2017 年 40 巻 12 号 p. 2015-2023
2017 年 40 巻 12 号 p. 2015-2023
Sialidase releases sialic acid residues from the ends of sugar chains. The sialidases are involved in many physiological processes including cell differentiation and proliferation and immune function as well as pathophysiological conditions such as various human cancers and infections. Therefore visualization of sialidase activities with high sensitivity could provide valuable insights into these isozyme’s activity. We developed novel fluorescent sialidase substrates, 2-benzothiazol-2-yl-phenol derivatives-based N-acetylneuraminic acid (Neu5Ac) (BTP-Neu5Ac) substrates, for highly sensitive and specific visualization of sialidase activity in living mammalian tissues and virus-infected cells. We found that BTP-Neu5Ac can visualize sialidase activities sensitively and selectively in rat tissues including brain slices. BTP-Neu5Ac can also clearly detect cancer cells implanted orthotopically in mouse colons and human colon cancers. In this review, I describe imaging of sialidase activity with BTP-Neu5Ac in animal tissues, detection of colon cancer, memory formation, detection of virus-infected cells, and application to drug-resistant influenza virus detection and separation.
The development of substrates for detection of sialidase activity is important for elucidating the roles of sialidases in many physiological processes and infections with pathogens.
4-Nitrophenyl derivative (PNP-Neu5Ac)1) and 5-bromo-4-chloro-3-hydroxyindole derivative (X-Neu5Ac),2) which are coloring substrates, 4-methylumbelliferone (4-MU) derivative (4MU-Neu5Ac)3) as a fluorescent substrate, and 1,2-dioxetane derivatives (NA-Star® and NA-TDX™)4) as chemiluminescent substrates are well-known substrates of sialidase (Table 1). PNP-Neu5Ac produces a yellow water-soluble reaction product (nitrophenol) by hydrolysis with sialidase. PNP-Neu5Ac is therefore not suitable for histochemical detection of sialidases. On the other hand, X-Neu5Ac yields a dark blue insoluble pigment (4-chloro-3-brom-indigo) by hydrolysis with sialidase and by subsequent air oxidation at the site of enzymatic activity. X-Neu5Ac can therefore be used for histochemical detection of sialidase activity. However, the chromogenic substrate has low sensitivity, and the sensitivity is not sufficient for measuring the activity of sialidase expressed in mammalian tissues. An improved method, which converts X into a fluorescent derivative by adding Fast Red Violet LB as a sensitizer to X-Neu5Ac, was developed for imaging of sialidase activity.5) However, this reaction is susceptible to pH conditions and requires two reaction steps. Additionally, the product does not show a sufficient Stokes shift. 4MU-Neu5Ac is frequently used as a reagent for fluorescence detection of sialidase activity. Sialidase hydrolyzes 4MU-Neu5Ac to Neu5Ac and a water-soluble fluorescent substance, 4-MU. 4MU-Neu5Ac is therefore unsuitable for histochemical fluorescent imaging of sialidase activity. The fluorescence of 4-MU is also strongly influenced by pH conditions. The fluorescence of 4-MU is attenuated under conditions more acidic than pH 8. The chemiluminescent substrate NA-Star ® is widely used for detection of activities of influenza virus sialidases. However, the product is also water-soluble and has a short emission half-life. This substrate is therefore not suitable for histochemical staining of sialidase.
| Substrates | ||||
|---|---|---|---|---|
| X-Neu5Ac (+FRVLB) | 4MU-Neu5Ac | NA-Star® | BTP-Neu5Ac | |
| Classification | Chromogenic | Fluorescent | Chemiluminescent | |
| Sensitivity | Low (high) | High | High | High |
| Specificity | Low (low) | High | High | High |
| Histochemical staining | Suitable | Unavailable | Unavailable | Suitable |
We therefore developed novel sialidase fluorescent substrates, 2-benzothiazol-2-yl-phenol derivatives-based N-acetylneuraminic acid (BTP-Neu5Ac) substrates (Chart 1). Although BTP is one of the classical molecules that were studied as organic electroluminescence materials,6) BTP is a water-insoluble molecule and emits fluorescence in a solid state. The fluorescence is not affected by pH conditions. BTP shows a large Stokes shift of 150 nm or more because the luminescence process of BTP is different from that of general fluorescent dyes. Fluorescence on and off is controlled by the presence or absence of a substituent on the phenolic hydroxyl group in BTP. BTP strongly emits light even under low pH conditions. Furthermore, fluorescence of BTP is not affected by excitation light or self-absorption (Fig. 1). For these reasons, BTP-Neu5Ac is useful for imaging of sialidase activity in animal tissues, detection of colon cancers and detection of virus-infected cells without fixation.7)

BTP-based sialic acid derivatives (BTP2-Neu5Ac, BTP3-Neu5Ac, and BTP4-Neu5Ac) were synthesized as described previously.7) Conditions: (a) dry MeOH, Amberlite IR-120(H+), overnight, 92% yield. (b) AcCl–AcOH, overnight, quant. (c) THF–DMF, BTP2–4, NaH, room temperature, overnight. (d) dry MeOH, NaOMe, room temperature, 6 h, then MeOH, NaOH aq., room temperature, 2 d.

