2017 Volume 40 Issue 6 Pages 830-836
2017 Volume 40 Issue 6 Pages 830-836
In the development of a drug for intra-articular administration, a sustained-release formulation is desirable since it is difficult to sustain the effects of conventional injections due to fast drug leakage from the joint cavity. In this study, we prepared sustained release gel formulations for intra-articular administration containing indocyanine green (ICG) as a model drug to follow its fate after intra-articular administration in rats with in-vivo imaging system (IVIS). ICG administered as an aqueous solution leaked from the joint cavity in a short time and was excreted out of the body within a day. On the other hand, ICG in the sustained-release formulations was retained and released in the joint cavity for a week. Next, we prepared a sustained-release formulation with hyaluronic acid (HA) as the gel base containing a pain-relief drug (Drug A). We had administered it and other formulations into the rat knee where we injected bradykinin to evaluate their walking distance after 1 and 3 d. The effect of an aqueous solution of Drug A disappeared on day 3. The HA gel formulation without Drug A was more effective than the aqueous solution. The HA gel formulation with Drug A was the most effective; the walking distance was about 85% of the baseline on day 3. This study showed that the gel formulations were effective to sustain the release of a drug in the knee joint, and that the combination of a pain-relief drug with HA gel was effective to improve the mobility of the acute pain model rats.
The joint pain accompanied by osteoarthritis and rheumatoid arthritis is caused by joint inflammation. The number of patients suffering from rheumatoid arthritis has been estimated to be 70 million worldwide.1) The Research Group of Rheumatology Research of the Ministry of Health, Labour and Welfare, Japan has reported that the number of patients with rheumatoid arthritis alone is 700000 in Japan and there are 15000 new cases per year.2) In osteoarthritis, cartilage of the joint, which absorbs impact of the knee or hip, is deformed by aging or intensive exercise, causing inflammation and pain. When symptoms progress, cartilage wears out and bones collide with each other, causing further pain. Rheumatoid arthritis is a collagen disease where an autoimmune mechanism attacks joints and cartilage, causing inflammation and pain.3,4)
The causes of joint pain are various. It has been established that the mechanism of pain in joints is inflammation; however, the mechanism of onset has yet to be clearly elucidated. To suppress joint inflammation, an oral preparation of methotrexate and a subcutaneous injection of adalimumab are clinically used for systemic drug therapy. Methotrexate inhibits nucleic acid synthesis by inhibiting dihydrofolate reductase and relieves joint pain by immune system activation.5,6) Adalimumab binds to tumor necrosis factor-α (TNF-α), which is abundantly released in the joint and induses inflammation. It inhibits the binding of TNF-α to the receptor and the transmission of inflammatory signals. In addition, adalimumab binds to cells producing TNF-α and causes cell death.6) Systemic drug therapy can delay the progression of the disease; however, side effects such as myelosuppression and severe infections are becoming a problem.3,4)
Reduction of inflammation by the topical administration of steroids such as prednisolone farnesylate and the intra-articular injection of hyaluronic acid (HA) is also carried out. HA is a substance present in the joint cavity, it is abundant in joint fluid, and makes joint movement smooth. It is also a component of joint cartilage and the meniscus, and it works to reduce impacts on joints. Examples of joint pain-relievers using HA include Synvisc®, Artz®, and Suvenyl®.
In parallel with investigating the cause of inflammation, the development of medicines leading to true remission is being carried out. In order to suppress systemic side effects to perform effective drug-based treatment, it is desirable to inject a drug directly into the intra-articular space. In this study, we prepared conventional gel formulations with a fluorescent drug to investigate the fate of the drug after administering them intra-articularly. We traced the fate of indocyanine green (ICG), which is clinically used as an agent for liver/circulatory function tests and has also been used in pharmacokinetic studies as a fluorescent substance in laboratories,7) after intra-articular administration in rats. ICG is highly soluble in water and is suitable as a tracer by fluorescence imaging. We prepared two sustained-release gel formulations with methyl cellulose (MC) and HA. MC is a long-chain-substituted cellulose ether of 50–1500 anhydroglucose containing 26–32% methoxy groups, and it is used as a gelling agent in pharmaceutical formulations.8) HA originally consists of a chain structure of N-acetylglucosamine and glucuronic acid. It has a marked water-holding capacity: 1g is said to hold 6 L of water.9,10) It is characterized by a very high viscosity and elasticity, which differ depending on the concentration and molecular weight of HA.
