and other groups were performed with the Dunnett’s test.
It has been reported that agitation or stirring reduces the thickness of the UWL on the surface of a Caco-2 cell monolayer.21–24) Based on these reports, we first evaluated the efficiency of pDNA transfection by cationic liposomes into a Caco-2 cell monolayer under mechanical agitation using a shaker. In this study, we prepared cationic liposomes consisting of DOTAP and cholesterol. DOTAP is one of the most frequently used cationic lipids to prepare cationic liposomes.25–27) The particle size and ζ-potential of cationic liposome/pDNA complexes (charge ratio at 1.0 : 2.3) used in this study were 516.8±18.5 nm and 53.1±3.8 mV, respectively (Table 1). As shown in Fig. 1A, the expression level of luciferase after pDNA transfer using cationic liposomes was increased significantly in a shaking rate-dependent manner, whereas luciferase activity in Caco-2 cells after naked pDNA transfection was unchanged with or without mechanical agitation. In addition, while the gene expression of cationic liposome/pDNA complexes without shaking was increased time-dependently, the level of luciferase expression under shaking had already reached a peak when Caco-2 cells were incubated with cationic liposome/pDNA complexes for 30 min (Fig. 1B).
| Cationic liposome/pDNA charge ratio | Particle size (nm) | ζ-Potential (mV) |
|---|---|---|
| 1.0 : 1.5 | 488.3±9.0 | 51.8±4.7 |
| 1.0 : 2.3 | 516.8±18.5 | 53.1±3.8 |
| 1.0 : 3.1 | 532.1±12.2 | 58.4±2.8 |
| 1.0 : 4.5 | 520.9±7.1 | 59.2±4.1 |
Each value represents the mean±S.D. (n=3).
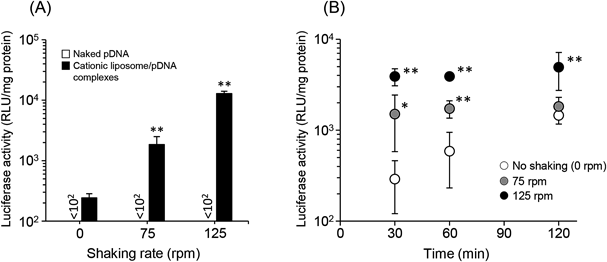
(A) Luciferase expression of naked pDNA and cationic liposome/pDNA complexes (charge ratio at 1.0 : 2.3) in Caco-2 cells. Culture plates were shaken for 30 min at 0, 75, or 125 rpm using a water bath shaker. Each value represents the mean+S.D. (n=4). ** p<0.01 compared with no shaking (0 rpm). (B) Luciferase expression in Caco-2 cells at 24 h after incubation of the cells with cationic liposome/pDNA complexes (charge ratio at 1.0 : 2.3) for 30, 60, and 120 min with or without mechanical agitation. Each value represents the mean±S.D. (n=4). * p<0.05; ** p<0.01 compared with the corresponding group without shaking (0 rpm).
Mechanical stimuli, such as ultrasound,28) electric pulse,29) and physical pressure,30) are known to enhance gene expression via transcriptional activation involving molecules such as activator protein-1 (AP-1) and NF-κB. Therefore, we examined whether shaking affected the mRNA expression of c-fos and c-jun, which are components of AP-1, in Caco-2 cells. As shown in Figs. 2A and B, c-fos/c-jun mRNA expression in Caco-2 cells was unchanged with or without shaking. In addition, we investigated the effect of shaking on the amount of phosphorylated p65, which is a component of NF-κB, in Caco-2 cells. As a result, the intracellular amount of phosphorylated p65 was not increased by shaking (Fig. 2C). These results indicate that mechanical agitation does not activate the transcriptional processes involved in AP-1- and NF-κB-mediated pathways.
