2017 年 40 巻 11 号 p. 1934-1940
2017 年 40 巻 11 号 p. 1934-1940
Long-term treatment with antiepileptic drugs (AEDs) is accompanied by reduced bone mass that is associated with an increased risk of bone fractures. Although phenytoin has been reported to adversely influence bone metabolism, little is known pertaining to more recent AEDs. The aim of this study was to evaluate the effects of gabapentin or levetiracetam on bone strength, bone mass, and bone turnover in rats. Male Sprague-Dawley rats were orally administered phenytoin (20 mg/kg), gabapentin (30 or 150 mg/kg), or levetiracetam (50 or 200 mg/kg) daily for 12 weeks. Bone histomorphometric analysis of the tibia was performed and femoral bone strength was evaluated using a three-point bending method. Bone mineral density (BMD) of the femur and tibia was measured using quantitative computed tomography. Administration of phenytoin significantly decreased bone strength and BMD, which was associated with enhanced bone resorption. In contrast, treatment with gabapentin (150 mg/kg) significantly decreased bone volume and increased trabecular separation, as shown by bone histomorphometric analysis. Moreover, the bone formation parameters, osteoid volume and mineralizing surface, decreased after gabapentin treatment, whereas the bone resorption parameters, osteoclast surface and number, increased. Levetiracetam treatment did not affect bone strength, bone mass, and bone turnover. Our data suggested that gabapentin induced the rarefaction of cancellous bone, which was associated with decreased bone formation and enhanced bone resorption, and may affect bone strength and BMD after chronic exposure. To prevent the risk of bone fractures, patients prescribed a long-term administration of gabapentin should be regularly monitored for changes in bone mass.
Epilepsy is a chronic neurological disorder characterized by recurrent seizures.1) Pharmacotherapy with antiepileptic drugs (AEDs) is the mainstay of treatment for epilepsy and is effective in the prevention of seizures. Patients with epilepsy usually require long-term treatment with AEDs; thus, the prevention of side effects is important in the continuation of medication.
The bone is a metabolically active organ that undergoes continuous remodeling from bone resorption by osteoclasts and bone formation by osteoblasts.2) Under healthy conditions, the balance between bone formation and bone resorption is always uniform; thus, bone strength and bone mass are maintained. Certain pathological states and drugs affect normal bone remodeling, which can induce skeletal disorders, such as osteopenia or osteoporosis.3) A serious side effect associated with long-term administration of AEDs is the elevated risk of bone fracture owing to a decrease in bone mass.4–7) Patients taking AEDs are approximately 2–6 times more at risk of bone fracture than healthy individuals are.8,9) Previous studies have demonstrated that treatment with phenytoin decreased bone mineral density (BMD) owing to enhanced bone resorption.10,11) Currently, newly developed AEDs, including gabapentin, topiramate, lamotrigine, and levetiracetam, have been approved for clinical use.12) Although traditional AEDs, such as phenytoin, continue to be used in patients with epilepsy, the prevalence of the new AEDs has increased.13) Several new AEDs have been permitted for not only combination therapy with traditional AEDs, but also for monotherapy in patients with epilepsy. Moreover, newer AEDs are increasingly used for non-seizure indications, including peripheral neuropathy, bipolar disorder, and migraine headaches. Although it is expected that clinical use of newer AEDs will increase in future, there are few reports describing the effects of these AEDs on bone metabolism. The retrospective cohort study of Lee et al.14) indicated a reduction in BMD in patients administered phenytoin, phenobarbital, and carbamazepine, but not in those administered gabapentin and levetiracetam. In contrast with those findings, a large retrospective cohort study revealed an elevated risk of bone fracture with the use of gabapentin for the treatment of epileptic patients.15) The prospective study showed a significant bone loss in the femoral neck of elderly male patients administered gabapentin.16) However, the mechanism of the effect of gabapentin on bone metabolism is uncertain and limited data are available with regard to the effects of the new AEDs on bone metabolism. Thus, in the present study, we investigated the effects of phenytoin, gabapentin, and levetiracetam on bone strength, bone mass, and bone turnover in rats.