In the present study, at first, we established our technique to administer the formulations precisely into the rat knee joint cavity. Then, we administered an aqueous formulation and two sustained-release gel formulations of ICG into the rat joint cavity to investigate the fate of ICG in the rat body.
We further investigated the pharmacological effect with “Drug A,” which is under development by a pharmaceutical company as a candidate drug to treat chronic inflammatory pain, acute pain, and autoimmune disease. Drug A is a compound with a molecular weight of about 500 and highly soluble in water. We verified the pharmacological effect of the sustained release gel formulation with HA with acute pain model rats prepared by injecting bradykinin solution into the knee joint.11–16)
This study showed that the gel formulations were effective to sustain the release of a drug in the knee joint and that the combination of a pain-relief drug with HA gel was effective to improve the movement of the acute pain model rats.
As a gel base, we used hyaluronic acid FCH-200 (HA, molecular weight: 1800–2200 kDa, Kikkoman Bio Chemifa Co., Ltd., Tokyo, Japan) and methyl cellulose 4000 cP grade (MC, molecular weight: about 88 kDa, Sigma-Aldrich, Tokyo, Japan). We used indocyanine green (ICG; Wako Pure Chemical Industries, Ltd., Osaka, Japan) as a fluorescent substance, water for injection (Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) as a dissolution liquid, and isoflurane (DS Pharma Animal Health Co., Ltd., Osaka, Japan) as an anesthetic. We used a 25-µL micro syringe (HAMILTON Co., Reno, NV, U.S.A.) equipped with a needle of 26 G×1/inner diameter 0.45×13 mm (TERUMO Co., Tokyo, Japan) for administration. The fluorescence of ICG administered to rats was detected with a real-time in-vivo imaging system (IVIS®; IVIS-SPECTRUM, Caliper Life Sciences, Hopkinton, MA, U.S.A.). The exposure time was set at 1 s. We used a 12.5 µM aqueous solution of bradykinin (Wako Pure Chemical Industries, Ltd.) as a pain-inducing agent.
AnimalsSprague-Dawley (SD) male rats weighing approximately 250 g (8 to 10 weeks old) purchased from Japan SLC, Inc. (Shizuoka, Japan) were used as the experimental animals. All animal experiments were carried out in accordance with the Guiding Principles for the Care and Use of Laboratory Animals approved by the Faculty of Pharmacy, Meijo University.
Establishment of Rat Knee Joint Cavity Administration ProcedureWe filled a 25-µL microsyringe with 20 µL of an aqueous solution of ICG for administration into the rat knee joint cavity under isoflurane inhalation anesthesia. We observed the behavior of ICG around the rat knees with IVIS. To visually confirm our technique to administer the solution precisely into the rat knee joint cavity, we sacrificed the rats and dissected the knees to observe the injection site.
Determination of Optimal ICG ConcentrationTo optimize the concentration of ICG solution, we prepared ICG solutions of 0.5, 1.0, and 2.0 mg/mL to inject into the right knee joint space of the rats. We measured the fluorescence intensity at the knee joint, liver, and small intestine with IVIS immediately after administration, and at 1, 3, and 6 h.
Preparation of Sustained-Release Gel FormulationWe added 75 mg of a gel base in small portions to 1.5 mL of a 1-mg/mL aqueous ICG solution at a room temperature. After adding the whole amount, we further agitated it under reduced pressure to prepare a uniform gel formulation without air bubbles.
Fate of ICG Administered into Knee Joint Cavity of RatsWe injected 20 µL of a 1.0 mg/mL aqueous ICG solution or each of the ICG-containing sustained release gel formulations into the rat right knee joint cavity. The fluorescence intensity around the knee joint, liver, and small intestine was measured with IVIS immediately after administration and at 1, 3, 6, 24, 72, and 168 h.
