2017 年 40 巻 6 号 p. 910-915
2017 年 40 巻 6 号 p. 910-915
In a study to find ways to prevent the side effects of indomethacin (IMC), we previously reported that magnesium ion (Mg2+) can prevent the onset of IMC-induced gastric mucosa in adjuvant-induced arthritis (AA) rats, a model for rheumatoid arthritis (RA). In this study we investigated whether the co-administration of IMC and Mg2+ prevents the formation and aggravation of intestinal ulcerogenic lesions in AA rats. The single oral administration of an excessive dose of IMC (40 mg/kg) induces hemorrhagic lesions and nitric oxide (NO) production via inducible nitric oxide synthase (iNOS) in the jejunal and ileal mucosa of AA rats, and the extent of the lesions, as well as iNOS and NO levels in AA rats are higher than in normal rats. On the other hand, the co-administration of 200 mg/kg Mg2+ attenuates intestinal ulceration and the elevation in the iNOS and NO levels in AA rats. Further, hemorrhagic lesioning and enhanced iNOS and NO levels in AA rats also result from the repetitive oral administration of 3 mg/kg IMC (therapeutic dose) for 42 d (once a day), and these changes are also prevented by the co-administration of 200 mg/kg Mg2+. In conclusion, the co-administration of Mg2+ suppresses the ulcerogenic response to IMC in the jejunal and ileal mucosa of AA rats, probably by preventing the elevation of iNOS and NO levels in the region.
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been to treat rheumatoid arthritis (RA)1); however, it is well known that the oral administration of these drugs leads to gastroenteropathy as a significant side effects,2) and that RA patients taking NSAIDs are more susceptible to NSAIDs-induced intestinal ulcerogenic lesions than other patients or control subjects.2–4) Therefore, the development of NSAIDs that do not cause gastroenteropathy or other significant side effects is highly desired.
It has been reported that a decrease in prostaglandins and excessive production of inducible nitric oxide synthase (iNOS) and nitric oxide (NO) are related to the formation and pathogenesis of intestinal ulceration induced by NSAIDs.4–11) In addition, we previously reported that the co-administration of magnesium ion (Mg2+) and NSAIDs (indomethacin (IMC) and loxoprofen) prevents the increase in iNOS and NO production in the gastric mucosa, resulting in the inhibition of the onset of NSAIDs-induced gastric lesions.12,13) Therefore, the co-administration of Mg2+ may also suppress the onset of intestinal ulcerogenic lesions by NSAIDs, and lead to develop a method for administering NSAIDs that will not lead to gastroenteropathy.
The changes in the biological characteristics of adjuvant-induced arthritis (AA) rats correspond to those that occur in human RA.2,4,14,15) Moreover, AA rats have been reported to show gastric and small intestinal mucosal lesions induced by IMC, naproxen, and aspirin.2,4) In addition, the gastric and small intestinal mucosal lesions in AA rats administered conventional NSAIDs are significantly aggravated as compared with normal rats.2,4) These findings suggest the AA rats may provide a useful model for studies on the suppression of NSAIDs-induced intestinal ulcerogenic lesions in RA. In this study, we investigated whether the co-administration of Mg2+ prevents the formation and aggravation of intestinal ulcerogenic lesion in IMC-administered AA rats.
Dark Agouti (DA, normal, male, 6–13 weeks of age) rats was provided by Shimizu Laboratory Supplies Co., Ltd. (Kyoto, Japan). IMC and carboxymethyl cellulose were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), Nacalai Tesque (Kyoto, Japan), respectively. RNA PCR Kit was given from TaKaRa Bio Inc. (Shiga, Japan), and LightCycler FastStart DNA Master SYBR Green I was purchased from Roche Diagnostics Applied Science (Mannheim, Germany). Bio-Rad Protein Assay Kit and heat-killed Mycobacterium butyricum were provided by BIO-RAD (California, U.S.A.), Difco (Detroit, MI, U.S.A.), respectively. All other chemicals used were of the highest purity commercially available. Fifty microliters of bayol F oil containing 10 mg/mL heat-killed Mycobacterium butyricum (adjuvant) was injected into the plantar region of the right hind foot and tail of DA rats to induce RA (AA rat). All animal experiments were performed in accordance with the Kindai University School of Pharmacy Committee for the Care and Use of Laboratory Animals.
