2017 年 40 巻 10 号 p. 1716-1723
2017 年 40 巻 10 号 p. 1716-1723
β-Adrenergic receptor (β-AR)-induction of collagen-I synthesis is partially mediated by the cardiac mineralocorticoid receptor (MR) system. However, it remains unclear whether the selective MR antagonist, eplerenone, inhibits collagen-I synthesis induced by β-AR stimulation. We investigated the effects of eplerenone on the responses to a non-selective β-AR agonist, isoproterenol, which induced collagen-I synthesis in primary cardiac fibroblasts (CFs) and the left ventricle. mRNAs encoding the MR and 11β-hydroxysteroid dehydrogenase type I (11β-HSD1) were evident in the left ventricle and primary CFs. mRNAs encoding the CYP family 11 subfamily B member 2 (CYP11-B2) were not detected, even after isoproterenol treatment. In vivo, isoproterenol induced collagenous fiber accumulation in the left ventricle. The phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2), 11β-HSD1 levels, and mRNA/protein levels of collagen-I increased upon exposure to isoproterenol, but these increases were inhibited by eplerenone co-treatment. In primary CFs, isoproterenol increased the phosphorylation of ERK1/2 and the expression levels of both 11β-HSD1 and collagen-I; these isoproterenol-attributable effects were inhibited by co-treatment with eplerenone and PD98059, a specific inhibitor of mitogen-activated protein kinase/ERK kinase activity. The results suggest that 11β-HSD1 but not CYP11-B2 is expressed in primary CFs. Eplerenone inhibited isoproterenol-induced ERK1/2 phosphorylation and expression of 11β-HSD1 and collagen-I in primary CFs, as well as the progression of cardiac fibrosis in the left ventricle. Therefore, eplerenone inhibited the isoproterenol-induced increases in 11β-HSD1 and collagen-I expression in primary CFs, and progression of cardiac fibrosis in the left ventricle.
Neurohumoral activation is associated with the development of cardiac fibrosis, which culminates in heart failure.1–3) It is thought that stimulation of the β-adrenergic receptor (β-AR) and the mineralocorticoid receptor (MR) independently trigger collagen-I accumulation in the ventricles, and ultimately, cardiac fibrosis.4–6) Of the various extracellular matrix components associated with fibrosis, collagen-I is thought to be the principal candidate of the extracellular matrix in the diseased heart.
Interestingly, β-AR and MR interact in a complex manner; their interaction extends beyond establishment of the pathology of cardiac fibrosis. For example, transgenic mice exhibiting sympathetic hyperactivity have elevated plasma aldosterone levels.7) The non-selective β-AR agonist isoproterenol increases aldosterone secretion in adrenal capsules, but this increase is potently inhibited by β-AR antagonists.8,9) In addition, chronic β-AR stimulation induces cardiac fibrosis (including collagen-I accumulation) in rats, which is inhibited by a non-specific MR antagonist.6,7,10,11)
Eplerenone is an aldosterone antagonist that is more specific for the MR than spironolactone, and exhibits low affinities for the progesterone, androgen, and glucocorticoid receptors.12) In several models of heart failure, eplerenone inhibits the progression of cardiac dysfunction and fibrosis, and reduces both the collagen content and the expression of collagen-I encoding mRNA in the left ventricle.13–18) Similarly, the nonspecific MR antagonists spironolactone and canrenoate inhibit cardiac fibrosis induced by β-AR stimulation in rats.4,10) However, it remains unclear whether the selective MR antagonist eplerenone inhibits collagen-I synthesis induced by β-AR stimulation in primary cardiac fibroblasts (CFs), and/or cardiac fibrosis in ventricles.