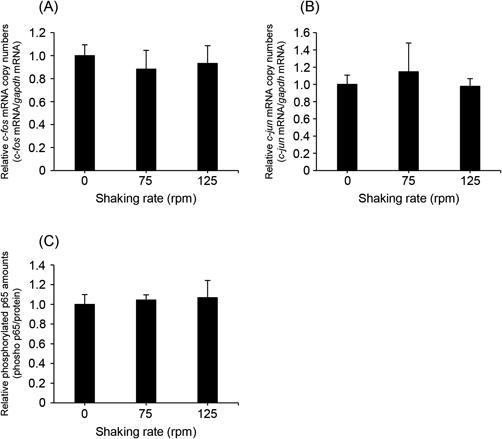
Culture plates were shaken for 120 min at 0, 75, or 125 rpm using a water bath shaker. Each value represents the mean+S.D. (n=4).
We next investigated the effect of the charge ratio (+/−) of cationic liposome/pDNA complexes on gene expression under mechanical agitation. The physicochemical properties of each complex are shown in Table 1. As shown in Fig. 3, the enhancement of luciferase expression by shaking was observed at all charge ratios of the complexes. The charge ratio at 1.0 : 2.3 resulted in the highest gene expression, whereas the lowest luciferase activity was observed at the charge ratio at 1.0 : 4.5.
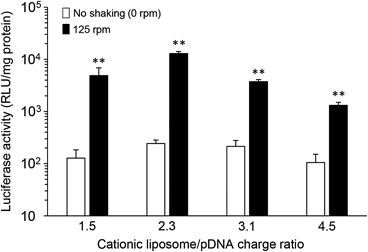
Culture plates were shaken for 30 min at 0 or 125 rpm using a water bath shaker. Each value represents the mean+S.D. (n=4). ** p<0.01 compared with the corresponding group without shaking (0 rpm).
In addition to the transfection experiments using cell culture plates, the transport of cationic liposome/pDNA complexes across a Caco-2 cell monolayer was investigated using Transwells. In this experiment, Caco-2 cells were seeded in the Transwell insert, and RAW264 cells were cultured in the bottom of plate (Fig. 4A). When the complexes (charge ratio at 1.0 : 2.3) were added to the insert and incubated under conditions of shaking, luciferase expression was increased significantly in Caco-2 cells. On the other hand, luciferase expression was not observed in the underlying RAW 264 cells (Fig. 4B).

(A) Schematic representation of the method for transport experiments using Transwells. (B) Luciferase expression of cationic liposome/pDNA complexes (charge ratio at 1.0 : 2.3) in Caco-2 and RAW264 cells. Culture plates with Transwell inserts were shaken for 30 min at 0, 75, or 125 rpm using a water bath shaker. Each value represents the mean+S.D. (n=4). ** p<0.01 compared with no shaking (0 rpm).
In addition to Caco-2 cells, MDCK cells have been used in absorption and permeability studies.8,9,15–18) Therefore, we assessed the transfection efficiency of pDNA into MDCK-I and MDCK-II cells using cationic liposomes (charge ratio at 1.0 : 2.3) under mechanical agitation. The expression levels of luciferase in both MDCK-I and MDCK-II cells were significantly high when the cells were agitated during transfection (Fig. 5). Furthermore, the increase in gene expression levels by shaking was lower in MDCK cells than in Caco-2 cells.

Luciferase expression of naked pDNA and cationic liposome/pDNA complexes (charge ratio at 1.0 : 2.3) in MDCK-I (A) and MDCK-II (B) cells. Culture plates were shaken for 30 min at 0, 75, or 125 rpm using a water bath shaker. Each value represents the mean+S.D. (n=4). ** p<0.01 compared with no shaking (0 rpm).
The intestinal epithelium is covered with mucus that forms and maintains the UWL.27–29) To transfer pDNA into intestinal epithelial cells using cationic liposomes and further penetrate below the epithelium, these complexes must proceed via the UWL.31–33) Hence, to develop oral pDNA delivery systems, it is vital to reveal the effect of the UWL on the permeability and transfection efficiency of cationic liposome/pDNA complexes into epithelial cells.