Oral Administration of IMC and Mg2+The IMC suspension for administration was prepared by suspending IMC in purified water (PW) containing 1.0% carboxymethyl cellulose. Two doses, an overdose (40 mg/kg) and a therapeutic dose (3 mg/kg), were used to demonstrate the preventive effects of Mg2+ on IMC-induced intestinal ulcerogenic lesions. In the study using the overdose (40 mg/kg), the IMC suspension was administered orally on day 14 after adjuvant injection. For the other dose (therapeutic dose, 3 mg/kg), rats were administered 3 mg/kg IMC (once a day) for 42 d after adjuvant injection. In this study, the water containing Mg2+ (magnesium water, MW) was prepared by using the MgSO4, and administrated orally to the rats. For the data shown in Fig. 2, MW (Mg2+, 200 mg/kg) was provided pre-, co-, post-IMC administration to the rats, with the dose interval for the pre- and post-administrations set at 30 min before and after IMC administration, respectively.
Evaluation of Intestinal Ulcerogenic LesionsRats were killed under deep isoflurane anesthesia 24 h after the last IMC administration.2,16) The small intestine (duodenum, jejunum and ileum) was excised, washed and fixed in 10% formalin solution, and the area of the intestinal ulcerogenic lesions was observed using Image J software (NIH). The lesion area in the total small intestine consisting of the jejunum and ileum is expressed as the percentage of the total area of each part. No ulcerogenic lesions in the duodenum were observed following the administration of either 3 mg/kg or 40 mg/kg IMC in this study. The duodenum, jejunum and ileum are defined as follows: duodenum, lower 2 cm of the stomach; jejunum (27.0±1.2 cm), 40% of the upper part of the small intestine except the duodenum; ileum (41.2±1.9 cm), 60% of the lower part of the small intestine except the duodenum (n=76).
Quantitative PCR (Real-Time PCR)Total RNAs in the epithelium of jejunum and ileum were extracted by the acid guanidium thiocyanate–phenol–chloroform extraction method,12,13) and the RT-PCR reaction was performed using an RNA PCR Kit and LightCycler FastStart DNA Master SYBR Green I according to the manufacturers’ instructions.12,13) Specific primers for iNOS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used: 5′-GGA GAG ATT TTT CAC GAC ACC C-3′ and 5′-CCA TGC ATA ATT TGG ACT TGC A-3′ for iNOS (NM 012611); and 5′-ACG GCA CAG TCA AGG CTG AGA-3′ and 5′-CGC TCC TGG AAG ATG GTG AT-3′ for GAPDH (NM 017008). The conditions for PCR were: 95°C for 10 min, 50 cycles of denaturing (10 s, 95°C), annealing (10 s, 60°C) and extension (5 s, 72°C). The PCR was performed using a LightCycler DX 400 (Roche Diagnostics Applied Science, Mannheim, Germany).
Measurement of NO LevelsThe epithelium of the jejunum and ileum were homogenized in saline on ice, and centrifuged at 10000 rpm (15 min, 4°C). The resultant supernatants were used for the measurement of NO levels. The experiment was performed according to our previous reports using an A-1-20-05 microdialysis probe and ENO-20 NO analyzer (Eicom, Kyoto, Japan).12,13) In this paper, the amounts of NO reflect the level of the NO2− metabolite, which is produced from NO. The data are presented as nmol/mg protein, and the protein levels were measured using a Bio-Rad Protein Assay Kit.
Statistical AnalysisAll values are expressed as the mean±standard error of the mean (S.E.M.). Statistical comparisons were evaluated by Dunnett’s multiple comparison. p<0.05 was considered significant.
