2017 Volume 40 Issue 12 Pages 2146-2152
2017 Volume 40 Issue 12 Pages 2146-2152
Bone marrow-derived mesenchymal stem cells (BMSCs) transplantation is one of the new therapeutic strategies for treating ischemic stroke. However, the poor survival rate of transplanted BMSCs in ischemic tissue limits the therapeutic efficacy of this approach. Oxidative stress is a major mechanism underlying the pathogenesis of brain ischemia and has a negative impact on the survival of transplanted BMSCs. Tetramethylpyrazine (TMP) has been reported to possess potent antioxidant activity. In the present study, we aimed to investigate the protective effects of TMP pretreatment on BMSCs survival of hydrogen peroxide (H2O2)-induced apoptosis in vitro and to elucidate the potential antiapoptotic mechanisms of TMP pretreatment on BMSCs. BMSCs were pretreated with TMP (10, 25, 50, 100, and 200 µmol/L) for 24 h and then exposed to 500 µmol/L of H2O2 for 24 h. We found that TMP pretreatment significantly increased cell viability and decreased cell apoptosis and intracellular reactive oxygen species (ROS) generation. Furthermore, the protective effects of TMP were related to increased Bcl-2 expression, attenuated Bax expression, and enhanced levels of phosphorylated Akt (p-Akt) and extracellular regulated protein kinases1/2 (p-ERK1/2). Further studies found that these beneficial effects of TMP were significantly blocked by wortmannin (an inhibitor of phosphoinositide-3 kinase (PI3K)) or PD98059 (an inhibitor of ERK1/2). In conclusion, our results confirm that TMP protects BMSCs against H2O2-induced apoptosis by regulating the PI3K/Akt and ERK1/2 signaling pathways, suggesting that TMP may be used in combination with BMSCs to improve cell survival for the treatment of ischemic stroke.
Ischemic stroke is one of leading causes of death and disability worldwide. Recently, basic and clinical studies have found that the transplantation of bone marrow-derived mesenchymal stem cells (BMSCs) is a promising therapeutic strategy for brain tissue repair following brain ischemia.1) However, the therapeutic efficacy of this procedure is greatly limited by the poor survival of transplanted BMSCs in the infarcted brain tissue. After transplantation, BMSCs face hostile microenvironments in the ischemic brain, including oxidative stress, hypoxia and inflammatory reactions, which promote cell apoptosis.2–4) Oxidative stress results in an excessive accumulation of reactive oxygen species (ROS), which is a major detrimental factor for the survival of engrafted BMSCs during stroke treatment. Therefore, a strategy that promotes the survival of BMSCs in ischemic microenvironments may augment the effectiveness of BMSC transplantation therapy.
Given that cell survival may greatly enhance the effectiveness of transplantation therapy, several remedial approaches have been suggested. Pharmacological pretreatment has been shown to be a rational approach to reinforce the cells to withstand the ischemic and reperfusion injury environment.5) Tetramethylpyrazine (TMP), a biologically active alkaloid extracted from Ligusticum chuanxiong HORT (Umbelliferae), has been widely used in China for the treatment of cardiovascular and cerebrovascular diseases.6) Its effects are mainly related to its antioxidative, anti-apoptosis, and anti-inflammatory actions.7) Recent studies have demonstrated that TMP can inhibit cell apoptosis under various conditions, such as hydrogen peroxide-induced oxidative damage in human umbilical vein endothelial cells8) and in PC12 cells,9) oxygen–glucose deprivation-induced neuronal apoptosis,10) hypoxia-induced myocardial cell apoptosis,11) and CoCl2-induced oxidative stress in PC12 cells.12) These findings suggest that TMP pretreatment may enhance the survival of transplanted BMSCs and promote brain repair after stroke.
