2017 Volume 40 Issue 2 Pages 128-134
2017 Volume 40 Issue 2 Pages 128-134
Biomacromolecules (>40 kDa) have been developed as drug delivery system (DDS) carriers of low-molecular weight drugs to promote these drugs’ uptake by cancer tissues via enhanced permeability and retention (EPR) effects. Human serum albumin (HSA) has been found to accumulate in cancer tissues via this EPR effect. HSA is the most abundant protein in serum, which performs essential physiological functions such as the transportation of many endogenous and exogenous ligands. Nitric oxide (NO) is a very small ligand of HSA; it is a unique and diffusible molecular messenger that plays a central role in mammalian physiology. Although the in vivo half-life of NO is extremely short, HSA could prolong the half-life of NO via S-nitrosation at the position of Cys-34. S-Nitrosated HSA (mono-SNO-HSA) is called an ‘Endogenous NO traffic protein,’ due to the highly stable S-nitroso form in circulating blood, and to the efficiency of S-transnitrosation in cells that require NO. Mono-SNO-HSA possesses a very strong cytoprotective action via the induction of heme oxygenase-1. On the other hand, HSA reinforced with approximately seven NO molecules (poly-SNO-HSA), which we developed by means of chemical modification, possesses multiple anticancer activities. Our previous data clarified that the high expression of protein disulfide isomerase on the surface of cancer cells plays a very important role in the anticancer action of poly-SNO-HSA. In this review, we focus on the advantage of poly-SNO-HSA in treating intractable cancers from the viewpoint of drug delivery systems and drug resistance.
A gas modulator, nitric oxide (NO), plays an important role in the human body.1–7) A small amount of NO is synthesized by endothelial and neuronal NO synthases. Its actions, including the relaxation of vascular smooth muscle, are pleiotropic.8,9) However, NO can sometimes be cytotoxic. For instance, a large amount of NO inhibits the growth of cancer cells and induces cell death via apoptosis.10,11) Many studies have identified that the apoptosis of cancer cells (directly) and the inhibition of cancer progression (indirectly) have relevance to NO.12) Unfortunately, the T1/2 of NO is so extremely short (<5 s) that NO without a carrier cannot be applied as a therapeutic biological agent. Thus, NO releasing compounds with pharmacological activity have been synthesized around the world.
For a long-acting and safe NO donor, we examined the possibility of developing a ‘NO traffic protein’ as a superior NO carrier. This ‘NO traffic protein’ would include (1) a NO binding protein with the superior efficiency of S-nitrosation, (2) superior stability of the S-nitroso form in human blood, and (3) the superior efficiency of NO transfer in selected cells which require NO. As a candidate for creating such a NO traffic protein, we selected human serum albumin (HSA), because HSA has high biocompatibility and good biodegradability, and because ‘endogenous’ S-nitrosothiols in serum are largely related to HSA.13,14) Endogenous HSA has one S-nitrosated site at Cys-34 thiol. The endogenous S-nitrosated HSA (SNO-HSA) was named ‘Mono-SNO-HSA,’ which has significantly superior stability compared with low molecular weight (LMW) S-nitrosothiols.
In many animal models, it is well established that Mono-SNO-HSA possesses many beneficial and cytoprotective actions. For example, Mono-SNO-HSA can inhibit the apoptosis of hepatic cells and bacteria growth. In an attempt to create highly effective SNO-HSA preparations, we synthesized a SNO-HSA that contained approximately 7 (S-nitroso mol/mol HSA) S-nitroso groups by chemical reaction with lysine residues on the surface of HSA, and named this ‘poly-SNO-HSA.’ Poly-SNO-HSA characteristics were compared in detail with those of a mono-SNO-HSA preparation, per their effectiveness against murine colon carcinoma (C26) and Human hepatoma (HepG2) cells.15) In the case of mono-SNO-HSA, the NO uptake of cells partly takes place via a LMW thiol, such as glutathione or cysteine, which cytoprotectively results in the development of actions via heme oxygenase-1 induction. In contrast to mono-SNO-HSA, the NO transfer from poly-SNO-HSA was more rapid and more pronounced. The cytoprotective action mainly occurs via the reaction of a protein disulfide isomerase (PDI) on the cell surface (Fig. 1). Thus, the excessive NO inflow resulted in apoptotic cell death caused by the induction of reactive oxygen species (ROS) production, caspase-3 activation and DNA fragmentation. We concluded that the site of S-nitroso groups on a carrier such as HSA is more important than an increased number of S-nitroso groups, and that it is difficult to enhance cellular responses to NO without inducing opposite actions. Here, we wish to focus on the possibility of using poly-SNO-HSA as a secure and effective multifunctional anticancer agent in biological systems.