The CYP family 11 subfamily B member 2 (CYP11-B2) is an aldosterone synthase that acts on 18-hydroxycorticosterone as a substrate.19) mRNAs encoding CYP11-B2 are expressed in the rat heart, and increases in the failing heart.15) However, CYP11-B2-encoding mRNAs are not found in the hearts of certain rat models of heart disease.20,21) Thus, any cardiac expression of CYP11-B2 remains controversial. Furthermore, as corticosterone binds to the rodent MR, corticosterone synthesized by 11β-hydroxysteroid dehydrogenase type I (11β-HSD1) may activate the MR.5,20,21) However, it remains unclear whether 11β-HSD1 is expressed in rat primary CFs, and whether β-AR stimulation plays any significant role in controlling CYP11-B2 or 11β-HSD1 expression in either CFs or the left ventricle.
In this study, we determined if β-AR stimulation affected expression of CYP11-B2 and 11β-HSD1 in the left ventricle and primary CFs. We also examined whether eplerenone influenced collagen-I synthesis in the left ventricle and primary CFs after β-AR stimulation.
This study followed the Guidelines for Institutional Laboratory Animal Care and Use of the School of Veterinary Medicine at Kitasato University, Japan (approval number: 10-024). This study was conducted in accordance with the experimental animal guidelines of the Japanese Ministry of Education, Culture, Sports, Science and Technology. Male Wistar Kyoto rats (4 weeks old) were obtained from the Charles River Lab (Yokohama).
ChemicalsAldosterone, (−)-isoproterenol, and eplerenone were purchased from Sigma-Aldrich (MO, U.S.A.). PD98059 was purchased from Calbiochem (La Jolla, CA, U.S.A.).
In Vitro StudyPrimary cultures of CFs from 4-week-old male Wistar Kyoto rats were established as described previously.22) Briefly, the left ventricle was excised, minced, and then added to a collagenase/protease digestion solution (Wako Pure Chemical Industries, Ltd., Osaka). After centrifugation, the cell suspension was transferred to cell culture flasks and the cells were incubated with Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich) supplemented with 10% foetal bovine serum and 1% antibiotic–antimycotic solution (penicillin, streptomycin, and amphotericin B; Sigma-Aldrich) under standard cell culture conditions (37°C, 5% CO2). Fibroblasts were easily characterised due to their spindle-shaped morphology. The cultures were >95% CFs, immunostaining strongly for vimentin and collagen-I, weakly for smooth muscle α-actin, and not at all for CD31.
After two or three passages (to >80% confluence), the cells were plated in 6-well plates at a density of 3.0×104 cells/well in DMEM with 10% (v/v) fetal bovine serum. After 24 h incubation, the medium was replaced with serum-free DMEM containing a 1% (w/v) antibiotic-antimycotic solution. Incubation continued for 48 h after which the cells were used in the experiments. Dimethyl sulfoxide (DMSO) was used to dissolve aldosterone, eplerenone, and PD98059; the final concentration per assay was <0.1%. Isoproterenol was dissolved in DMEM. All of the test chemicals were immediately mixed with serum-free DMEM prior to treatment.
In Vivo StudyRats were divided into three groups: placebo, isoproterenol, and isoproterenol-plus-eplerenone (n=6 for each group). Isoproterenol was dissolved in 0.9% (w/v) saline and administered intraperitoneally (i.p.) at 4.5 mg/kg/d for 3 d.23) Control rats were given 0.9% (w/v) saline alone. Simultaneously, eplerenone dissolved in DMSO was given orally (100 mg/kg/d) to rats in the isoproterenol-plus-eplerenone group.24) DMSO served as the vehicle control for both the placebo and isoproterenol groups.
After 3 d of treatment, the rats were euthanized via anesthesia with pentobarbital (60 mg/kg i.p.) and the hearts were removed. The whole hearts and left ventricles were weighed (yielding HWs and LVWs) to determine relevant mass indices (the HW/body weight and LVW/body weight ratios). The heart was cut into coronal sections at the level of the papillary muscles; the base of the heart was fixed with 10% paraformaldehyde for histopathological analysis, and the apex was plunged into liquid nitrogen for Western blotting and PCR.