In the present study, we investigated whether pretreatment with TMP protected BMSCs against hydrogen peroxide (H2O2)-induced apoptosis and elucidated the antiapoptotic mechanism of TMP against oxidative stress. Our present study demonstrated for the first time that TMP protected BMSCs from H2O2-induced apoptosis by enhancing the resistance of BMSCs against oxidative injury. TMP exerts its antiapoptotic effects by activating the phosphoinositide 3-kinase (PI3K)/Akt and the extracellular signal-regulated protein kinases 1/2 (ERK1/2) signaling pathways, suggesting that TMP pretreatment could be a promising approach to increase cell survival in cell transplantation therapy for ischemic brain injury.
Male Sprague-Dawley (SD) rats (3 weeks, 80±10 g) were purchased from Sino-British SIPPR/BK Laboratory Animal Ltd. (Shanghai, China, Certificate number: SCXK 2013-0016). All the experimental procedures were performed according to the animal guidelines of Laboratory Animal Research Center, Zhejiang Chinese Medical University. TMP was purchased from Aladdin (Shanghai, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), sodium dodecyl sulfate (SDS), Tris, and dimethyl sulfoxide (DMSO) were purchased from Sigma (MO, U.S.A.). Dichlorofluorescein diacetate (DCFH-DA) and the SDS-polyacrylamide gel electrophoresis (PAGE) kit were purchased from Beyotime (Shanghai, China). Fetal bovine serum (FBS) and the penicillin/streptomycin solution were obtained from Gibco-BRL (NY, U.S.A.). Dulbecco’s modified Eagle’s medium (DMEM), 0.05% pancreatin and 0.02% ethylenediaminetetraacetic acid (EDTA) (Trypsin) were purchased from Genom (Hangzhou, China). Hydrogen peroxide was purchased from Yocom (Tianjin, China). Annexin V fluorescein isothiocyanate (FITC)/propidium iodide (PI) was obtained from BD Biosciences (CA, U.S.A.). The terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) apoptosis assay kit was purchased from Roche (U.S.A.). Wortmannin was purchased from Selleck Chemicals (Houston, U.S.A.). PD98059 was purchased from Gene Operation (Michigan, U.S.A.).
Cell Culture and TreatmentRat BMSCs were cultured as we have previously described.13) Briefly, the bone marrow was flushed out with no serum DMEM/F12. The cell suspension was plated in DMEM/F12 medium plus 10% FBS, 1% penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2 and 95% O2. Non-adherent cells were removed by replacing the medium every 3 d. When the cell adherence reached 85–90%, the cell culture was trypsinized and subcultured at the ratio of 1 : 2. The primary BMSCs were cultured in the culture dish displayed short bar shapes initially. Passage 3 (P3) BMSCs showed typical spindle-shaped cell morphology.13) P3 BMSCs were used for the following experiments. The cells were pretreated with TMP (10, 25, 50, 100, and 200 µmol/L) for 24 h and then treated with H2O2 (500 µmol/L) for 24 h.14,15) Wortmannin (an inhibitor of PI3K, 200 nmol/L) and PD98059 (an inhibitor of ERK1/2, 25 µmol/L) were incubated for 1 h after TMP pretreatment.
Cell Viability AssayCell viability was assessed using the MTT assay. Cells were plated onto 96-well plates at a density of 1×104 cells per 100 µL per well. After H2O2 treatment, 10 µL of MTT tetrazolium salt solution at a final concentration of 5 mg/mL was added to each well and the mixture was incubated for 4 h at 37°C. MTT reagent was then replaced with 150 µL of DMSO to dissolve the formazan crystals. After the mixture was shaken at 37°C for 15 min, the optical density (OD) value was determined at 490 nm using a microplate reader (Bio-Tek, U.S.A.).
Annexin V-FITC/PI AssayThe apoptosis was determined with the Annexin V-FITC/PI apoptosis detection kit. Briefly, BMSCs were collected and washed twice with cold PBS buffer, resuspended in 200 µL of binding buffer, and incubated with 5 µL of Annexin V-FITC conjugated to FITC and 5 µL of PI for 15 min at room temperature. Then, 300 µL binding buffer was added after incubation and the cells were analyzed by flow cytometry (Beckman-Coulter Inc., CA, U.S.A.). The cells treated with DMSO were used as the negative control. The experiment was repeated 4 times.