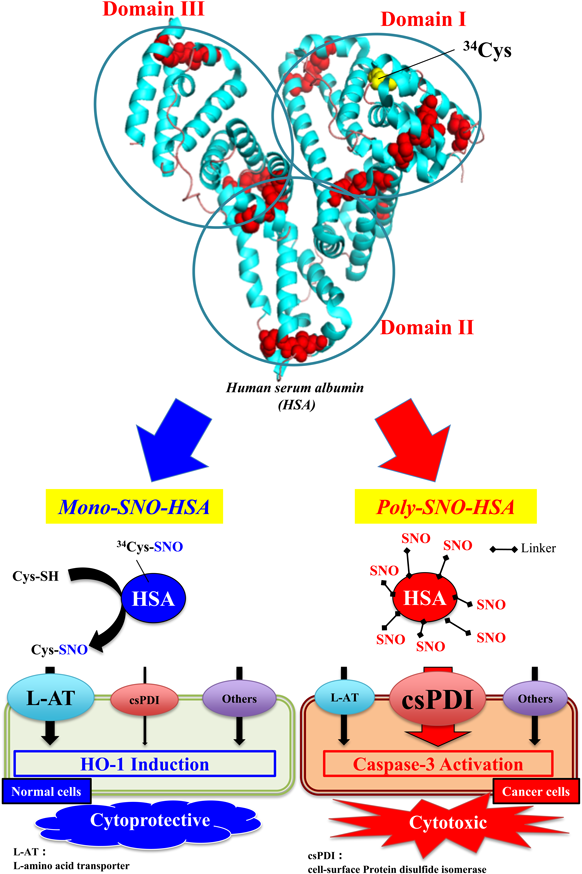
Mono-SNO-HSA slows the entry of NO into the normal cell, an effect partly mediated by the L-amino acid transporter (L-AT) via S-transnitrosation reaction with free LMW thiol. On the other hand, poly-SNO-HSA can react with cell-surface protein disulfide isomerase (cs-PDI) to accelerate the entry of NO into cancer cells. The entry of NO from mono-SNO-HSA is relatively slow, and results in cytoprotective activity via HO-1 induction. On the other hand, the entry of NO from poly-SNO-HSA is very rapid, and results in cell death caused by apoptosis.
Previously, poly-SNO-HSA was incubated with various cancer cells, and we observed that this HSA form had the ability to induce the apoptosis of cancer cells.16–18) Via activation of the intrinsic apoptosis pathway, apoptosis occurred in various cancer cells such as murine colon cancer, C26 cells, and a variant of Yoshida sarcoma, LY-80 cells, both in vivo and in vitro.
The apoptotic pathway was induced as follows. First, poly-SNO-HSA increased intracellular NO via PDI on the surface of the cancer cells. Subsequently, the elevated NO concentration induced ROS. Then, mitochondrial function and membrane potential were damaged by ROS. Finally, caspase-3, which is a critical instigator of apoptosis, was activated, causing cells to undergo morphological changes, such as chromatin condensation and the engulfment of apoptotic bodies.
Next, the anticancer actions of poly-SNO-HSA were investigated in vivo: C26-bearing mouse and LY-80-bearing rat were given an intravenous injection of poly-SNO-HSA. Interestingly, cancer growth in the poly-SNO-HSA treated animals was reduced to 30% of that observed in the control or HSA-treated animals. These findings indicate that poly-SNO-HSA has anticancer actions both in vitro and in vivo by its ability to induce apoptosis. Similar results were observed when we performed the same experiments using mice bearing other cell types, such as SW480 cells, indicating that poly-SNO-HSA has anticancer action against various cancers.
One barrier in various cancer therapies has been well characterized: the development of multidrug resistance by cancer cells. To overcome this problem, various methods have been performed, including the use of agents that inhibit P-glycoprotein (P-gp) through targeted drug delivery, and by changing the cell membrane. However, while some approaches have succeeded in mouse and rat models, clinical applications remain limited. Hence, we propose that NO is an interesting and promising compound in the search for treatments to combat the multidrug resistance of cancer.