A, Fluorescent appearance of 5 mM BTP2, BTP3, and BTP4 in artificial cerebrospinal fluid (upper) and on filter papers under 365 nm UV light (lower) as described previously.7) B–D, Excitation and emission spectra of BTP2 (B), BTP3 (C) and BTP4 (D) were determined in artificial cerebrospinal fluid (pH 7.3). The numbers represent wavelengths (nm) at peak fluorescence intensity. Abbreviations: rF, relative fluorescence intensity.
We succeeded for the first time in detecting bacterial sialidase blotted on a polyvinylidine difluoride (PVDF) membrane using BTP-Neu5Ac. We then visualized mammalian sialidase activity in rat brain tissues and whole fetuses. In addition, colon cancer tissue in model mice and human colorectal cancer tissue that was removed in a surgical operation were clearly stained with BTP-Neu5Ac. On the other hand, cells infected with influenza viruses were detected with TP-Neu5Ac regardless of the neuraminidase (NA) subtype of the virus.8) Furthermore, cells infected with paramyxoviruses, human parainfluenza virus (hPIV), mumps virus (MuV), Sendai virus (SV), or Newcastle disease virus (NDV) were rapidly and clearly visualized with BTP-Neu5Ac.9–12) As described above, the present fluorescent probe is also useful for detection of viruses that exhibit weak or no cytopathic effect and plaque formation or have a low rate of proliferation. We also found that drug-resistant viruses and cells that they infected were selectively detected by combining an NA inhibitor and BTP3-Neu5Ac.
Among the four sialidase isoforms (Neu1–Neu4 in rats, NEU1–NEU4 in humans) intracellular localization of sialidases in mammalian tissues is known to be varied under different conditions of cells.13–15) Neu1, Neu2, and Neu3 show enzyme activity on the extracellular surface of the plasma membrane. Neu1 mainly exists in lysosomes, and it hydrolyzes oligosaccharides and glycoproteins. Neu1 is also localized to the outer membrane of the nucleus. Neu1 migrates from the lysosome to the plasma membrane in accordance with activation of human T cells and differentiation of human monocytes. Neu1 also activates the phagocytosis in macrophages and dendritic cells. Neu2 is mainly localized in the cytoplasm and hydrolyzes glycoproteins and gangliosides under a neutral pH condition. Neu2 also exists in plasma membranes of the mouse brain and thymus. Neu3 is mainly localized in the plasma membrane. Neu3 is also located in the nuclear envelope. Neu3 hydrolyzes gangliosides, which are major components of cranial nerve tissue, as substrates. Neu3 regulates neurite elongation and promotes regeneration of neurites in rat hippocampal neurons. Neu3-regulated cell membrane gangliosides play an important role in the regulation of insulin signaling. Neu4 is present in lysosomes and inner membranes of mitochondrial cells. Neu4, unlike other sialidases, has broad substrate specificity. There are two isoforms (Neu4L and Neu4) of human Neu4. Neu4L is expressed specifically in the brain and is involved in apoptosis of nerve cells. Thus since the sialidase isoforms (Neu1–Neu4) have different substrate specificities, the functions of the sialidases in cells and tissues might be different.
At first, we tried to stain a bacterial sialidase with BTP4-Neu5Ac.