Histopathological StudyTransverse sections (4 µm) were cut and stained with picrosirius red and Azan stain using routine methods. Each stained left ventricular section was examined under ×200 or ×400 magnification using a light microscope. All of the images were imported into NIH Image software (National Institutes of Health, Bethesda, MD, U.S.A.) for quantitative analysis. A minimum of 10 fields from each left ventricular section at 200× magnification were stored. The percentage of left ventricular fibrosis was calculated by determining the total fibrotic region/total area. A minimum of 100 cells from each section was examined at 400× magnification. The myocyte cross-sectional area of each cell was measured by computed planimetry.
Western Blot AnalysisLeft ventricles were homogenised or primary CFs were harvested in lysis buffer. Protein extracts (4 µg) were separated on 10% sodium dodecyl sulfate-polyacrylamide gels, and the proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon RP, Millipore, Bedford, MA, U.S.A.). The membranes were incubated with primary monoclonal antibodies: anti-collagen-I, 1 : 500 (Rockland Immunochemicals, Gilbertsville, PA, U.S.A.); anti-phospho-p44/42 extracellular signal-regulated kinase (ERK1/2) and anti-p44/42 ERK1/2, each 1 : 1000 (all from Cell Signaling Technology, Danvers, MA, U.S.A.); anti-11β-HSD1 antibody, 1 : 500 (abcam, Tokyo, Japan); and anti-β-actin, 1 : 1000 (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.). The membranes were subsequently incubated with horseradish peroxidase-labelled secondary antibody (Santa Cruz Biotechnology) at a dilution of 1 : 10000–20000. The proteins were visualised with peracid and luminol (ECL Western blotting detection reagents; GE Healthcare, Piscataway, NJ, U.S.A.). Protein expression was quantified using Alpha Ease FC (Alpha Innotech Corp., San Leandro, CA, U.S.A.).
PCRThe TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.A.) was used to extract DNA-free total RNA from the left ventricles or CFs, according to the manufacturer’s protocol. First-strand cDNA was synthesised by reverse transcription with 1.0 µg total RNA per 13 µL reaction mixture using the Transcript First-Strand cDNA synthesis kit (Roche Diagnostics, Tokyo, Japan) with random primers.
RT-PCR was performed using the Go Taq Green Master Mix (Promega, Madison, U.S.A.) in a PC-801 machine (ASTEC, Fukuoka, Japan). PCR products were separated via 1.5% agarose gel electrophoresis and visualised under UV illumination following ethidium bromide staining.
For Real-Time PCR, StepOnePlus Real-Time PCR system (Applied Biosystems by Life Technologies, Austin, TX, U.S.A.) was used to amplify and quantify cDNA. The SYBR Green PCR Master Mix (Applied Biosystems) was used in all reactions, and regular relative standard curves were plotted. Relative mRNA levels were calculated based on glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression. The primer sequences for RT-PCR and real-time PCR are provided in Table 1.
| Gene Bank | Forward primer | Reverse primer | Product length | |
|---|---|---|---|---|
| RT-PCR | ||||
| MR | M36074.1 | 5′-TCTGTGAGAGATGCCGAGTACAC-3′ | 5′-GACCTTGAGCCTCTGTTTTCGAC-3′ | 413 bp |
| CYP11-B2 | U14908.1 | 5′-GGGCCAAGAGAACCTACACC-3′ | 5′-TGGATGAACTTCAGGCTACCAG-3′ | 498 bp |
| 11β-HSD1 | BC078865.1 | 5′-AGCCCATGTGGTATTGACTG-3′ | 5′-AGGACACAGAGAGTGATGGA-3′ | 471 bp |
| 11β-HSD2 | BC087023.1 | 5′-CAGAGGATATCAGCCGTGTT-3′ | 5′-TAGTCACTGCCTCTGTCTTG-3′ | 390 bp |
| GAPDH | BC087743.1 | 5′-GCCATCACTGCCACTCAGAAG-3′ | 5′-GTGGTCCAGGGTTTCTTACTCC-3′ | 486 bp |
| Real time-PCR | ||||
| Collagen-I | NM_053304 | 5′-ATCAGCTGGAGTTTCCGT-3′ | 5′-TTCCCCATCATCTCCGTT-3′ | 190 bp |
| 11β-HSD1 | BC078865.1 | 5′-AAGGTCAACGTGTCCATCACTC-3′ | 5′-TTGCGCAGAACTGTGCCTTT-3′ | 146 bp |
| 11β-HSD2 | BC087023.1 | 5′-CAGTGGTAACTTTCCGCGAATG-3′ | 5′-AGCAGTGCAATAGCTGCCTT-3′ | 184 bp |
| GAPDH | BC087743.1 | 5′-AGCGGCATCTTCTTGTGCAGT-3′ | 5′-GGTCGTTGATGGCAACAATGTCC-3′ | 144 bp |
CYP11-B2, CYP family 11 subfamily B member 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MR, mineralocorticoid receptor; 11β-HSD1, 11β-hydroxysteroid dehydrogenase type I; 11β-HSD2, 11β-hydroxysteroid dehydrogenase type II.