Male Sprague–Dawley rats (weight: 150–160 g) were purchased from CLEA Japan Inc. (Tokyo, Japan). All animals were maintained at 22±2°C, 55±5% humidity, under a 12-h light-dark cycle with ad libitum access to water and standard chow (MF; Oriental Yeast Co., Tokyo, Japan). All procedures were approved by the Animal Research Committee of Niigata University of Pharmacy and Applied Life Sciences (NUPALS) in accordance with the Japanese Government Animal Protection and Management Law, Japanese Government Notification on Feeding and Safekeeping of Animals, and the Guidelines for Animal Experiments in NUPALS.
DrugsCommercially available tablets of phenytoin (Sumitomo Dainippon Pharma, Tokyo, Japan), gabapentin (Pfizer Japan Inc., Tokyo, Japan), and levetiracetam (UCB Japan Co., Ltd., Tokyo, Japan) were obtained, uniformly pulverized, and suspended in 0.2% carboxymethylcellulose sodium (CMC-Na; Sigma-Aldrich, St. Louis, MO, U.S.A.) solution.
Experimental ProcedureThe animals were divided into six groups of nine animals each: control (vehicle; 0.2% CMC-Na solution), phenytoin 20 mg/kg, gabapentin 30 mg/kg, gabapentin 150 mg/kg, levetiracetam 50 mg/kg, and levetiracetam 200 mg/kg. Drug doses were selected based on previous reports relevant to phenytoin,17,18) gabapentin,19,20) and levetiracetam.21) Each drug was administered orally at a volume of 0.1 mL/100 g body weight once per day for 12 weeks. Twenty-four hours after the final drug administration, the rats were anesthetized with CO2 and whole blood was collected. Serum was obtained by centrifugation of the blood sample at 1500×g for 10 min and stored at −80°C until measurement. The femur and tibia were dissected and soft tissues were removed.
Bone Strength AnalysisThe bone strength of the femoral mid-diaphysis was evaluated by a three-point bending method using a mechanical testing machine (EZ-S; Shimadzu, Tokyo, Japan). The femur was positioned on two supports placed 15 mm apart. The bending load was vertically applied to the mid-diaphysis with a crosshead speed of 1.0 mm/min until bone fracture occurred. The load deformation curves were calculated using Operation software (Trapezium X; Shimadzu, Tokyo, Japan), and the maximum load, breaking energy, and stiffness were calculated directly from the load deformation curve.
Measurement of BMDThe whole femur and tibia were measured using quantitative computed tomography (LaTheta LCT-100; Aloka, Tokyo, Japan) with a pixel size of 250×250 µm and a slice thickness of 1 mm. Total BMD, cortical BMD, and trabecular BMD were calculated by using LaTheta software (ver. 1.31; Aloka, Tokyo, Japan).
Measurement of Serum Biochemical MarkersSerum calcium levels were measured by using a commercial reagent (Denka Seiken, Tokyo, Japan) and an automatic analyzer (Hitachi7180; Hitachi, Tokyo, Japan). Serum 25-hydroxy vitamin D (25(OH)D) levels were measured by using the 25(OH) vitamin D direct day enzyme-linked immunosorbent assay (ELISA) kit (Immundiagnostik GmbH, Bensheim, Germany). Serum parathyroid hormone (PTH) levels were measured by using the Rat Intact PTH ELISA kit (Immutopics, San Clement, CA, U.S.A.). The levels of serum osteocalcin, a bone formation marker, were measured by using the Osteocalcin ELISA system for rats (General Electric Healthcare Japan, Tokyo, Japan). The levels of serum tartrate-resistant acid phosphatase-5b (TRAP), a bone resorption marker, were measured using the Rat TRAP assay (SBA-Sciences, Oulu, Finland).