Sialidase derived from Arthrobacter ureafaciens (AUSA) was blotted onto a PVDF membrane and incubated with 10 µM BTP4-Neu5Ac. As shown Fig. 2, AUSA was stained in a concentration-dependent manner with BTP4-Neu5Ac. The sensitivity of BTP4-Neu5Ac was 1000 times higher than that of X-Neu5Ac. Next, brains of male Wistar rats were excised and coronal sections of 400 µm in thickness were incubated in ACSF containing BTP4-Neu5Ac.

A–C, Sialidase activity was visualized in acute coronal slices of adult rat brains with BTP-based sialic acid derivatives as described previously.7) BTP2-Neu5Ac (A), BTP3-Neu5Ac (B) and BTP4-Neu5Ac (C) were used at pH 7.3. Brain slices were stained with different concentrations of BTP4-Neu5Ac (D). E, Sialidase activity of the slices with 10 mM BTP4-Neu5Ac visualized with or without 1 mM DANA, a sialidase inhibitor.
The sections were observed with a fluorescence microscope. The corpus callosum, hippocampus, encapsulation, and outer capsule were strongly stained in the slices (Fig. 2). The staining was remarkably suppressed by pretreatment with 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (DANA).7)
Fetuses of Wistar rats were frozen at –20°C and sliced to a thickness of 300 µm. The sections were incubated with BTP4-Neu5Ac and observed with a fluorescence microscope (data not shown). In the slices of rat fetuses, the skin, gastrointestinal tract, spinal cord, and brain were strongly stained with BTP4-Neu5Ac. Slices of a rat fetus pretreated with glutaraldehyde were hardly stained with BTP4-Neu5Ac. The results indicate that BTP-Neu5Ac can sensitively and selectively visualize sialidase activity in acute rat brain and fetus slices. BTP4-Neu5Ac could contribute to study of the functions of mammalian sialidases.
The minimum size of cancers detected by fluorescence endoscopy is about 1 mm, which is smaller than the sizes of tumors (about 5–20 mm) detected by positron emission tomography (PET), computed tomography (CT), and magnetic resonance imaging (MRI). If it were possible to use a fluorescent imaging probe with high sensitivity in endoscopic examination of cancer, the probe would certainly contribute to early detection and rapid treatment of cancer. Colon cancer has been reported to exhibit strong enzyme activity of a plasma membrane-associated sialidase. We prepared colon cancer model mice and attempted to detect colon cancer cells with BTP4-Neu5Ac in living colon tissues. Colon 26 NL-17 cells with high metastatic potential were orthotopically implanted in mice, and colon tissues were resected after 1 week and stained with 100 µM BTP4-Neu5Ac. Strong fluorescence was observed in the colon transplanted with the colon cancer cells, but fluorescence was not detected in inflammatory or normal colons (Fig. 3). Regions that showed intense fluorescence in colon transplanted with the colon cancer cells were confirmed to have cancer by immunohistochemical staining.7) The results indicate that mouse colon cancer can be readily distinguished from normal tissues by staining with BTP-Neu5Ac.

A, living colon tissues were visualized with BTP4-Neu5Ac after orthotopic colon implantation of Colon 26 NHL-17 cells as described previously.7) Arrowhead indicates the cancer areas. B and C, Inflammatory (B) or normal (C) colons were also visualized with BTP4-Neu5Ac. Left, middle and right panels in panel A–C show bright field, merged and fluorescent views, respectively. D and E, Enlarged image of cancer (D) and normal (E) regions visualized with BTP4-Neu5Ac. F, Background fluorescence image of panels D and E is observed with PBS. G, Colon 26 NHL-17 cells were confirmed by immunohistochemical staining with rabbit anti-CD71 antibody. Scale bar in panel C stands for 2.5 mm and is the same in panels A and B. Scale bar in panel F stands for 0.5 mm and is the same in panels D and E. Scale bar in panel G stands for 0.2 mm.