All numerical data are expressed as means±standard error of the mean (S.E.M.) Statistical analyses among three or four groups were performed using one-way ANOVA followed by Tukey’s multiple comparison test. Comparisons between the two groups were performed using the Student’s t-test. Differences were considered significant at p<0.05.
mRNAs encoding the MR, CYP11-B2, 11β-HSD1, and 11β-HSD2 were sought by RT-PCR (Fig. 1). MR-encoding mRNAs were detected in the left ventricle and the CFs. Although CYP11-B2 mRNA was detected in the adrenal gland (a positive control), it was not detected in the left ventricle or CFs. By contrast, mRNAs encoding 11β-HSD1 and 11β-HSD2 were detected in the left ventricle and CFs.

mRNA was detected by RT-PCR in the rat left ventricle and cardiac fibroblasts; the adrenal gland served as the positive control (n=3). AG, adrenal gland; CF, cardiac fibroblasts; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LV, left ventricle; M, molecular marker.
To explore whether β-AR stimulation affected the expression of CYP11-B2, 11β-HSD1, and 11β-HSD2, we performed real-time PCR on material from the left ventricles and CFs that treated (or not) with isoproterenol. Even after isoproterenol treatment, CYP11-B2-encoding mRNA was not detected in either the left ventricle or the CFs (data not shown). By contrast, the levels of 11β-HSD1-encoding mRNA significantly increased after isoproterenol treatment in both the left ventricle and the CFs (Fig. 2). Although the level of 11β-HSD2-encoding mRNA in the left ventricle was unchanged after isoproterenol treatment, the level in CFs fell significantly after such treatment.
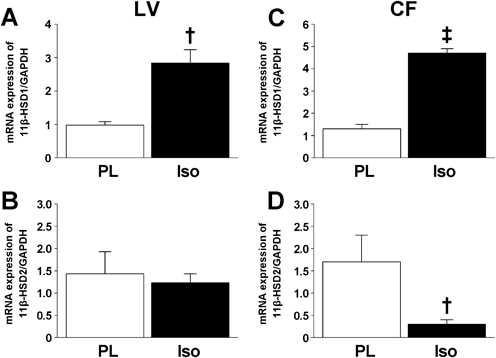
Quantification of mRNAs encoding 11β-HSD1 and 11β-HSD2 in the rat left ventricle (A and B; n=4) and CFs (C and D; n=3). In the in vivo study, isoproterenol (4.5 mg/kg/d) or 0.9% (w/v) saline was injected intraperitoneally for 3 d. In the in vitro study, the cells were incubated with or without 10−5 M isoproterenol for 24 h. CF, cardiac fibroblasts; LV, left ventricle; PL, vehicle control; Iso, isoproterenol. † p<0.01 vs. vehicle control, ‡ p<0.001 vs. vehicle control.