Bone Histomorphometric AnalysesWe prepared undecalcified specimens from the proximal tibia metaphysis. Briefly, the tibia was fixed with 70% ethanol for 7 d, stained with Villanueva Bone Stain (basic fuchsin, fast green, orange G, and azure II; Merck, Darmstadt, Germany) in 70% methanol for 7 d, and embedded in methyl methacrylate resin. The resin blocks were sliced to a thickness of 5-µm by using a microtome (Leica RM2255; Leica Inc., Nussloch, Germany). The measurements of all bone histomorphometric parameters were conducted in the secondary spongiosa region. To exclude the primary spongiosa, the measurement region was 0.7–2.7 mm distal to the lowest point of the growth plate and 0.2 mm from the lateral cortex. The bone histomorphometric measurements were performed by using a semiautomatic image analyzing system (Histometry RT CAMERA; System Supply, Nagano, Japan) at 250× magnification. The following bone structural parameters were obtained: bone volume per tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp). The following bone formation parameters were obtained: osteoid surface per bone surface (OS/BS), osteoid volume per bone volume (OV/BV), and osteoblast surface per bone surface (Ob.S/BS). The following bone resorption parameters were obtained: eroded surface per bone surface (ES/BS), osteoclast number per bone surface (N.Oc/BS), and osteoclast surface per bone surface (Oc.S/BS). The dynamic parameters were measured using a double fluorescent labeling technique. For labeling, all rats were injected subcutaneously with 25 mg/kg tetracycline (Sigma-Aldrich) and 10 mg/kg calcein (Wako Pure Chemical Industries, Ltd., Osaka, Japan) at 5 and 2 d before sacrifice, respectively. The labeled surface, which reflected the calcification front at the time of tetracycline and calcein administration, was clearly visualized by using a fluorescent microscope (Olympus BX50; Olympus America Inc., Center Valley, PA, U.S.A.). The parameters of single- and double-labeled surface (sLS and dLS, respectively) and inter-labels thickness and times (Ir.L.Th and Ir.L.t, respectively) were used in the calculation of the mineralizing surface per bone surface [MS (dLS+sLS/2)/BS)] and the mineral apposition rate (MAR; Ir.L.Th/Ir.L.t). The standard bone histomorphometric nomenclature, symbols, and units were based on those described in the report of the American Society for Bone and Mineral Research (ASBMR) Histomorphometry Nomenclature Committee.22)
Statistical AnalysisThe data are presented as the mean±standard error of the mean (S.E.M.). The differences in the mean values between each of the groups were analyzed with one-way ANOVA followed by Dunnett’s method of multiple comparisons. A value of p<0.05 was considered statistically significant.
The following parameters of the femoral mid-diaphysis significantly decreased in the phenytoin-treated group compared with that in the control group: maximum load (16%), breaking energy (20%), and stiffness (16%) (Table 1). There were no significant differences in the bone strength parameters in the gabapentin- and levetiracetam-treated groups compared with the control group.
| Control | Phenytoin | Gabapentin | Levetiracetam | |||
|---|---|---|---|---|---|---|
| 20 mg/kg | 30 mg/kg | 150 mg/kg | 50 mg/kg | 200 mg/kg | ||
| Maximum load (N) | 175±7.93 | 147±9.99* | 170±5.09 | 168±6.06 | 167±6.41 | 165±3.17 |
| Breaking energy (N.mm) | 200±8.52 | 160±8.45* | 185±10.4 | 181±9.86 | 185±10.4 | 180±10.1 |
| Stiffness (N/mm) | 222±8.01 | 187±7.57* | 216±4.14 | 208±5.28 | 213±5.34 | 214±4.83 |
Data represents the mean±S.E.M. of nine rats. * p<0.05 vs. Control.
The phenytoin-treated group exhibited significantly decreased cortical BMD (2.3, 1.9%), trabecular BMD (5.2, 5.3%), and total BMD (5.2, 4.3%) of the whole femur and tibia, respectively, compared with that in the control group (Table 2). However, no statistically significant differences in BMD were observed after treatment with gabapentin or levetiracetam compared with that in the control group.
| Control | Phenytoin | Gabapentin | Levetiracetam | |||
|---|---|---|---|---|---|---|
| 20 mg/kg | 30 mg/kg | 150 mg/kg | 50 mg/kg | 200 mg/kg | ||
| Whole femur | ||||||
| Cortical BMD (mg/cm3) | 1166±2.96 | 1139±5.16* | 1163±3.70 | 1159±5.19 | 1166±2.15 | 1164±4.88 |
| Trabecular BMD (mg/cm3) | 600±3.89 | 569±3.18* | 593±5.71 | 586±4.53 | 599±8.70 | 602±11.2 |
| Total BMD (mg/cm3) | 893±6.76 | 846±7.07* | 885±16.9 | 874±7.85 | 894±10.7 | 892±7.50 |
| Whole tibia | ||||||
| Cortical BMD (mg/cm3) | 1190±1.81 | 1167±3.81* | 1189±3.89 | 1182±6.31 | 1189±2.66 | 1190±2.78 |
| Trabecular BMD (mg/cm3) | 499±4.93 | 472±7.66* | 496±6.99 | 488±4.55 | 497±4.26 | 495±6.19 |
| Total BMD (mg/cm3) | 916±7.32 | 877±8.55* | 913±8.18 | 904± 6.57 | 915±12.0 | 916±11.4 |
Data represents the mean±S.E.M. of nine rats. * p<0.05 vs. Control.