TUNEL AssayThe cell apoptosis was detected by the TUNEL assay. BMSCs staining was performed according to the manufacturer’s instructions. TUNEL-positive cells were visualized with a fluorescence microscope (Leica, Germany) using an excitation wavelength in the 450–500 nm range and a detection wavelength in the 515–565 nm range (green). The percentage of apoptotic cells was calculated by dividing the number of TUNEL-positive cells by the total number of cells visualized in the same field. Three digitized images with similar total cell numbers were selected from each cover slip for counting and averaging. Three independent experiments were analyzed.
Detection of ROSIntracellular ROS were measured using DCFH-DA. DCFH-DA was dissolved in serum-free medium to a concentration of 10 µmol/L. After treatment, the cells were washed with PBS 3 times and then stained with DCFH-DA for 30 min at 37°C in the dark. The cells were then detected by flow cytometry with excitation and emission wavelengths of 488 and 525 nm, respectively.
Quantitative (q)RT-PCRThe mRNA expression levels of Bcl-2 and Bax were detected by qRT-PCR. Total RNA was extracted from cultured cells using the Trizol method according to the manufacturer’s instruction. The purity of the RNA was confirmed by the ratio of optical densities at 260 and 280 nm. cDNA was produced from the total RNA using PrimeScript RT Master Mix (TaKaRa, Tokyo, Japan). Real-time PCR was carried out using the SYBR Premix Ex Taq Kit (TaKaRa) on an iQ5 multiplex real-time fluorescence quantitative PCR instrument (Bio-Rad, Hercules, CA, U.S.A.). The primers for target genes are shown in Table 1. The cycling conditions were as follows: 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 30 s. The fluorescence signal was detected during the extension step in each cycle. The data were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the 2−ΔΔCT method was used to calculate target gene expression. The result was represented as the relative value compared with the control.
| Gene | Primer sequence | Product length (bp) |
|---|---|---|
| Bcl-2 | Forward 5′-GGTGGACAACATCGCTCTG-3′ | 365 |
| Reverse 5′-ACAGCCAGGAGAAATCAAACA-3′ | ||
| Bax | Forward 5′-GCCCTTTTGCTTCAGGGTTT-3′ | 140 |
| Reverse 5′-TCCAATGTCCAGCCCATGAT-3′ | ||
| GAPDH | Forward 5′-ACAGCAACAGGGTGGTGGAC-3′ | 252 |
| Reverse 5′-TTTGAGGGTGCAGCGAACTT-3′ |
Cellular protein was collected and lysed in lysis buffer. The proteins (50 µg) were subsequently separated by 10% SDS-PAGE gel and transferred onto the polyvinylidene difluoride membranes (Bio-Rad). After blocking with 5% skim milk, the membranes were incubated with primary antibodies Bcl-2, Bax, ERK1/2, p-ERK1/2, Akt and p-Akt (Cell Signaling Technology, U.S.A.), and β-actin (Santa Cruz Technology, U.S.A.) overnight at 4°C, followed by sequential incubation with horseradish peroxidase-conjugated secondary antibodies (ZSGB-BIO, China) for 2 h. The protein bands were visualized by an enhanced chemiluminescence detection kit (ECL, Amersham, IL, U.S.A.) and exposed to a gel imaging system. The intensities of the bands were assessed using Quantity One Software (Bio-Rad).
Statistical AnalysisAll values are expressed as mean±standard deviation (S.D.). Differences among the groups were tested by a one-way ANOVA. The level of statistical significance was defined as p<0.05.