To explore the in vivo effects of eplerenone, rats were treated with isoproterenol and/or eplerenone for 3 d. Compared to the placebo, isoproterenol significantly increased the HW/body weight (BW) ratio and the LVW/BW ratio (Table 2). Co-treatment with eplerenone inhibited these effects. Histopathologically, isoproterenol increased the numbers of collagenous fibers, and induced myocyte hypertrophy, in the left ventricle (Figs. 3A–F). The fibrotic area and the myocyte cross-sectional area were significantly increased by isoproterenol treatment compared to the placebo (Figs. 3G, H). In contrast, co-treatment with eplerenone significantly decreased the isoproterenol-induced these effects. Finally, isoproterenol triggered significant ERK1/2 phosphorylation and 11β-HSD1 expression, both of which were markedly decreased upon co-treatment with eplerenone (Figs. 4A–C). Similarly, isoproterenol significantly increased collagen-I mRNA/protein expression, which was significantly inhibited by co-treatment with eplerenone (Figs. 4A, D, E).
| PL | Iso | Iso+Epl | |
|---|---|---|---|
| n | 6 | 5 | 5 |
| BW (g) | 103±4 | 105±3 | 105±2 |
| HW/BW (mg/g) | 3.9±0.1 | 4.7±0.2‡ | 4.3±0.1 |
| LVW/BW (mg/g) | 2.6±0.1 | 3.3±0.1‡ | 2.9±0.1 |
BW, body weight; HW/BW, ratio of the heart weight to the body weight; Iso, isoproterenol; Iso+Epl, isoproterenol plus eplerenone; LVW/BW, ratio of the left ventricular weight to the body weight; PL, placebo. Data are presented as means±S.E.M. ‡ p<0.001 vs. PL.

Representative left ventricular sections of the placebo (A and D, n=6), isoproterenol (B and E, n=5), and isoproterenol-plus-eplerenone groups (C and F, n=5) are shown. The upper panels (A–C) were stained with Picrosirius Red and examined at 200× magnification (scale bars=100 µm). The bottom panels (D–F) were stained with Azan and were examined at 400× magnification (scale bars=50 µm). Comparison of left ventricular fibrotic area and myocyte cross-sectional area among the groups are shown (G and H). Data are presented as the mean±S.E.M. Iso, isoproterenol; Iso+Epl, isoproterenol plus eplerenone; MCSA, myocyte cross-sectional area; PL, placebo. ‡ p<0.001 vs. vehicle control, # p<0.001 vs. isoproterenol.

Representative Western blot images showing ERK1/2, 11β-HSD1, collagen-I, and β-actin levels in the left ventricle (A). Quantification of the levels of phosphorylated ERK1/2 and 11β-HSD1 in the left ventricle (B and C, respectively). Quantification of the levels of mRNA encoding collagen-I, and the protein expression levels, in the left ventricle (D and E, respectively). Data are presented as the mean±S.E.M. AU, arbitrary units; Iso, isoproterenol; Iso+Epl, isoproterenol plus eplerenone; pERK1/2, phosphorylated ERK1/2; PL, placebo; tERK1/2, total tERK1/2. † p<0.01 vs. PL, ‡ p<0.001 vs. PL, § p<0.05 vs. Iso, # p<0.01 vs. Iso.
The aldosterone/MR signaling pathway plays a significant role in fibroblast collagen-I synthesis.17,25,26) To explore whether eplerenone affected aldosterone-induced collagen-I expression in primary CFs, the cells were treated with aldosterone and/or eplerenone. After pre-incubation with 10−5 M eplerenone for 2 h, the cells were incubated with or without 10−7 M aldosterone for 24 h. Aldosterone induced significant increases in the expression levels of collagen-I and phosphorylated ERK1/2 compared to those of vehicle-treated cells (Fig. 5); these effects were significantly attenuated by co-treatment with eplerenone.

Representative Western blot images and quantification of ERK1/2 phosphorylation (A) and collagen-I expression (B) in cell lysates. After pretreatment with 10−5 M eplerenone for 2 h, the cells were further incubated with or without 10−7 M aldosterone for 24 h. Data are presented as means±S.E.M., n=4 per group. AU, arbitrary units; pERK1/2, phosphorylated ERK1/2; tERK1/2, total tERK1/2. † p<0.01 vs. vehicle control, § p<0.01 vs. aldosterone.