The measurements of serum biochemical markers are summarized in Table 3. There were no significant differences in serum calcium levels, 25(OH)D levels, and PTH levels between all groups. However, serum TRAP levels significantly increased in the phenytoin-treated group compared with that in the control group. In the 150-mg/kg gabapentin-treated group, serum osteocalcin levels significantly decreased, whereas serum TRAP levels significantly increased compared with control values.
| Control | Phenytoin | Gabapentin | Levetiracetam | |||
|---|---|---|---|---|---|---|
| 20 mg/kg | 30 mg/kg | 150 mg/kg | 50 mg/kg | 200 mg/kg | ||
| Calcium (mg/dL) | 10.3±0.10 | 10.1±0.08* | 10.0±0.15 | 10.1±0.18* | 10.3±0.10 | 10.2±0.07 |
| Osteocalcin (ng/dL) | 41.7±1.54 | 38.9±2.26* | 36.5±1.26 | 32.7±1.82* | 40.1±1.84 | 39.2±1.19 |
| TRAP (U/L) | 13.3±1.68 | 26.4±2.49* | 17.0 ±1.32 | 21.2±2.67* | 13.7±0.64 | 14.1±1.13 |
| 25(OH)D (mol/L) | 215±10.2 | 190±12.6* | 209±11.5 | 211±13.6* | 215±17.8 | 216±20.1 |
| PTH (pg/mL) | 41.5±7.00 | 49.7±7.34* | 41.2±8.22 | 45.3±7.12* | 41.7±5.44 | 43.5±4.80 |
Data represents the mean±S.E.M. of nine rats. * p<0.05 vs. Control. TRAP: tartrate-resistant acid phosphatase-5b, 25(OH)D: 25-hydroxy vitamin D, PTH: parathyroid hormone.
The bone structural parameters, according to bone histomorphometry of the proximal tibia metaphysis, are shown in Fig. 1. The phenytoin-treated and 150 mg/kg gabapentin-treated groups showed significantly reduced BV/TV (39, 18%), Tb.Th (29, 13%), and Tb.N (32, 14%) and increased Tb.Sp (32, 20%). The bone formation and bone resorption parameters based on bone histomorphometry are shown in Fig. 2. In the phenytoin-treated group, the bone resorption parameters ES/BS, Oc.S/BS, and N.Oc/BS significantly increased by approximately 69, 85, and 61%, respectively. In the 150-mg/kg gabapentin-treated group, the bone formation parameters OS/BS, OV/BV, Ob.S/BS, MS/BS, and MAR significantly decreased by approximately 31, 26, 14, 18, and 13%, respectively, whereas ES/BS, Oc.S/BS, and N.Oc/BS significantly increased by approximately 41, 49, and 40%, respectively, compared with that in the control group. Typical micrographs of the slices (Fig. 3) were assessed by bone histomorphometric analysis of the control and 150-mg/kg gabapentin-treated groups. It was observed that bones in the 150 mg/kg-gabapentin-treated group had a decreased osteoid surface, osteoid volume, and inter-label thickness compared with that in the control group.