Figure 1 shows the preventive effect of Mg2+ on intestinal ulcers induced by a single overdose of IMC. The extent of the IMC-induced hemorrhagic lesions in the both jejunal and ileal mucosa of AA rats was reduced by the co-administration of Mg2+. Although hemorrhagic lesions in the jejunal mucosa were attenuated more as the dose was increased from 50 to 200 mg/kg Mg2+, a similar degree of attenuation was observed at Mg2+ doses of 200 and 400 mg/kg. On the other hand, the hemorrhagic lesions in the ileal mucosa were attenuated more as the Mg2+ dose increased from 50 to 400 mg/kg Mg2+. Figure 2 shows changes in intestinal ulcers induced in AA rats by a single overdose of IMC when MW (Mg2+, 200 mg/kg) was administered 30 min before (pre-), at the same as (co-), or 30 min after (post-) IMC administration. No significant differences were observed based on when the Mg2+ was administered. Figures 3 and 4 show changes in lesion area of the small intestine (Fig. 3), and iNOS and NO production (Fig. 4) in rats administered a single overdose of IMC with or without MW. The single overdose of IMC caused intestinal ulcers, and the co-administration of MW significantly decreased the extent of the intestinal ulcerogenic lesions. The iNOS mRNA and NO levels in AA rats administered a single overdose of IMC were higher than in AA rats not administered IMC, and the enhanced iNOS mRNA and NO levels were also attenuated by the co-administration of MW.
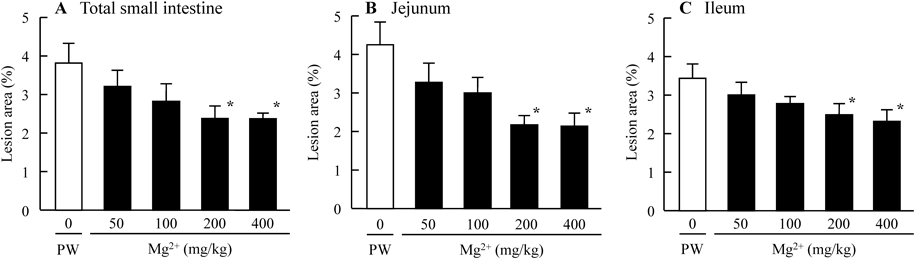
AA rats were co-administrated 0–400 mg/kg Mg2+ and 40 mg/kg IMC. A–C: lesion scores in the total small intestine (A), jejunum (B) and ileum (C) of IMC-administered AA rats. Open columns (PW groups): AA rats co-administered PW and IMC; closed columns: AA rats co-administered MW and IMC. n=7–12. * p<0.05, vs. PW groups for each category.
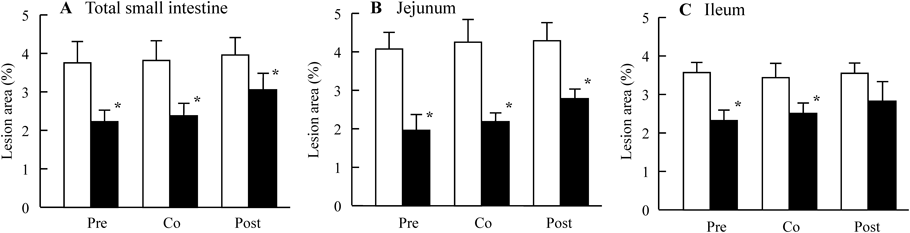
IMC was administered to AA rats 14 d after adjuvant injection. were pre-, co-, post-treated with MW (Mg2+, 200 mg/kg) was administered 30 min before (Pre), together with (Co), or 30 min after (Post) IMC administration. A–C: lesion scores in the total small intestine (A), jejunum (B) and ileum (C) of IMC-administered rats. Open columns: AA rats administered PW and IMC; closed columns: AA rats administered MW and IMC. n=7–10. * p<0.05, vs. AA rats administered PW and IMC for each category.