Next, to determine whether eplerenone affected isoproterenol-stimulated collagen-I expression in primary CFs, the cells were treated with isoproterenol and/or eplerenone. After pre-incubation with 10−5 M eplerenone for 2 h, the cells were incubated with or without 10−5 M isoproterenol for 24 h. Compared to the vehicle control, the levels of ERK1/2 phosphorylation and 11β-HSD1 expression was significantly increased by isoproterenol, but was significantly attenuated by co-treatment with eplerenone (Figs. 6A, B). Similarly, isoproterenol-enhanced collagen-I expression was significantly attenuated by co-treatment with eplerenone (Fig. 6C).

Representative Western blot images and quantification of phosphorylated ERK1/2, 11β-HSD1, and collagen-I in cell lysates (A, B, and C, respectively). Following pretreatment with 10−5 M eplerenone for 2 h, the cells were incubated further with or without 10−5 M isoproterenol for 24 h. Data are presented as means±S.E.M., n=4 per group. AU, arbitrary units; pERK1/2, phosphorylated ERK1/2; tERK1/2, total tERK1/2. † p<0.01 vs. vehicle control, § p<0.01 vs. isoproterenol.
Because mitogen-activated protein kinase/ERK kinase is the upstream activator of ERK1/2, to determine whether isoproterenol-stimulated phosphorylation of ERK1/2 affected 11β-HSD1 expression in primary CFs, cells were incubated with or without 10−5 M PD98059 (Nagai Y.),17) a specific inhibitor of mitogen-activated protein kinase/ERK kinase activity, for 24 h. Isoproterenol-stimulated ERK1/2 phosphorylation was inhibited by simultaneous treatment with PD98059 (Fig. 7A). Moreover, isoproterenol-stimulated 11β-HSD1 expression and collagen-I expression were significantly attenuated by simultaneous treatment with PD98059 (Figs. 7B, C).

Representative Western blot images and quantification of phosphorylated ERK1/2, 11β-HSD1, and collagen-I in cell lysates (A, B, and C, respectively). After pretreatment with 10−5 M PD98059 for 2 h, the cells were further incubated with or without 10−5 M isoproterenol for 24 h. Data are presented as means±S.E.M., n=4 per group. AU, arbitrary units; pERK1/2, phosphorylated ERK1/2; tERK1/2, total tERK1/2. † p<0.01 vs. vehicle control, § p<0.01 vs. isoproterenol.
Previous studies have reported transregulation between the sympathetic nervous system and the renin angiotensin aldosterone system in cardiac remodeling. In vivo studies, increased sympathetic activity causes cardiac remodeling, which leads to concomitant activation of the cardiac angiotensin II synthesis.27,28) Further, β-AR and MR interact in a complex manner; their interaction extends beyond establishment of the pathology of cardiac fibrosis. Recent studies have shown that a non-selective MR antagonist inhibited cardiac fibrosis induced by β-AR stimulation in rats; β-AR stimulation-induced collagen-I and collagen-III accumulation was reduced and cardiac dysfunction was ameliorated.4,6,10,11) Thus, we speculate that specific MR blockade with eplerenone may have anti-fibrotic effects during β-AR-induced cardiac fibrosis. The novel findings in this study include the fact that eplerenone prevented isoproterenol-induced collagenous fiber accumulation and collagen-I expression. Furthermore, the increase in collagen-I expression induced by isoproterenol in primary CFs was attenuated by co-treatment with eplerenone. Together, our results indicate that β-AR activation stimulates collagen-I expression via the MR in primary CFs, which at least in part, contributes to progression of cardiac fibrosis in the left ventricle.
Aldosterone is synthesized by CYP11-B2 and contributes to various cellular activities by binding to the cytoplasmic MR. CYP11-B2 mRNA was found in neonatal rat cardiomyocytes29) and its expression was upregulated in failing hearts.15) However, cardiac expression of CYP11-B2 remains controversial; CYP11-B2 mRNA is not reproducibly detected in certain rat models of heart disease.20,21) Our observations confirm previous findings that showed that CYP11-B2 is not expressed in the heart, rendering it possible that aldosterone synthesis stimulated by β-AR is not reflected in the left ventricle and primary CFs.