Extraction and Measurement of Residual Drug A in Rat Knee JointWe prepared aqueous solution and sustained-release gel formulations with MC and HA of Drug A as described for ICG formulations. Each formulation was administered into the rat knee joints. At 1, 2, 3, 5, 7 10, and 14 d, the rats were sacrificed and the knee joints were excised. Each knee joint was cut open and placed in a 50-mL Falcon tube, to which 4 mL of methanol was added and ultrasonic wave application or stirring was carried out for 30 min. Then, the knee joint was taken out of the Falcon tube and washed with 4 mL of saturated sodium sulfate solution, to which 4 mL of ethyl acetate was added and further stirred. Ten minutes later, the sodium sulfate/methanol aqueous solution was centrifuged at 10000 rpm for 10 min. Three milliliters of ethyl acetate was added to the supernatant and it was stirred for 10 min, and then centrifuged to recover the supernatant; this was repeated twice. Ten milliliters of the recovered solution were added to a tube placed in a heat block at 50°C and dried in a nitrogen stream. The residue was dissolved in 1 mL of methanol and the Drug A concentration was determined by an HPLC system (Agilent Technologies, Inc., U.S.A.) consisting of a quaternary pump (G1311A), degasser (G1322A), UV-Vis detector (G1314B), column oven (G1316A), and auto sampler (G1329A). The mobile phase was composed of acetonitril and 10 mM ammonium acetate (4 : 1, volume ratio). The flow rate was set at 1.0 mL/min. The column (YMC-Triart C18, 50×4.6 mm i.d.; YMC Co., Ltd., Kyoto, Japan) was heated at 35°C. The injection volume was 100 µL. The UV absorbance of each sample was measured at 230 nm.
A calibration curve of the drug was made with Drug A solutions prepared by adding different amounts of Drug A into the sodium sulfate/methanol aqueous solution obtained from the Drug A-free knee joint. It was confirmed that the UV absorbance was correlated linearly with the amount of Drug A added between 0.2 and 200 µg.
Pharmacological Effect of Sustained-Release Gel Formulation of Drug AWe examined the effect of the sustained-release gel formulation of Drug A by a simple pharmacological test.
We administered water for injection, 20 µL of an aqueous solution of Drug A, or 20 µL of HA gel formulations with or without Drug A into the right knee joint cavity. One and 3 d later, we applied the walking test described below. Then, we administered a 12.5 µM aqueous solution of bradykinin to the knee joint cavity of the rats, and applied the walking test again to evaluate the pain-relief effect of Drug A in a solution or gel formulation.
Walking TestWe evaluated the mobility of the rats by tracing their walking distance on placing them in a test case with a length of 55 cm and a width of 110 cm for 3 min.
Statistical AnalysisStatistical comparisons were made with a one-way ANOVA. Comparisons of means were performed with the least significant difference test. The significance level was set at p<0.05 or 0.01.
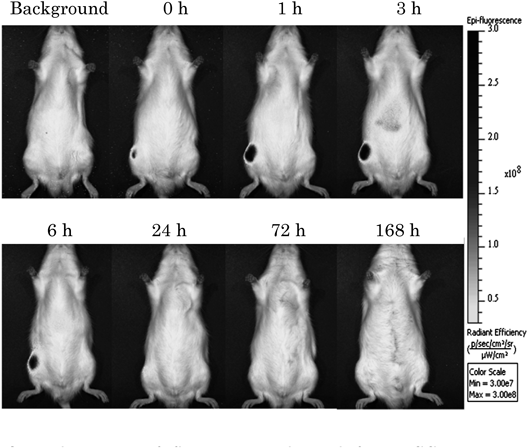
After training, we were able to identify the position of the rat knee joint cavity by touching it with our fingers from the surface of the skin where we were going to inject the ICG solution (Fig. 1). We confirmed that the dose of 20 µL was adequate and did not leak out from the joint cavity. In the following experiment, the dose administered in the rat knee joint cavity was fixed at 20 µL.

An ICG solution was injected through the surface of the skin into the knee joint (a), and this was visually confirmed by dissecting the knee (b, c).
We administered 20 µL of aqueous ICG solutions of 0.5, 1.0, and 2.0 mg/mL into the knee joint cavity of the rats and observed fluorescence with IVIS for 6 h (Fig. 2). It was confirmed that ICG injected into the knee joint cavity migrated to the liver and small intestine by bile excretion. While the fluorescence intensity observed in rats administered 2.0 mg/mL solution was higher than those administered 0.5 and 1.0 mg/mL, any of the three concentrations was applicable to monitor the fate of ICG in the body of the rats. In the following experiment, the concentration of ICG was fixed at 1.0 mg/mL.