Before testing whether TMP had a protective effect on BMSCs in vitro, we first examined the cytotoxic effects of TMP on BMSCs viability using the MTT assay. The results showed that TMP (10, 25, 50, 100, and 200 µmol/L) treatment alone had no significant effect on BMSCs viability (Fig. 1A). Subsequently, BMSCs were exposed to various concentrations of H2O2 (0, 50, 100, 200, 300, 400, and 500 µmol/L) for 12, 24, and 48 h, and cell viability was measured by the MTT assay. H2O2 reduced cell viability in a concentration- and time-dependent manner (Fig. 1B). The treatment with 500 µmol/L of H2O2 for 24 h caused approximately 40% cell death, so this concentration was chosen for the subsequent experiments.15) Pretreatment with TMP (25, 50, and 100 µmol/L) significantly increased the viability of BMSCs injured by H2O2. The concentration of 50 µmol/L was the most effective, and this concentration was used for the following experiments (Fig. 1C). Furthermore, the BMSCs were incubated in wortmannin (an inhibitor of PI3K, 200 nmol/L) or PD98059 (an inhibitor of ERK1/2, 25 µmol/L) for 1 h after pretreatment with TMP (50 µmol/L) for 24 h and then exposed to 500 µmol/L H2O2 for 24 h. Interestingly, the protective effect of TMP against H2O2-induced BMSC death was blocked by wortmannin and PD98059 (Fig. 1D).
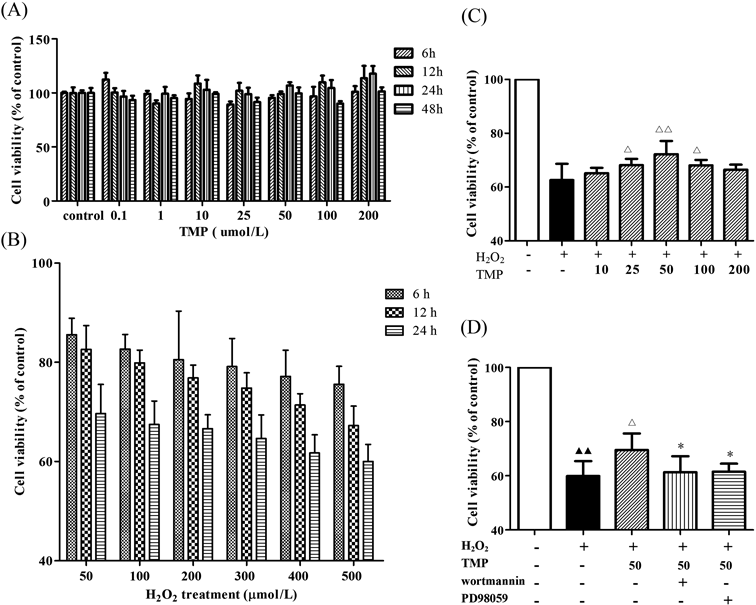
Cell viability was determined by the MTT assay. (A) BMSCs were pretreated with different concentrations of TMP for different time durations. (B) BMSCs were incubated with different concentrations of H2O2 for different time durations. (C) BMSCs were preincubated with different concentrations of TMP for 24 h prior to exposure to 500 µmol/L H2O2 for 24 h. (D) BMSCs were incubated in wortmannin (200 nmol/L) or PD98059 (25 µmol/L) for 1 h after pretreated with TMP (50 µmol/L) for 24 h, and then exposed to 500 µmol/L H2O2 for 24 h. BMSCs cultured in complete medium under normal condition were used as a control. Mean±S.D. of six independent experiments; ▲▲ p<0.01 vs. the control, △ p<0.05 and △△ p<0.01 vs. only H2O2-treated group, * p<0.05 vs. the group treated with H2O2 and TMP.
The present study determined whether TMP protected BMSCs from apoptosis by annexin V-FITC/PI staining. Flow cytometric analysis results indicated that H2O2 induced apoptosis in BMSCs. Following treatment with TMP (50 µmol/L), the proportion of apoptotic cells decreased significantly from (39.05±1.45)% to (27.78±1.18)% (Figs. 2A, C). However, wortmannin or PD98059 partially abolished the protection of TMP in H2O2-induced BMSCs apoptosis. To further investigate the apoptotic ratio of TMP-treated BMSCs, TUNEL staining was performed. TMP appeared to significantly attenuate H2O2-induced BMSCs apoptosis (Figs. 2B, D), which was partially upregulated by wortmannin or PD98059. These results suggested that TMP suppressed BMSCs apoptosis, at least in part, via the regulation of the PI3K/Akt and ERK1/2 signaling pathways.