The expression level of Neu3 mRNA in human colon cancer has been reported 3–100-times higher than that in normal tissue. Therefore we also attempted to detect human colon cancer with BTP4-Neu5Ac. Human colon cancer viable tissues were incubated with 200 µM BTP4-Neu5Ac. Strong fluorescence was observed in the tumor area but not in the normal area. Using hematoxylin–eosin staining, it was confirmed that regions exhibiting intense fluorescence and non-fluorescence were cancer tissue and normal tissue, respectively. Cancers classified as T3 and T4 in human colon tissue were also detected with BTP4-Neu5Ac (Fig. 4). Although it is necessary to evaluate the toxicity of BTP4-Neu5Ac, BTP4-Neu5Ac could be used not only for pathological examination but also for cancer detection for noninvasive diagnosis.7)
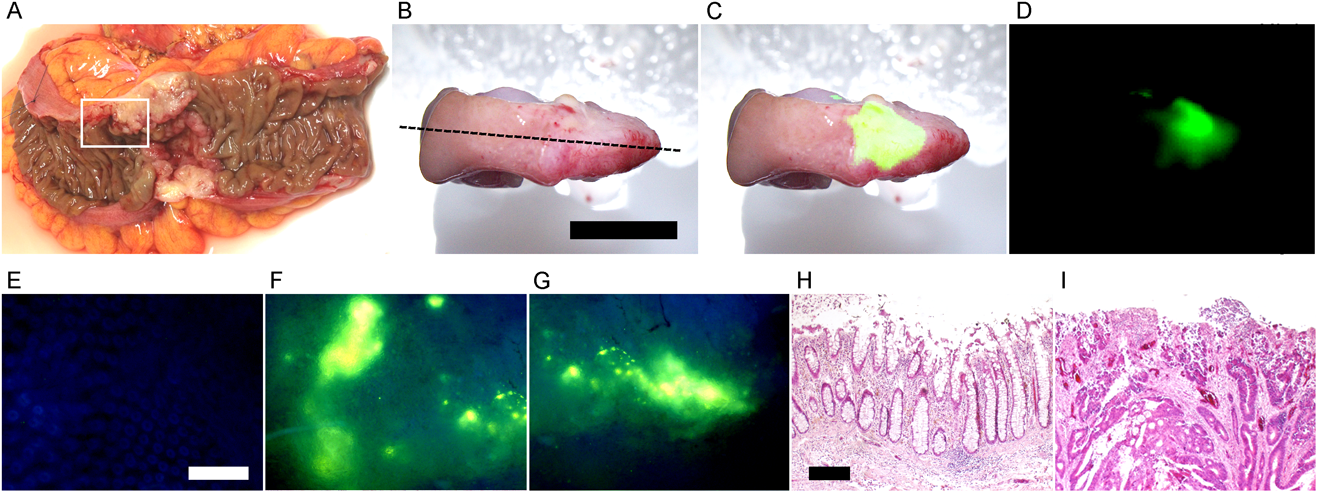
A, A human colon cancer specimen (region enclosed with a white line) was obtained from surgical cancer tissue (UICC T classification: T3) as described previously.7) B–D, The colon tissue was visualized with BTP4-Neu5Ac. B, C and D represent photographic, merged the two and fluorescent images, respectively. E–G, Magnified fluorescence images of normal (E) and cancer (F and G) regions with a fluorescent microscope. Non-fluorescence (H) and fluorescence (I) regions in panel C were stained with hematoxylin–eosin, respectively.
Exogenous sialidase applied extracellularly to the hippocampus affects many neurological functions including memory and synaptic plasticity. Endogenous sialidase also plays a decisive role in hippocampal memory formation on the membrane surface. We investigated changes in sialidase activity during memory processing with BTP3-Neu5Ac. We found that sialidase activity in the rat CA 3-layer lucidium immediately increases in response to LTP-induced high-frequency stimulation.16,17) To elucidate the spatiotemporal dynamics of sialidase activity during LTP induction, abrupt changes in sialidase activity in response to LTP-induced high-frequency stimulation were determined using BTP3-Neu5Ac. When the hippocampal CA3 region was stained with BTP3-Neu5Ac at pH 7.3, the lamellar mucus showed relatively strong fluorescence in the CA3 region (Fig. 5). BTP3-Neu5Ac did not produce fluorescence in the presence of DANA. The results indicate that BTP3-Neu5Ac specifically detected sialidase activity in the hippocampal CA3 region.
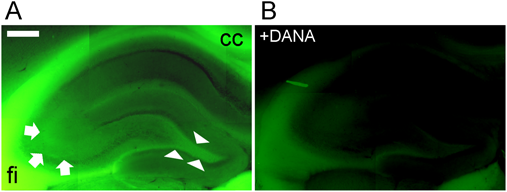
Sialidase activity of the rat hippocampus and the surrounding region was visualized with 100 µM BTP3-Neu5Ac (A) or with 100 µM BTP3-Neu5Ac +10 mM DANA (B) at pH 7.3 as described previously.16) cc: corpus callosum, fi: hippocampal fimbria. Arrows, CA3 striatum lucidum; arrowheads, hilus. Scale bar, 0.5 mm.