When the gel base was dissolved in aqueous ICG solution at a normal pressure, fine bubbles entered the gel and made it turn white. On the other hand, the gel prepared under a reduced pressure had no bubbles and was considered to be suitable as a gel formulation to administer in the knee joint cavity of rats.
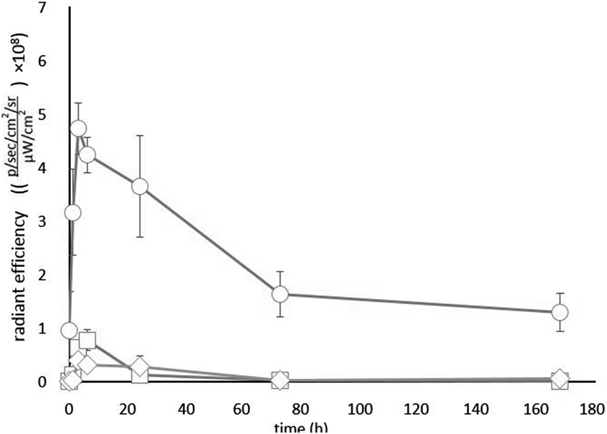
Fate of ICG Administered into Knee Joint Cavity of RatsWe administered 20 µL of ICG solution at 1.0 mg/mL into the rat right knee joint cavity and measured the fluorescence intensities around the knee joint, liver, and small intestine using IVIS immediately after administration and at 1, 3, 6, 24, 72, and 168 h (Fig. 3). The fluorescence intensity around the knee joint peaked at 1 h after administration. The leakage from the knee joint cavity of ICG was rapid; almost no fluorescence could be observed at 24 h, and almost all ICG had disappeared within 72 h (Fig. 4).

Knee (○), liver (□), and intestine (◇). Each value represents the mean±standard deviation (S.D.) (n=3).
Next, we administered MC and HA gel formulations. The fluorescence intensity at the knee peaked at 3 h after administration, and gradually decreased until 72 h thereafter for both formulations. Fluorescence was observed around the knee even after a week (Figs. 5–8), suggesting that the simple gel formulations with MC and HA were effective to suppress the rapid leakage of ICG from the knee cavity.


Knee (○), liver (□), and intestine (◇). Each value represents the mean±S.D. (n=3).

Knee (○), liver (□), and intestine (◇). Each value represents the mean±S.D. (n=3).
Because Drug A has no fluorescence that can be monitored by IVIS, we extracted it from the rat knee to determine it by HPLC. It was confirmed that Drug A in the gel formulations remained for longer in the knee joint compared with that in aqueous solution (Fig. 9). The HA formulation could retain Drug A for a long time compared with the MC formulation.

Each value represents the mean±S.D. (n=3).
The effects of 4 formulations, water for injection, an aqueous solution of Drug A, and HA gel formulations with or without Drug A, were compared by the walking test (Table 1). Rats administered water for injection experienced pain after the administration of bradykinin and hardly walked. The walking distance of the rats was markedly decreased by about one-third by the administration of bradykinin on days 1 and 3, being a significantly shorter distance than those for the other 3 formulations. The aqueous solution of Drug A was effective on day 1; the walking distance was two-thirds of that before the injection of bradykinin. However, the effect disappeared on day 3; the walking distance was decreased to less than half, being a significantly shorter distance than those for the HA gel formulations with or without Drug A. The HA gel formulation without Drug A was more effective than the aqueous solution of Drug A. The walking distance was more than 70% of that before the administration of bradykinin on days 1 and 3. However, they were considered to feel pain. They were lazy, walked more slowly, and showed poorer movements than those administered sustained-release gel formulations. The most effective formulation was the HA gel formulation with Drug A; the walking distance of the rats was about 85% on days 1 and 3. On day 3, the decreases in walking distance with the 4 formulations were significantly different: the order of the walking distance was the HA gel formulations with Drug A>HA gel formulations without Drug A>the aqueous solution of Drug A>water for injection.