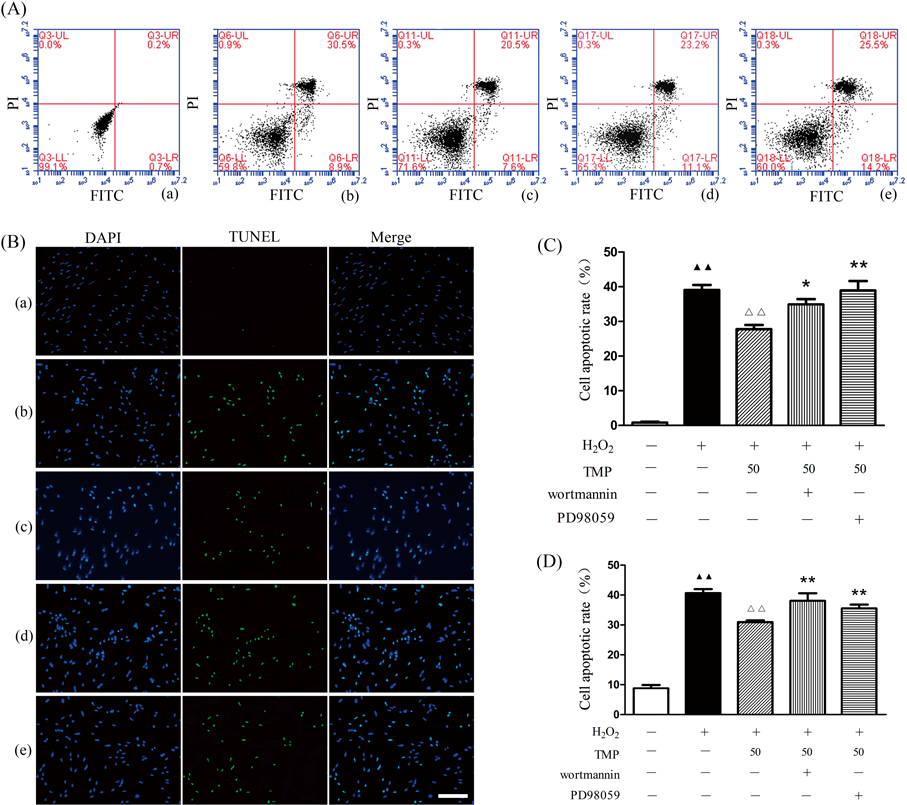
BMSCs were incubated in wortmannin (200 nmol/L) or PD98059 (25 µmol/L) for 1 h after pretreated with TMP (50 µmol/L) for 24 h, and then exposed to 500 µmol/L H2O2 for 24 h. Annexin V-FITC/PI assay ((A) and (C)) and TUNEL assay ((B) and (D)) were used to evaluate the apoptosis. (a)–(e) represented normal control, H2O2, TMP+H2O2, TMP+wortmannin+H2O2, TMP+PD98059+H2O2. ▲▲ p<0.01 vs. the control, △△ p<0.01 vs. only H2O2-treated group, * p<0.05 and ** p<0.01 vs. the group treated with H2O2 and TMP. Scale bar=100 µm.
To determine whether TMP attenuates the cell death of BMSCs by reducing the generation of ROS, the intracellular ROS level was examined using the DCFH-DA assay. In the present study, H2O2 significantly increased the intracellular ROS level in BMSCs, and pretreatment with TMP (50 µmol/L) markedly blocked the generation of ROS. However, the reduction of ROS generation by TMP was reversed by wortmannin or PD98059 (Fig. 3). These findings indicated that TMP inhibited intracellular ROS formation, at least partially, by inhibiting the PI3K/Akt and ERK1/2 signaling pathways.