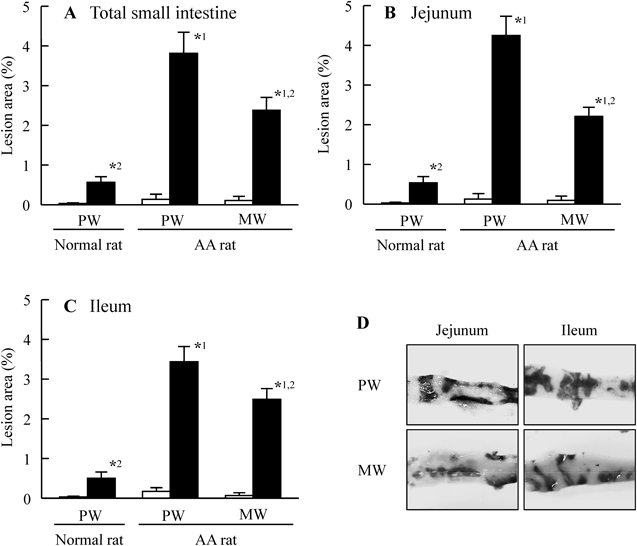
Rats were co-administered either PW or MW (Mg2+, 200 mg/kg) at the time of IMC administration. A–C: lesion scores in the total small intestine (A), jejunum (B) and ileum (C) of normal and AA rats. D: intestinal images of the jejunum and ileum of IMC-administered AA rats treated with or without MW. Open columns: vehicle-administered rats treated with PW or MW (no IMC); closed columns: IMC-administered rats treated with PW or MW. n=6–9. *1 p<0.05, vs. PW group in IMC-administered normal rats for each category. *2 p<0.05, vs. PW group in IMC-administered AA rats for each category.
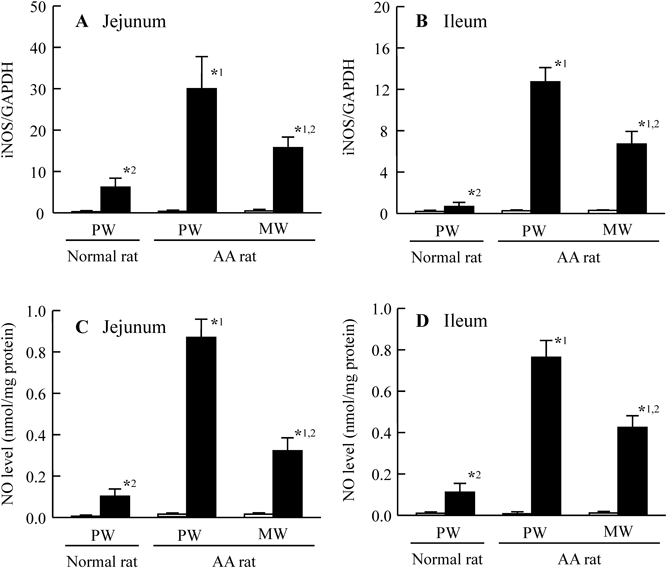
Rats were co-administered with PW or MW (Mg2+, 200 mg/kg) and IMC (40 mg/kg). A, B: iNOS mRNA levels in the jejunum (A) and ileum (B) of normal and AA rats. C, D: NO levels in the jejunum (C) and ileum (D) of normal and AA rats. Open columns: vehicle-administered rats treated with PW or MW (no IMC); closed columns: IMC-administered rats treated with PW or MW. n=6–9. *1 p<0.05, vs. PW group in IMC-administered normal rats for each category. *2 p<0.05, vs. PW group in IMC-administered AA rats for each category.
Figure 5 shows the changes in the lesion area of the jejunum and ileum in AA rats treated with repetitive daily therapeutic doses of 3 mg/kg IMC. Although hemorrhagic lesions developed in AA rats in response to the repetitive administration of 3 mg/kg IMC, the lesion area was smaller than in the acute model involving a single administration of 40 mg/kg IMC. The lesion area in AA rats receiving repetitive therapeutic doses of IMC was sharply reduced by the co-administration of MW, and the lesion area of in the total small intestine in AA rats receiving the co-administration of IMC and MW was 1.68% as compared with rats treated with IMC alone. Figure 6 shows the changes in iNOS mRNA level and NO production in repetitively IMC-administered AA rats treated with or without MW. Increases in iNOS mRNA and NO levels in AA rats were also observed following the repetitive administration of 3 mg/kg IMC, and the co-administration of MW attenuated these increases in the jejunum and ileum.