Influenza is an important infectious disease with seasonal outbreaks occurring every year and pandemics occurring once every several decades. The outbreaks and pandemics have great social and economic impacts. Influenza A viruses are detected in a wide range hosts including humans, birds, pigs, and horses.18,19) Epidemics of oseltamivir-resistant viruses were reported worldwide from 2007 to 2008.20,21) Thus strengthening of monitoring for anti-influenza drug-resistant virus strains is needed for reducing the risk of resistance acquisition and maintaining effectiveness of drugs against the viruses. A new method for simple and rapid detection of influenza viruses would contribute greatly to viral research and surveillance.
We attempted to detect virus-infected cells by imaging the sialidase activity of NA, which is abundantly expressed on the surface of cells infected with influenza A or B virus. At first, we confirmed that influenza virus, which was dot-blotted on a PVDF membrane, can be detected with BTP3-Neu5Ac. Next, Madin–Darby canine kidney (MDCK) cells that had been infected with influenza virus A/WSN/1933 (H1N1) strain were cultured for 12 h. The virus-infected cells were histochemically detected by incubation with 10 µM BTP3-Neu5Ac at 37°C for 10 min. Furthermore, we confirmed that staining of BTP3-Neu5Ac in the virus-infected cells was significantly suppressed by pretreatment with zanamivir (data not shown). After immobilizing the virus-infected cells with 4% paraformaldehyde, the sialidase activity remained at about 70% of that of unfixed cells. Therefore the virus-infected cells were immunostained with an anti-NA monoclonal antibody after fixation with paraformaldehyde. The fluorescence imaging with BTP3-Neu5Ac in the infected cells almost overlapped with that of anti-NA monoclonal antibody staining.8)
Influenza A viruses have a broad host range and many subtypes. We demonstrated that cells that had been infected with several subtypes of influenza A virus isolated from different species could be detected with BTP3-Neu5Ac. MDCK cells infected with A/PuertoRico/8/1934 (H1N1), A/WSN/1933 (H1N1), A/Memphis/1/1971 (H3N2), A/Shizuoka/833/2009 (H1N1pdm), or B/Lee/1940 were fluorescently imaged with BTP3-Neu5Ac. In addition, COS-7 cells expressing each NA gene of A/chicken/Miyazaki/S4/2011 (H5N1), A/mandarin duck/Miyazaki/22 M-765/A/chicken/Shimane/1/1918 (H1N1), A/Anhui/1/2013 (H7N9), or A/Shanghai/1/2013 (H7N9) were also detected with BTP3-Neu5Ac.8)
A plaque assay is generally used for detection and isolation of influenza viruses. However, it is often difficult to detect clinical isolates by a plaque assay because the viruses make small plaques or no plaque. We showed that viral strains can be isolated from virus-infected cell populations (focus) by live imaging with BTP3-Neu5Ac (Fig. 6). In 2007, an epidemic of an oseltamivir-resistant H1N1 type virus occurred mainly in northern Europe, then the oseltamivir-resistant H1N1 virus expanded worldwide from 2008 to 2009.20,21)
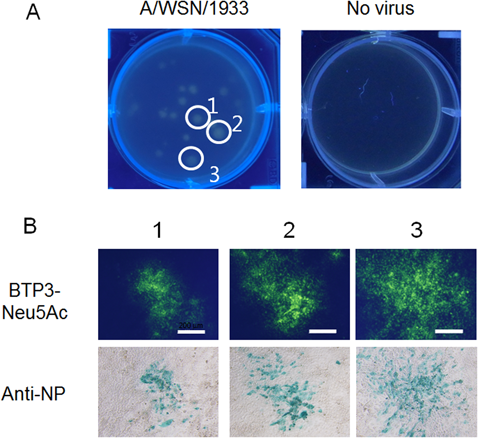
(A) MDCK cells infected with influenza A virus strain A/WSN/1933 (H1N1) were visualized with 200 µM BTP3-Neu5Ac dropped onto the overlaid agarose-containing medium. The plate was observed under UV irradiation at 354 nm after incubation for 15 min. (B) MDCK cells were infected with three isolates obtained from fluorescent focuses using live-focus fluorescence imaging. The cells were double-stained by anti-NP antibody and BTP3-Neu5Ac.