Besides inducing apoptosis, poly-SNO-HSA could affect the multidrug resistance of cancer. In practice, we reported that poly-SNO-HSA (0.5–10 µM) significantly reduced the resistance to doxorubicin in human leukemia multidrug resistant (K562/doxorubicin) cells. The inhibitory actions of doxorubicin and poly-SNO-HSA on the growth of K562/doxorubicin cells were synergistic. These data strongly indicate that poly-SNO-HSA possesses not only the ability to inhibit cancer cell growth, but also to attenuate resistance to doxorubicin in vitro.19)
To evaluate the action of poly-SNO-HSA on chemo-resistance in vivo, we investigated the role of poly-SNO-HSA using K562/doxorubicin-bearing mice. After the cancer tissues had reached the size of about 150 mm3, doxorubicin and/or poly-SNO-HSA was injected to the mice biweekly. As a result, in the K562/doxorubicin mice, doxorubicin had no significant action on cancer growth. Intriguingly, a combination treatment of both doxorubicin and poly-SNO-HSA in the K562/doxorubicin mice resulted in a decrease in cancer volume, to 30% that of the mice treated with each alone. This result indicates that poly-SNO-HSA attenuates doxorubicin resistance in vivo. Our findings demonstrate that poly-SNO-HSA has potential for use as a secure and powerful multifunctional anticancer agent. Concerning clinical application, a limitation of the current nanoparticles used for targeted drug delivery is that these carriers also accumulate in non-target organs, inducing serious side actions and side effects. In the case of our carrier, HSA has good biodegradability and low accumulation in non-target organs; therefore, serious side effects would not be encountered. Additionally, our carrier presents a unique opportunity for treating cancers without significant side effects because almost no NO is released from poly-SNO-HSA near cancer tissues. The current tool of drug delivery to cancers, based on nanotechnology, can provide significant therapeutic benefit through understanding the basic mechanisms of drug actions, including that of therapeutic NO.20)
Recent clinical trials support the hypothesis that the use of antiangiogenic drugs, which target abnormal blood vessels in cancer, can inhibit cancer progression.21) However, longer term clinical responses to angiogenesis inhibitors do not always lead to success. For instance, in clinical trials, approximately 50% of cancers progressed after an initially successful treatment using bevacizumab in glioblastomas,22) consistent with the development of resistance to antiangiogenic therapy, indictating a low prognosis and low response to valuable treatments developed thus far.23) Some clinical data indicate that cancer hypoxia and hypoxia-mediated resistance to antiangiogenic therapy are enhanced via the devascularization induced by antiangiogenic treatment.24) Importantly, hypoxia activates autophagy, which is a lysosomal degradation pathway which may accelerate the survival of cancer cells.25,26) One possible mechanism has been proposed in which BCL2/adenovirus E1B 19 kDa protein-interacting protein 3, a hypoxia inducible factor (HIF)-1α downstream target gene, is essential to hypoxia-mediated autophagy.27,28) Hence, autophagy inhibitors could be candidates for the prevention of resistance to antiangiogenic therapy. In our previous studies, we demonstrated that poly-SNO-HSA has anti-autophagy activity via the inhibition of HIF-1α in cancer cells in vitro and in vivo.29) We are conducting further studies to clarify the issue of whether poly-SNO-HSA can also be used as a specific and potent autophagy inhibitor.
Cancer rejection via binding to natural killer group 2, member D (NKG2D), can be highly effective during early phases of cancer proliferation.30–33) However, the expression of MICA on the surface of cancer cells, and the shedding of soluble MICA by late phase cancer progression, negatively imprints on the systemic immune response. Thus, cancer cells facilitate the cancer immune escape response.34–36)
MICA on the surface of cancer cells is accompanied by endoplasmic reticulum protein 5, which, identically to PDI, usually helps in the folding of many proteins in the endoplasmic reticulum.37) Interestingly, a previous report demonstrated that the function of cell-surface endoplasmic reticulum protein 5 is required for MICA shedding.37) The shedding of MICA is regarded as an important step in human cancer progression and metastasis in order to escape the immunosurveillance mediated by NKG2D. MICA shedding not only decreases the density of MICA on the surface of cancer cells, but also produces soluble MICA, which has been determined to down-regulate systemic NKG2D on cytotoxic effector cells, thus expanding the immunosuppressive microenvironment in the cancerous region.34,36,38) Therefore, inhibition of MICA shedding is a promising strategy to therapeutically improve anticancer immunity.
Our previous reports clearly showed that the transfer of NO from poly-SNO-HSA into cancer cells was induced by cell-surface PDI. Although it is well known that PDI is similar to ERp5, the exact action of poly-SNO-HSA on MICA shedding by ERp5 remains unknown. To clarify this, the human breast cancer cell line MCF-7, which expressed MICA, was incubated with poly-SNO-HSA. As a result, poly-SNO-HSA significantly increased the expression of MICA on the surface of MCF-7 cancer cells, and the soluble MICA in medium was decreased compared with the control. On the other hand, Phorbol 12-myristate 13-acetate (PMA), which stimulates the shedding of MICA, actually decreased the expression of MICA on the surface of MCF-7 and increased the soluble MICA in medium (Fig. 2). These data indicate that poly-SNO-HSA plays an important role as an inhibitor of immune escape mechanisms of cancers (Fig. 3). Therefore, poly-SNO-HSA is a promising component in the development of targeted tumor immunotherapy.