(A) Intracellular ROS was visualized by flow cytometry. (B) Quantitative analysis of fluorescence intensity in Fig. 3(A). BMSCs were incubated in wortmannin (200 nmol/L) or PD98059 (25 µmol/L) for 1 h after pretreated with TMP (50 µmol/L) for 24 h, and then exposed to 500 µmol/L H2O2 for 24 h. (a)–(e) represented normal control, H2O2, TMP+H2O2, TMP+wortmannin+H2O2, TMP+PD98059+H2O2. Mean±S.D. ▲▲ p<0.01 vs. the control, △△ p<0.01 vs. only H2O2-treated group, ** p<0.01 vs. the group treated with H2O2 and TMP.
The mRNA expression levels of Bcl-2 and Bax were determined by qRT-PCR. H2O2 treatment significantly increased pro-apoptotic Bax gene expression and decreased anti-apoptotic Bcl-2 gene expression. Treatment with TMP (50 µmol/L) significantly decreased Bax mRNA expression and increased Bcl-2 mRNA expression. Based on the above mRNA expression results, the effect of TMP on the protein expression levels of Bax and Bcl-2 was detected by Western blotting. The results showed that H2O2 treatment significantly increased the expression of Bax and decreased the expression of Bcl-2. Pretreatment with TMP (50 µmol/L) significantly attenuated the H2O2-induced downregulation of Bcl-2 protein expression and the upregulation of Bax protein expression. However, the effects of TMP were reversed by wortmannin or PD98059 (Fig. 4).

BMSCs were incubated in PD98059 (25 µmol/L) or wortmannin (200 nmol/L) for 1 h after pretreated with TMP (50 µmol/L) for 24 h, and then exposed to 500 µmol/L H2O2 for 24 h. Mean±S.D. ▲▲ p<0.01 vs. the control, △ p<0.05 and △△ p<0.01 vs. only H2O2-treated group, * p<0.05 and ** p<0.01 vs. the group treated with H2O2 and TMP.
As mentioned above, TMP can protect BMSCs against H2O2-induced apoptosis, increasing cell viability and attenuating ROS formation, which were reversed by wortmannin or PD98059. We speculated that the anti-apoptotic effect of TMP was linked to the PI3K/Akt and the ERK1/2 signaling pathways. Therefore, Western blotting analysis was used to assess the phosphorylation of Akt (Figs. 5A, B) and ERK1/2 (Figs. 5A, C). The results showed that H2O2 significantly decreased the relative protein expression of p-Akt and p-ERK1/2 on BMSCs. Treatment with TMP (50 µmol/L) significantly increased the relative protein levels of p-Akt and p-ERK1/2, which were blocked by wortmannin and PD98059, respectively (Fig. 5).

BMSCs were incubated in wortmannin (200 nmol/L) or PD98059 (25 µmol/L) for 1 h after pretreated with TMP (50 µmol/L) for 24 h, and then exposed to 500 µmol/L H2O2 for 24 h. (A) The expression of p-Akt, Akt, p-ERK1/2 and ERK1/2 were detected by Western blotting. β-Actin was used as loading control. (B) The quantitative analysis of p-Akt and Akt. (C) The quantitative analysis of p-ERK1/2 and ERK1/2. Mean±S.D. ▲▲ p<0.01 vs. the control, △ p<0.05 and △△ p<0.01 vs. only H2O2-treated group, * p<0.05 and ** p<0.01 vs. the group treated with H2O2 and TMP.
BMSCs, which are capable of self-renewal, differentiation and the secretion of growth factors,16,17) are considered an ideal source of cells for tissue repair. BMSCs transplantation has shown great promise for the treatment of ischemic stroke. However, the poor survival rate of transplanted BMSCs in ischemic tissue limits the therapeutic efficacy of these cells.18,19) Oxidative stress is one of the important mechanisms underlying the pathogenesis of ischemic stroke. Small amounts of ROS are constantly produced as a byproduct of mitochondrial respiration and are necessary for cell survival and proliferation.20) Excess ROS could result in the loss of mitochondrial transmembrane potential, the release of proapoptotic molecules and the initiation of apoptosis.21) In this study, we first examined the cytotoxic effects of TMP on BMSCs viability, and then used H2O2 to imitate the poor microenvironment of the ischemic brain into which BMSCs were transplanted. Our results showed that TMP treatment alone had no significant effect on BMSCs viability, and H2O2 reduced the cell viability of BMSCs in a concentration- and time-dependent manner and induced BMSCs apoptosis and intracellular ROS generation.