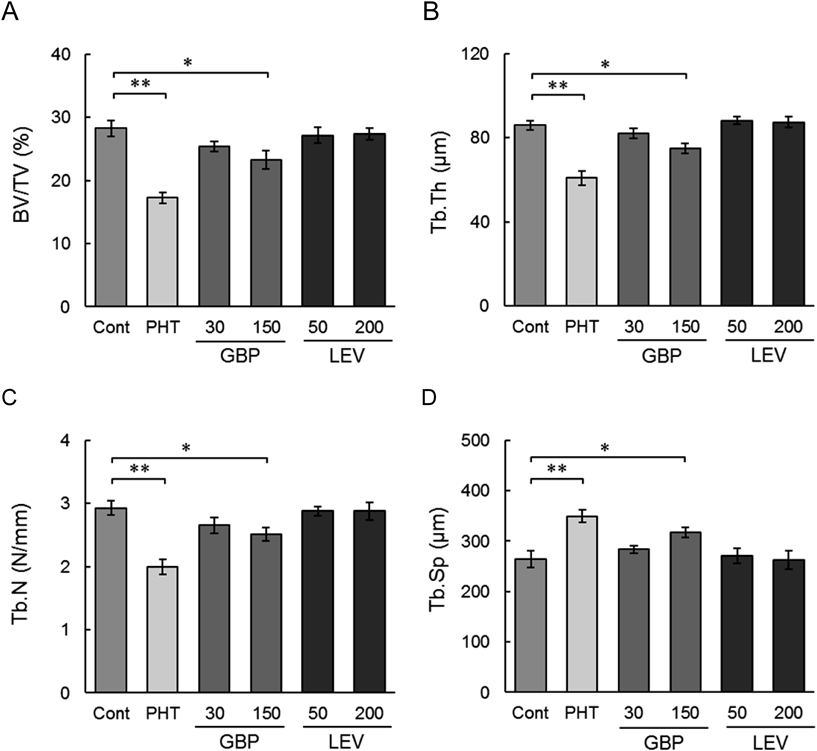
Phenytoin (PHT; 20 mg/kg), gabapentin (GBP; 30, 150 mg/kg), and levetiracetam (LEV; 50, 200 mg/kg) were orally administered once per day for 12 weeks. The control group (Cont) was treated with vehicle (0.2% CMC-Na solution). Data represent the mean±S.E.M. of nine rats. * p<0.05, ** p<0.01 vs. Control. BV: bone volume, TV: tissue volume, Tb.Th: trabecular thickness, Tb.N: trabecular number, Tb.Sp: trabecular separation.

Phenytoin (PHT; 20 mg/kg), gabapentin (GBP; 30, 150 mg/kg), and levetiracetam (LEV; 50, 200 mg/kg) were orally administered once per day for 12 weeks. The control group (Cont) was treated with vehicle (0.2% CMC-Na solution). Data represent the mean±S.E.M. of nine rats. * p<0.05, ** p<0.01 vs. Control. OS: osteoid surface, BS: bone surface, OV: osteoid volume, BV: bone volume, Ob.S: osteoblast surface, MS: mineralizing surface, MAR: mineral apposition rate, ES: eroded surface, Oc.S: osteoclast surface, N.Oc: osteoclast number.

In the upper photos, the osteoid surface is indicated by the black arrows. In the lower photos, the labeled surface with tetracycline and calcein are indicated by the yellow arrow and the green arrow, respectively (Villanueva Bone Stain, ×200).
Long-term treatment with traditional AEDs has been shown to result in the decreased bone mass of patients with epilepsy and, consequently, an increased risk of bone fractures.4–7) Indeed, it has been reported that long-term treatment with phenytoin resulted in decreased BMD,10,11) but few studies have examined the effects of the more recently developed newer AEDs on bone metabolism. In present study, we showed that the daily administration of phenytoin 20 mg/kg to rats for 12 weeks resulted in a significant decrease in bone strength and BMD, which was associated with enhanced bone resorption. Although treatment with gabapentin or levetiracetam in same experimental conditions resulted in no significant alteration of bone strength and BMD, gabapentin at a dose of 150 mg/kg induced the rarefaction of cancellous bone in the proximal tibia metaphysis, which was associated with the suppression of bone formation and the enhancement of bone resorption. Previous studies assumed that one of the mechanism for bone loss by AEDs promoted the catabolism of 25(OH)D and 1,25(OH)D.23–25) The resulting decrease in serum 25(OH)D and 1,25(OH)D levels leads to reduced calcium absorption, with consecutive secondary hyperparathyroidism, increased bone resorption, and accelerated bone loss.26) In support of this hypothesis, several clinical studies have reported the elevation of serum PTH levels27) and the reduction of serum 25(OH)D levels in patients treated with enzyme-inducing AEDs.28) In contrast to this finding, several studies have observed no significant differences in these parameters.29,30) In this study, we investigated the effect of AEDs on vitamin D metabolism, and found no significant difference in serum calcium, 25(OH)D, and PTH levels between the control group and the AED-treated groups. Although these results indicated that the AED-induced bone loss was not related to an abnormality in vitamin D metabolism, factors such as sex differences, species differences, and effects of duration of treatment need to be considered and investigated in the future.