(A) MCF-7 cells were treated with poly-SNO-HSA (25 µM) or PMA (1 µg/mL) for 12 h. The expression of MICA on the surface of MCF-7 was detected immunohistochemically in paraffin-embedded sections, as described above, using anti-MICA with the corresponding fluorescence labeled second antibodies. (B) The density of the bands for MICA was quantitatively analyzed using NIH Image J Software. (C, D) Soluble MICA in the conditional medium was determined by FACS analysis using anti-MICA antibody-FITC. (E) The expression of MICA and PDI were co-localized on the surface of MCF-7 cells. Data are expressed as the mean±S.D. (n=3). ** p<0.01, compared with control.

The cell-surface PDI function is required for MICA shedding. The cell-surface PDI function is also required for NO release from poly-SNO-HSA. Poly-SNO-HSA oxidized thiols of the cell-surface PDI via NO transfer from poly-SNO-HSA. As a result, MICA shedding was inhibited. Therefore, poly-SNO-HSA enhances the anti-cancer action of NK cells via NKG2D, and decreases the expansion of immunosuppressive T cells via soluble MICA.
As described above, poly-SNO-HSA functions as an apoptosis inducer, a multidrug resistance suppressor, an autophagy inhibitor and a MICA-shedding inhibitor (Fig. 4). However, the in vivo half-life of S-nitroso in poly-SNO-HSA was approximately 20 min in cancer-bearing models. These data suggest that poly-SNO-HSA is not a reliably long-lasting NO donor.39) To construct a superior NO delivery carrier, we investigated the factors involved in the stability of S-nitroso on poly-SNO-HSA. Previous findings demonstrated that a polyethylene glycol (PEG) elevated the stability of S-nitroso compounds, because the PEG acts as a stabilizer of S-nitroso bonding.40) We confirmed that this action can be applied to construct more useful poly-SNO-HSA preparations.41) Furthermore, the problem of drug targeting to cancers should be addressed, in addition to the in vivo stability of S-nitroso. From the point of view of cancer targeting, the enhanced permeability and retention (EPR) effect is very important. This effect is a unique and well-known phenomenon in the solid cancer-selective delivery of nanoparticles, liposomes or large proteins, including antibodies.42) Matsumura and Maeda demonstrated that various long-lasting pharmaceutical nanocarriers larger than 40 kDa are able to spontaneously accumulate in solid cancers.43) Additionally, Kataoka’s group indicated that approximately 30 nm micelles, but not 60 nm micelles, are able to enter very low permeable pancreatic cancers.44) These reports strongly indicate that nanoparticle therapeutics ranging in size from 40 kDa to less than 30 nm can serve as efficient drug delivery carriers to even poorly permeable cancers, such as pancreatic cancers. To clarify this possibility, we constructed a HSA dimer (20 nm) produced by Pichia pastoris using a method of genetic engineering. In our previous study, we reported that HSA dimer has a longer blood circulation time than HSA monomer in rats and mice.45) Therefore, we speculated that HSA dimer could be promising clinically as a novel DDS material with superior serum retention as well as increased cancer-specific accumulation. And in fact, the dimerization of poly-SNO-HSA did result in improved anticancer activity via more efficient delivery of NO in C26-bearing mice.41) The PEGylation product of poly-SNO-HSA also possesses good biocompatibility and biodegradability. Furthermore, nanoparticles of PEGylated poly-SNO-HSA-dimer (about 30 nm) are potentially more efficient carriers of anticancer drugs. In the near future, we hope that the promising PEGylated poly-SNO-HSA dimer and its nanoparticles will be developed as a superior anticancer drug with reduced side effects (Fig. 5).

As direct effects, the rapid transfer of NO from poly-SNO-HSA into cancer cells induces apoptosis and reverts drug resistance, partly by activating a cGMP dependent pathway. As indirect effects, NO inhibits the expression of HIF-1α and P-gp. As a consequence, poly-SNO-HSA attenuates drug resistance and hypoxia-induced autophagy. In addition, poly-SNO-HSA inhibits MICA shedding via the dysfunction of cell-surface PDI.

PEGylation, dimerization, and multimerization are useful methods for developing poly-SNO-HSA as a superior antitumor nanomedicine.
This review of the author’s work was written by the author upon receiving the 2016 Pharmaceutical Society of Japan Award for Young Scientists.
I would like to acknowledge Dr. Masaki Otagiri, Dr. Toru Maruyama and current laboratory members at the Department of Biopharmaceutics, Graduate School of Pharmaceutical Sciences, Kumamoto University, for their valuable contributions to this work. I wish to thank Dr. H. Maeda, Institute of DDS Research, Sojo University and Dr. T. Akaike, Graduate School of Medicine, Tohoku University, for their valuable advice and kind consideration. I also thank Dr. Victor T. G. Chuang for editing the manuscript.
The author declares no conflict of interest.