It is likely that, in rodents, MR activation lies upstream of 11β-HSD1 transcription/translation, and that 11β-HSD1 upregulates corticosterone synthesis.5,20,21) mRNA encoding 11β-HSD1 is reportedly upregulated in the failing heart,20,30,31) and eplerenone inhibits such upregulation and the development of heart failure.20) However, the localization of 11β-HSD1 in primary CFs, and the regulation of 11β-HSD1 expression by β-AR in the heart remain unclear. The novel findings of this study include the demonstration that 11β-HSD1 mRNA/protein was expressed in primary CFs and the left ventricle. The isoproterenol-induced increases in this expression were inhibited by co-treatment with eplerenone and PD98059, consistent with the collagen-I expression. These results indicate that β-AR stimulation may upregulate 11β-HSD1 expression in both the left ventricle and primary CFs. In addition, MR and ERK1/2 signaling may, at least in part, contribute to the observed increases in such expression.
Corticosterone exhibits an affinity for the MR, and may stimulate MR activity via a positive feedback loop.20) Deoxycorticosterone increased the systolic blood pressure and induced cardiac fibrosis in rats, whereas eplerenone inhibited these pathological changes.32) Because our primary aim was to explore the effects of eplerenone on β-AR-stimulated collagen-I expression, further studies are necessary to clarify the signaling pathway through which 11β-HSD1 regulates collagen-I expression in CFs.
ERK1/2 phosphorylation contributes to the regulation of various cellular functions. It is commonly thought that the MR and β-AR contribute independently to ERK1/2 phosphorylation and extracellular matrix remodeling.4,6,11,25,26,33) ERK1/2 phosphorylation induced by β-AR stimulation regulates collagen-I synthesis in both cardiomyocytes and CFs.34,35) Similarly, stimulation of ERK1/2 phosphorylation by aldosterone triggers cellular hypertrophy, proliferation, and collagen-I synthesis in several cell types.17,33,36) Furthermore, inhibition of ERK1/2 attenuated the aldosterone-stimulated increase in collagen-I mRNA expression in renal fibroblasts.17) In this study, we confirmed that PD98059 inhibited isoproterenol-stimulated 11β-HSD1 and collagen-I expression in primary CFs, which indicate that β-AR-dependent ERK1/2 phosphorylation is the upstream activator of 11β-HSD1 in cardiac fibroblasts. Additionally, eplerenone inhibited isoproterenol-stimulated ERK1/2 phosphorylation and collagen-I expression in primary CFs. This suggest that MR-dependent ERK1/2 phosphorylation is a mediator of β-AR-induced collagen-I expression in cardiac fibroblasts.
Our study had several limitations. First, we could not evaluate CYP11-B2 or 11β-HSD activities, or measure aldosterone and corticosterone concentrations, because of technical limitations. Second, because we did not evaluate the signaling pathway from β-AR to MR in the present study, the relationship between the β-AR and MR should be interpreted with caution. Finally, we found that isoproterenol significantly reduced the level of mRNA encoding 11β-HSD2 in CFs, but not in the left ventricle. By contrast, level of mRNA encoding 11β-HSD2 in the heart was elevated in a rat model of myocardial infarction.37) Although we cannot definitively explain this between-study difference, it is possible that methodological variation among models of heart failure may have affected the results obtained.
We have shown, for the first time, that 11β-HSD1 but not CYP11-B2 is expressed in primary CFs. Isoproterenol increased 11β-HSD1 expression in both primary CFs and the left ventricle. Furthermore, eplerenone inhibited the isoproterenol-induced increases in 11β-HSD1 and collagen-I expression in primary CFs, and progression of cardiac fibrosis in the left ventricle. These results suggest that the effects of eplerenone on collagen-I expression mediated by β-AR stimulation involve the MR/11β-HSD1 signaling pathway.
This study was supported by a Grant-in-Aid for Scientific Research (C) (No. 24580463) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a Kitasato University Research Grant for Young Researchers (No. 3148).
The authors declare no conflict of interest.