In Japan, four types of medicine (NA inhibitors), zanamivir, oseltamivir, peramivir, and raninamivir, are used for treatment of influenza.22)
Since the sialidase activity of drug-resistant viruses is retained in the presence of an NA inhibitor, we demonstrated that selective and efficient fluorescence imaging of drug-resistant virus-infected cells can be achieved by combining each NA inhibitor and BTP3-Neu5Ac (Fig. 7). Furthermore, we showed that drug-resistant viruses can be directly isolated from the virus-infected cell population (focus) stained with BTP3-Neu5Ac in the presence of NA inhibitors.23)
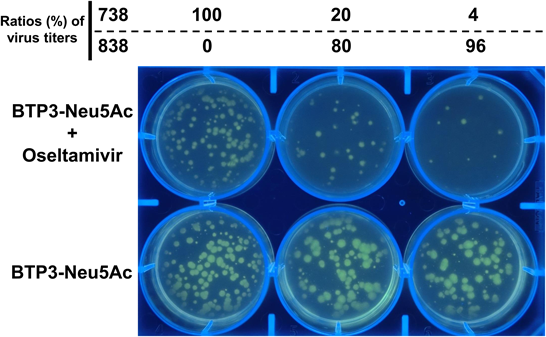
MDCK cells on a 6-well plate were inoculated with mixtures of oseltamivir-resistant 738 and oseltamivir-sensitive 838 at various ratios (%) as described previously.23) MDCK cells were visualized with 2 mM BTP3-Neu5Ac dropped onto the overlaid agarose-containing SFM with or without 1000 nM oseltamivir after incubation at 37°C for 2 d. Fluorescent images of the plate were observed under UV irradiation at 365 nm.
Human parainfluenza virus type 1 (hPIV1) and type 3 (hPIV3), which belong to the genus Respirovirus, family Paramyxoviridae, are important human pathogens causing upper and lower respiratory disease in infants and young children.24) hPIV1 generally shows no visible plaques in common cell lines including Lewis lung carcinoma-monkey kidney (LLC-MK2) cells, MDCK cells, and human epidermoid cancer (HEp-2) cells, which are generally used for cultivation, isolation, and quantification of paramyxoviruses.25,26) Therefore it is difficult to isolate hPIV1 by the conventional plaque-forming method. The authors previously established a method to visualize plaques at 7–9 d after infection of hPIV1 using an initial overlay medium of bovine serum albumin-free Eagle’s minimum essential medium containing agarose and acetylated trypsin for 4–6 d followed by a second overlay staining medium containing agarose and neutral red for 2–3 d.27) However, this method requires many days for plaque formation. We attempted to visualize an hPIV1-infected cell population (focus) by live imaging with BTP3-Neu5Ac. LLCMK2 cells were infected with hPIV1 and overlaid a serum-free medium containing agarose and then acetylated trypsin and incubated for 2 d. Two hundred micromolar BTP3-Neu5Ac was dropped on the agar medium and the cells were incubated at 37°C overnight to increase the sensitivity of fluorescence detection because the hemagglutinin-neuraminidase (HN) spike glycoprotein of hPIV1 shows low sialidase activity under neutral conditions. We successfully detected the focus with BTP3-Neu5Ac and isolated the virus by picking up the fluorescence focus (data not shown).9)
MuV belongs to the family Paramyxoviridae, the subfamily Paramyxovirinae, and genus Rubulavirus. Epidemic parotitis (mumps) is caused by MuV infection. Epidemic parotitis develops after a latency period of 2–3 weeks and is characterized by swelling of one or both parotid salivary glands. The most common complication is meningitis, and the viral infection also causes meningoencephalitis, orchitis, ovarianitis, hearing loss, and pancreatitis.28) MuV plaque formation by conventional methods takes more than 6 d after infection. MuV clinical strains, which show little induction of apoptosis or formation of multinucleated giant cells in virus-infected