Bone strength is defined by bone mass and bone quality31); the maintenance of bone mass requires a certain balance between bone formation by osteoblasts and bone resorption by osteoclasts.2,32) The term “bone quality” refers collectively to elements other than bone mass that affect bone fractures and is mainly defined by bone microstructure, bone turnover, microdamage, and bone mineralization.31) A meta-analysis concluded that the reduction in BMD was too small to explain the increased risk of bone fracture observed in patients with epilepsy who were prescribed traditional AEDs.33) The assessment of bone quality is important in the evaluation of the effects of AEDs on bone metabolism. In this study, we performed an analysis of the cancellous bone microstructure and the abilities of the bone mineralization by the bone histomorphometry in order to evaluate the effect of AEDs on bone quality. As treatment with phenytoin and gabapentin at a dose at 150 mg/kg significantly decreased BV/TV, Tb.N, and Tb.Th associated with increased Tb.Sp, it was suggested that these drugs caused deterioration of the cancellous bone microstructure. Bone resorption parameters, such as ES/BS, Oc.S/BS, and N.Oc/BS, significantly increased in the phenytoin-treated and 150 mg/kg gabapentin-treated group. In contrast, bone formation parameters, such as OS/BS, OV/BV, Ob.S/BS, MS/BS, and MAR, significantly decreased in the 150 mg/kg gabapentin-treated group. OS/BS and OV/BV are an indication of the ratio of uncalcified bone volume, whereas MS/BS and MAR are considered an index of osteoblast differentiation and proliferation.34) Therefore, these results suggested that gabapentin may reduce the abilities of osteoblast and mineralization. In this study, the effects of AEDs on bone resorption and bone formation were also observed through the evaluation of serum biochemical markers. Collectively, we have demonstrated that phenytoin significantly decreased bone mass owing to the enhancement of bone resorption without influencing bone formation, which was in accordance with previous reports.10,11) Furthermore, it was suggested that gabapentin significantly decreased bone volume owing to the uncoupling of bone turnover by the suppression of bone formation and the enhancement of bone resorption.
In our study, treatment with levetiracetam did not affect BMD or bone metabolism. This was in agreement with a recent clinical study on the effect of levetiracetam on bone metabolism, which indicated that it did not affect bone strength or bone metabolism in epileptic patients.35) In contrast to phenytoin and gabapentin, levetiracetam does not affect bone metabolism. Therefore, the difference in mechanism of action of AEDs and the differences in their effects on bone metabolism should be clarified in the future.
The limitation of this study was the short treatment period that was used to evaluate the effects of gabapentin on bone metabolism in rats. There was no evidence that gabapentin affected bone strength or BMD in this study. However, the drug was associated with deterioration of the cancellous bone microstructure owing to decreased bone formation and increased bone resorption after 12 weeks, which may lead to a long-term effect on bone strength or BMD. Therefore, future studies are needed to assess the long-term effects of gabapentin.
In conclusion, we demonstrated that the administration of phenytoin 20 mg/kg to rats for 12 weeks significantly decreased bone strength and BMD, which was associated with enhanced bone resorption, whereas treatment with newer AEDs, such as gabapentin or levetiracetam, did not significantly affect bone strength and BMD. However, it was clear that the in vivo administration of gabapentin led to an imbalance in bone remodeling, in part by the suppression of bone formation and the enhancement of bone resorption, which led to the rarefaction of cancellous bone. In the current therapy of epilepsy, gabapentin is generally used in combination with another AED, such as phenytoin, and the drugs are prescribed for an extended period. Therefore, the effects of gabapentin on bone metabolism may additively increase the bone fragility induced by another AED. Hence, these findings suggest that patients receiving gabapentin over a prolonged period should be regularly monitored for changes in bone mass to prevent the risk of bone fractures.
The authors declare no conflict of interest.