TMP, one of the most important active ingredients of the Chinese herb Chuan Xiong, is widely used in the treatment of ischemic stroke. Previous studies have reported that TMP possesses antioxidative and anti-apoptosis activities in many cellular systems.8,22,23) However, the protective effects of TMP on BMSCs remain unclear. In the present study, we found that pretreatment with TMP (25, 50, and 100 µmol/L) significantly increased BMSCs cell viability after H2O2 treatment, the results were similar to previously published articles.11,24) Then we demonstrated that pretreatment with TMP (50 µmol/L) significantly suppressed H2O2-induced BMSCs apoptosis and intracellular ROS generation, indicating that TMP could protect BMSCs from H2O2-induced apoptosis through its antioxidant activity.
Given the above results, we further detected whether TMP could regulate the expression of apoptosis-related proteins. The Bcl-2 family is an important apoptosis-regulating family that includes the genes for the anti-apoptotic molecule Bcl-2 and the pro-apoptotic molecule Bax.25) Numerous studies have demonstrated that Bcl-2 and Bax are associated with the mitochondrial membrane.26) Bax exhibits extensive amino acid homology with Bcl-2 and can form heterodimers with Bcl-2, effectively antagonizing Bcl-2 function and promoting apoptosis.25) In the current study, the expression levels of Bcl-2 and Bax were detected by qRT-PCR and Western blot analysis. The results showed that H2O2 treatment significantly decreased Bcl-2 expression and increased Bax expression on BMSCs. However, pretreatment with TMP (50 µmol/L) could effectively reverse the unfavorable changes in the expression of Bcl-2 and Bax, suggesting its anti-apoptotic effects.
Several studies have demonstrated that the PI3K/Akt and ERK1/2 signaling pathways play pivotal roles in many physiological processes, such as cell metabolism, proliferation, migration and survival.27,28) The activation of PI3K reportedly leads to the phosphorylation and activation of Akt, which promotes cell survival by enhancing the expression of anti-apoptotic proteins and inhibiting the activity of pro-apoptotic proteins.29) Phosphorylated ERK1/2 moves from the cytoplasm across the nuclear membrane, activates associated proteins, induces Bcl-2-associated death promoter (BAD) phosphorylation, and activates a variety of nuclear transcription factors to play a role in anti-apoptosis.30,31) In our experiments, TMP pretreatment increased the expression levels of p-Akt and p-ERK1/2 in H2O2-treated BMSCs. We speculated that TMP could modulate the activity of p-Akt and p-ERK1/2 to adjust the expression of apoptosis-related proteins. To determine whether TMP modulated the expression of apoptosis-related proteins through the PI3K/Akt and ERK1/2 signaling pathways, wortmannin and PD98059 were used in this study. The upregulation of the Bcl-2 protein and the downregulation of the Bax protein by TMP were blocked with the application of wortmannin or PD98059. These results suggested that the PI3K/Akt and ERK1/2 signaling pathways were involved in the anti-apoptotic activity of TMP in H2O2-induced BMSCs apoptosis.
In conclusion, our results indicated that TMP exerted protective effects against H2O2-induced apoptosis in BMSCs through the PI3K/Akt and ERK1/2 signaling pathways, suggesting that TMP could be a promising approach to increase cell survival in cell replacement therapy for ischemic brain injury.
This study was supported by the National Natural Science Foundation of China (81274113), Public Welfare Technology Application Research Project of Zhejiang Province (2016C33185), and the Science and Technology Innovation Team foundation from college of Basic Medical Science (JCIT2016-2).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.