cells, apparently do not form apparent plaques even 6 d or more after virus infection.29,30) MuV HN maintains relatively high sialidase activity at pH 4.0 to 7.0. Therefore MuV-infected Vero cells and HN-expressed COS-7 cells were incubated with BTP3-Neu5Ac under neutral conditions that are easy to handle with good applicability to cultured cells for 15 min. The focus of MuV-infected cells was clearly live-imaged with BTP3-Neu5Ac 4 d post-infection, and the virus was isolated by picking up the fluorescence focus (Fig. 8). HN-expressed COS-7 cells were also detected with BTP3-Neu5Ac.10)
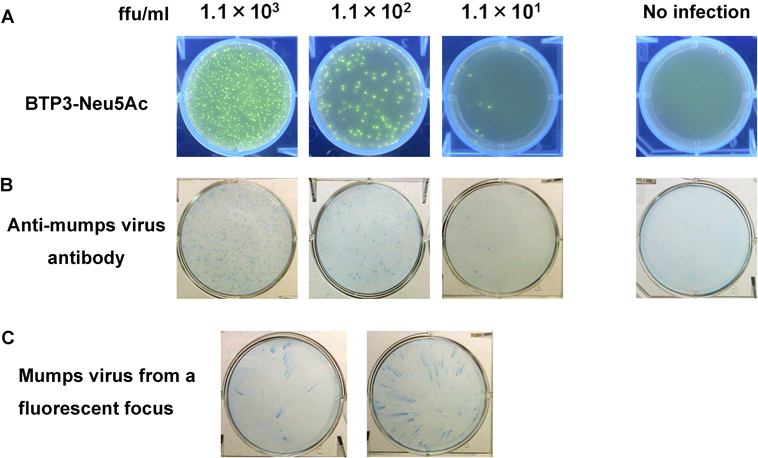
(A) Fluorescent visualization of mumps virus-infected cells. Vero cells inoculated with mumps virus were visualized with BTP3-Neu5Ac dropped onto the overlay medium after incubation at 37°C for 6 h as described previously.10) (B) Mumps virus-infected Vero cells (A) were immunostained with rabbit anti-mumps virus antibody and HRP-labeled Protein A. Mock infection was used as a negative control. (C) Two fluorescent focuses in (A) were picked up and a new monolayer of Vero cells was infected with each focus. Virus replication from each focus was confirmed by immunostaining with rabbit anti-mumps virus antibody after incubation at 37°C for 48 h.
SV, which belongs to the genus Respirovirus, is a murine respiratory virus. SV can be artificially generated from genes alone by a reverse genetics system. An SV vector has therefore been extensively studied for application to gene therapy for Fabry disease or Alzheimer’s disease and as a vaccine vector for human pathogenic respiratory syncytial virus, hPIV, or AIDS virus.31–34) NDV which belongs to the genus Avulavirus, is widely distributed in wild birds. NDV has been studied as a tumor necrosis virus and for viral vectors.35–37) We demonstrated the usefulness of BTP3-Neu5Ac for histochemical fluorescent staining of SV- or NDV-infected cells and animal tissues. We succeeded in histochemical fluorescent staining of SV and NDV both in vitro and in vivo (data not shown).11,12) BTP3-Neu5Ac will contribute to applied research with the viruses.
BTP3-Neu5Ac has already been commercialized. By improving the efficiency of detection of sialidase activity and providing a new analytical method for research on viral and animal sialidases, it is expected to make a great contribution to academic research.
I would like to acknowledge people in my laboratory who contributed to this work. I would also like to acknowledge Professor Kiyoshi Ikeda and Dr. Tadamune Otsubo of Hiroshima International University, Professor Naoto Oku, Dr. Kosuke Shimizu, and Professor Hiroaki Kanazawa of the University of Shizuoka, Dr. Yoshihiro Kawaoka of the University of Tokyo and the University of Wisconsin-Madison, Dr. Takehiko Saito and Dr. Yuko Uchida of the National Institute of Animal Health, National Agriculture and Food Research Organization, and Dr. Mark von Itzstein and Dr. Robin Thomson of the Institute for Glycomics, Griffith University. I am very grateful to Professor Yasuo Suzuki for recommending me for the PSJ Award for Divisional Scientific Contribution, the Pharmaceutical Society of Japan.
The author declares no conflict of interest.
This review of the author’s work was written by the author upon receiving the 2017 Pharmaceutical Society of Japan Award for Divisional Scientific Contribution.