It has been estimated by an in vitro study that the thickness of the UWL on the surface of the intestinal epithelium is much larger than that under in vivo physiological conditions,11,13) because of the peristaltic movement of the intestine, which effectively reduces the UWL. Several in vitro studies indicate that mechanical agitation, such as magnetic stirring, shaking, or air bubbling, reduces the thickness of the UWL.21–24) Naruhashi et al. have also shown that the results of drug permeability obtained from in vitro experiments under agitation are well correlated with those from in vivo experiments.22) Based on these reports, we performed mechanical agitation using a shaker during in vitro transfection of pDNA via cationic liposomes to evaluate the effect of the UWL on cationic liposome permeability in a Caco-2 cell monolayer.
As shown in Fig. 1, the efficiencies of pDNA transfection via DOTAP/cholesterol cationic liposomes were much higher when mechanical agitation was applied during transfection. These results suggest that the UWL restricts access of cationic liposome/pDNA complexes to the surface of a Caco-2 cell monolayer. Similar results were obtained in transfection experiments of pDNA into Caco-2 cells using other cationic liposomes, such as 1,2-di-O-octadecenyl-3-trimethylammonium propane (DOTMA)/cholesterol liposomes and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)/DC-cholesterol liposomes (Fig. S1). These observations indicate that the transfection efficiency of pDNA by cationic liposomes is greatly affected by mechanical agitation, regardless of the lipid composition of cationic liposomes. It is well known that the transepithelial transport of lipophilic compounds is greatly affected by a UWL.21–24) Yano et al. showed that the transport of heptyl paraben across a Caco-2 cell monolayer is the most affected by stirring compared with other shorter chain alkylparabens (butyl, amyl, and hexyl parabens).24) Because cationic liposomes are composed of a cationic lipid (DOTAP) and cholesterol, it is conceivable that cationic liposomes are also poorly diffusable across a UWL.
In addition, we found that luciferase expression had already reached a plateau when Caco-2 cells were incubated with cationic liposome/pDNA complexes for 30 min (Fig. 1B). This observation is similar to the result concerning the uptake of cationic liposomes by Caco-2 cells under shaking conditions (Fig. S2). We speculate that these phenomena occur because of the saturation of cationic liposome/pDNA complexes adhered on the surface of the Caco-2 cell monolayer. Because the particle size of the cationic liposomes used in this study is relatively large (approximately 500 nm), a large proportion of the surface of the Caco-2 cell monolayer would be covered by cationic liposome/pDNA complexes at an early stage of the uptake experiment (within 30 min). These adhered complexes on the cell surface appear to prevent further adhesion and internalization of additional complexes. This effect may be why the transfection efficiency meditated by cationic liposome/pDNA complexes had already reached a peak at 30 min after the start of shaking and did not increase at 60 or 120 min. Un et al. have also demonstrated that cellular uptake of large bubble cationic liposomes/pDNA complexes (particle size: 550 nm; ζ-potential: 47.9 mV) reaches a plateau at 30 min after the start of uptake.34) This result is consistent with our present observation.
We speculate that mechanical agitation influence pDNA transfection efficiency by cationic liposomes via two mechanisms. First, shaking enhances the cellular internalization of cationic liposome/pDNA complexes by reducing the interaction of the complexes with the UWL. Second, shaking facilitates gene expression in cells via transcriptional activation. To confirm that mechanical agitation enhances the gene expression of cationic liposome/pDNA complexes by a reduction in the thickness of the UWL, it is necessary to evaluate the effects of shaking on transcriptional processes. Mechanical stimulation has been reported to promote gene expression by activation of several transcription factors. Un et al. demonstrated that ultrasound exposure induces activator protein-1 (AP-1) and NF-κB, resulting in enhancement of gene expression.28) In addition, Pazmany et al. reported that an electric pulse activates several transcription factors such as AP-1 and Sp1.29) Therefore, we evaluated the activation of transcription factors AP-1 and NF-κB in Caco-2 cells. As shown in Fig. 2, both the mRNA expression of c-fos/c-jun and the amount of phosphorylated p65 in Caco-2 cells were unchanged by shaking, indicating that shaking did not affect transcriptional processes in Caco-2 cells. These results suggest that the stimulus intensity of mechanical agitation in cells is milder than that of conventional mechanical stimuli such as ultrasound and electric pulsing. Based on these observations, it is reasonable that mechanical agitation enhances the gene expression of cationic liposome/pDNA complexes by reducing the interaction of the UWL with the complexes, not induction of transcriptional activation.