| Formulation | Bradykinin administration (day 1) | Bradykinin administration (day 3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before administration | After administration | Before administration | After administration | ||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| Water | Walking distance (cm) | 713 | 649 | 675 | 193 | 248 | 211 | 516 | 721 | 648 | 229 | 285 | 174 |
| Decrease in walking distance (%) | 27.1 | 38.2 | 31.3 | 44.4 | 39.5 | 26.9 | |||||||
| Average±S.D. (%) | 32.2±5.6 | 36.9±9.1 | |||||||||||
| Aqueous Drug A solution | Walking distance (cm) | 708 | 637 | 598 | 426 | 468 | 403 | 694 | 773 | 747 | 309 | 330 | 356 |
| Decrease in walking distance (%) | 60.2 | 73.5 | 67.4 | 44.5 | 42.7 | 47.7 | |||||||
| Average±S.D. (%) | 67.0±6.7 | 45.0±2.5 | |||||||||||
| HA Gel formulation without Drug A | Walking distance (cm) | 708 | 752 | 734 | 610 | 518 | 481 | 675 | 709 | 693 | 456 | 536 | 498 |
| Decrease in walking distance (%) | 86.2 | 68.9 | 65.5 | 67.6 | 75.6 | 71.9 | |||||||
| Average±S.D. (%) | 73.5±11.1 | 71.7±4.0 | |||||||||||
| HA Gel formulation with Drug A | Walking distance (cm) | 829 | 785 | 763 | 682 | 640 | 675 | 802 | 661 | 696 | 697 | 589 | 608 |
| Decrease in walking distance (%) | 82.3 | 81.5 | 88.5 | 86.9 | 89.1 | 87.4 | |||||||
| Average±S.D. (%) | 84.1±3.8 | 87.8±1.2 | |||||||||||
On day 1, the decrease in walking distance with Water was significantly less than those with the other 3 formulations (p<0.01) and that with Aqueous Drug A solution was significantly less than that for the HA Gel formulation with Drug A (p<0.05). On day 3, the decrease in walking distance with Water was significantly less than those with the other 3 formulations (p<0.01), that with Aqueous Drug A solution was significantly less than those for the HA Gel formulations with or without Drug A (p<0.01), and that for the HA Gel formulation without Drug A was significantly less than that for the HA Gel formulation with Drug A (p<0.01).
In this study, it was confirmed that the residence time of the solution administered into the rat knee joint cavity was extremely short. The fluorescence intensity of ICG injected into the knee joint cavity reached a peak at 1 h and almost no ICG remained at 24 h. Since fenestrated blood vessels are distributed in the synovial membrane in the joint, it is considered that the drug injected into the synovial fluid quickly moves into the blood.10) The accumulation of ICG in the aqueous solution was limited to 24 h due to the rapid leakage of ICG from the knee joint cavity, which suggested that the development of a sustained-release formulation is necessary for effective drug treatment of rheumatoid arthritis. To maintain the concentration of a pain-relief drug in the knee joint cavity high enough to suppress inflammation, the release rate of the drug from the formulation into the synovial fluid needs to exceed the disappearance rate of the drug from the fluid.
The sustained-release gel formulations we prepared employed MC and HA, swelling and dissolving additives mainly used for sustained-release oral formulations. The gel formulation is simple and also expected to have joint pain-relief properties due to the viscoelasticity of the gel. Indeed, injections of HA for intraarticular and subcutaneous local administration have been clinically used. It has been reported that HA has affinity for cartilage and protects it from destruction.8,15,16) However, HA has rarely been clinically used as a drug carrier. There are also few data on monitoring the fate of drugs administered as a gel formulation inside the joint space.
Three hours after administration of the MC and HA gel formulations, the ICG concentration around the knee reached maximum contrary to our expectation that it would have decreased right after administration. To give an insight into this phenomena, we measured the fluorescence intensity of aqueous ICG solutions of 0.01 to 10000 µg/mL. The intensity was maximized at 30 µg/mL and it decreased according to the increase in the ICG concentration. The fluorescence intensity of 1 mg/mL ICG solution, which was equivalent to the ICG concentration of the present gel formulations, was only one-twentieth of that of 30 µg/mL solution. We guess that the increase in fluorescence intensity around the knee up to 3 h would suggest the release of ICG from the gels. The fluorescence intensity around the knee of rats administered the aqueous ICG solution reached maximum in 1 h, which suggested rapid release of ICG from the solution.