We also demonstrated augmentation of the luciferase expression of cationic liposomes/pDNA complexes by mechanical agitation, regardless of their charge ratio (Fig. 3). On the other hand, the lowest luciferase activity was observed at the charge ratio at 1.0 : 4.5. Sakurai et al. reported that a high charge ratio (+/−) restricts the release of pDNA from complexes in cells, resulting in low transfection efficiency.35) Considering these findings, the internalization of cationic liposome/pDNA complexes (charge ratio at 1.0 : 4.5) into Caco-2 cells is likely to increase by reducing the UWL thickness under mechanical agitation. However, effective gene expression was not achieved because of the limitation of the release of pDNA from the complexes.
In addition to the transfection efficiency of pDNA via cationic liposomes into Caco-2 cells, we attempted to clarify the characteristics of cationic liposome transport across a Caco-2 cell monolayer. To evaluate the effects of mechanical agitation on the transport of cationic liposome/pDNA complexes, we used a co-culture model that mimicked the intestinal epithelium (Fig. 4A). A significant increase in the luciferase expression of Caco-2 cells was induced by shaking, whereas luciferase activity in the underlying RAW264 cells was not detected with or without shaking (Fig. 4B). This result suggests that cationic liposome/pDNA complexes are internalized by Caco-2 cells, while transcytosis does not occur. Bannunah et al. reported that positively charged nanoparticles are effectively taken up by Caco-2 cells, but transepithelial transport hardly occurs.36) This observation is in accordance with our results. For cationic liposomes to traverse across a Caco-2 cell monolayer, it would be necessary to modify the physicochemical properties of the liposomes, such as the particle size and surface charge.36–38)
Similar to Caco-2 cells, MDCK cells form a monolayer, differentiate into columnar epithelium with a brush border, and form tight junctions.8,9) Therefore, an MDCK cell monolayer has also been used as an in vitro model of the intestinal epithelium. There are two MDCK cell lines, MDCK-I and MDCK-II. MDCK-I cells form a tight monolayer and exhibit high transepithelial electrical resistance, whereas the MDCK-II cell monolayer is leaky.8,39) In the present study, we investigated the effect of agitation on the transfection efficiencies of pDNA via cationic liposomes into MDCK-I and MDCK-II cell monolayers. As shown in Fig. 5, an increase in luciferase expression was induced by shaking in both MDCK-I and MDCK-II cells. This result indicates that both MDCK-I and MDCK-II cell monolayers form a UWL on their surface, and mechanical agitation reduces the thickness of the UWL, resulting in augmentation of the contact between cationic liposome/pDNA complexes and the MDCK cell surface. On the other hand, the effect of shaking on the luciferase expression in MDCK cells was much less than that in Caco-2 cells. This observation suggests that the effect of the UWL on the uptake of cationic liposome/pDNA complexes into Caco-2 cells is greater than that in MDCK cells.
In conclusion, we demonstrated that a UWL greatly restricts the uptake of cationic liposome/pDNA complexes by Caco-2 and MDCK cells in vitro. This effect of the UWL can be mitigated by mechanical agitation using a shaker during transfection.
This study was supported in part by a Grant from the Strategic Research Foundation Grant-aided Project for Private Universities, a Grant-in-Aid for Young Scientists (B) (Grant Number 15K18851) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Uehara Memorial Foundation, the Ritsumeikan Global Innovation Research Organization (R-GIRO) project at Ritsumeikan University, and the Program for Research of Young Scientists from Ritsumeikan University.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.