ICG in the MC and HA gel formulations prepared in the present study remained in the joint cavity even 3 d after the intraarticular administration in rats. The simple gel formulations successfully sustained ICG release, and the drug was released for a week after administration. In addition, a pain-relief drug, Drug A, in the gel formulations remained for longer in the knee joint compared with that in the aqueous solution, suggesting that the gel formulation was effective to maintain the pain-relief effect of Drug A administered in the knee cavity.
The viscosity of the gel generally influences sustained release. Although the viscosity of the MC and HA gels used in this study was measured using a rotational viscometer with a measurement range of 3 to 30000 mPa·s, the viscosity of the gels exceeded the upper measurement limit. When injecting the gel formulations into the knee joint cavity of rats, we felt stronger resistance with the HA gel than the MC gel, suggesting that the viscosity of the HA gel might be higher than that of the MC gel. However, we consider that the viscosity of the MC and HA gels was not a critical factor in this study because the in-vivo release of ICG from these gels were almost equivalent. We further examined the effect of molecular weight of HA on the fate of ICG by administering a gel formulation prepared with Synvisc®, a commercially available gel injectable containing 8 mg/mL of cross-linked HA with a molecular weight of 6 million in rat knee. However, there was no significant difference in the transition of the fluorescence intensity after administration in rat knee joint cavity (data not shown).
Drug A is a candidate anti-arthritis drug and its efficacy has been confirmed in animals and humans through oral administration by a pharmaceutical company, but systemic side effects are a concern. Therefore, pain therapy and the suppression of inflammation were attempted by the administration of an aqueous solution of Drug A intra-articularly. However, the effect on joint inflammation was not confirmed with an aqueous intra-articular injection formulation in animals. It is thought that Drug A administered as a solution did not stay inside the joint cavity but flowed out because the flow of joint fluid out of the joint cavity was fast enough to clear it from inside the joint cavity.17) As a result, it is considered that the effective concentration of Drug A in the joint cavity was not maintained for a sufficient time.
In the present study, Drug A in the HA formulation was retained for a longer time than that in the MC formulation as well as the aqueous solution. Based on this finding, we employed HA as the gel base in the following pharmacological study with acute pain model rats prepared by injecting bradykinin solution into the knee joint. Contrary to Drug A, the release of ICG from the MC and HA gels was almost equivalent. Drug A and ICG are water soluble and their molecular weights, about 500 and 775, respectively, are close to each other. We could not explain the difference between ICG and Drug A based on water solubility or molecular weight. We speculate that other factors such as interaction between the drug and polymer would affect the different release pattern.
The walking test showed that the pain-relief effect of the HA gel formulation containing Drug A was increased and prolonged compared with the aqueous solution of Drug A. The HA gel formulation without Drug A was also significantly effective compared with the water for injection, which is in accordance with the fact that HA gel formulations have been used clinically for rheumatoid arthritis patients. The decreases in the walking distance of the rats administered the HA gel formulation without Drug A on days 1 and 3 were almost the same, suggesting that the HA gel remained in the knee cavity for 3 d. On day 3, the HA gel formulation with Dug A was significantly more effective than that without Drug A and the aqueous Drug A solution, suggesting that synergistic effects of the combination of Drug A with HA gel worked to improve the mobility of the acute pain model rats. Although we did not examine the systemic side effects of Drug A intra-articularly administered into the knee joint as the HA gel formulation, we expect them to be weaker than those orally administered because the leakage of Drug A should be suppressed by the gel formulation. Since pain due to bradykinin does not reproduce rheumatoid inflammation, it should be noted that permeability of a substance from the joint to the blood vessel may be different in actual rheumatism. However, it is considered that the HA gel formulation is effective for sustained release of a substance in actual rheumatism. Since the gel formulation is a simple prescription, it is considered to be suitable for clinical development.
From these results, we believe that an intra-knee joint sustained-release gel formulation can be conveniently prepared and is a useful preparation capable of a slow release. However, based on the time–course of the fluorescence intensity of ICG and concentration of Drug A administered in the rat knee joint as the HA gel formulations, there is still room to improve the formulation for a prolonged action and controlled release. Now, we are investigating an HA gel formulation containing ICG-microspheres to meet these requirements.18–24)
The authors declare no conflict of interest.