AA rats were administered a therapeutic dose (3 mg/kg) of IMC once a day for 42 d after adjuvant injection. None, non-treated AA rat. Vehicle, vehicle-administered AA rat treated with PW. IMC, IMC-administered AA rat treated with PW. MW, IMC-administered AA rat treated with MW (Mg2+, 200 mg/kg). A–C: lesion scores of the total small intestine (A), jejunum (B) and ileum (C) of AA rats. D: intestinal images of the jejunum and ileum of IMC-administered AA rats treated with PW or MW. n=6–9. *1 p<0.05, vs. none for each category. *2 p<0.05, vs. vehicle for each category. *3 p<0.05, vs. IMC for each category.
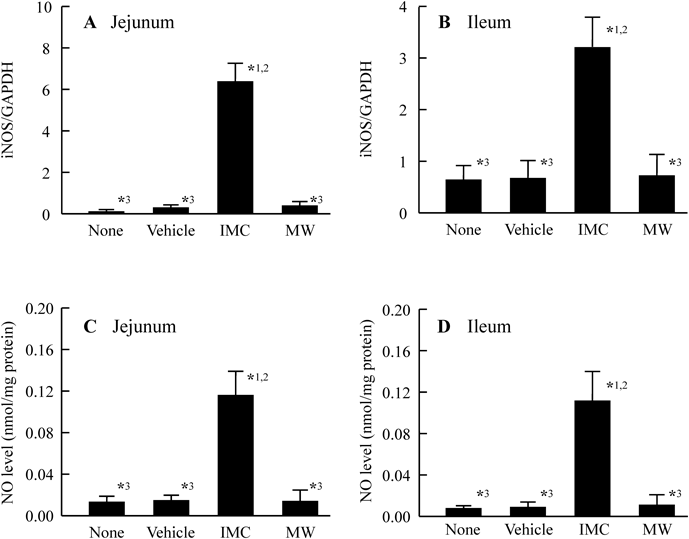
AA rats were administered a therapeutic dose (3 mg/kg) of IMC once a day for 42 d after adjuvant injection. None, non-treated AA rat. Vehicle, vehicle-administered AA rat treated with PW. IMC, IMC-administered AA rat treated with PW. MW, IMC-administered AA rat treated with MW (Mg2+, 200 mg/kg). A, B: iNOS mRNA levels in the jejunum (A) and ileum (B) of IMC-administered AA rats. C, D: NO levels in the jejunum (C) and ileum (D) of IMC-administered AA rats. n=6–9. *1 p<0.05, vs. none for each category. *2 p<0.05, vs. vehicle for each category. *3 p<0.05, vs. IMC for each category.
The purpose of this study was to find a method for administering IMC that does not lead to gastroenteropathy. We show that the co-administration of Mg2+ acts to decrease intestinal ulcerogenic lesions in AA rats receiving orally administered with IMC.
First we demonstrated that Mg2+ prevents the excessive intestinal ulceration induced in AA rats administered a single overdose of IMC (40 mg/kg). In normal rats 14 d after adjuvant injection, few ulcerogenic lesions in the duodenum were observed following the oral administration of a single overdose of IMC. On the other hand, in AA rats, the oral administration of a single overdose of IMC induced hemorrhagic lesions in the jejunal and ileal mucosa, and the area of the hemorrhagic lesions area in the IMC-administered AA rats was significantly larger than in IMC-administered normal rats (Fig. 3). It is known that gastrointestinal toxicity is the main adverse effect of IMC, and it has been reported that patients with RA are more susceptible to NSAIDs-induced intestinal ulcerogenic lesions than other NSAIDs users.2–4,15) In addition, it has been reported that the gastric and small intestinal mucosal lesions in AA rats administered conventional NSAIDs are significantly aggravated as compared with normal rats.2,4) These results show that AA rats can be used to investigate the effect of Mg2+ on IMC-induced intestinal ulcerogenic lesions.
Next, we investigated that the relationship between IMC-induced intestinal ulcers and Mg2+ dosage. MW attenuated IMC-induced intestinal ulcers in proportion to increasing Mg2+ dose (Fig. 1), and the preventive effect of MW reached a plateau at 200 mg/kg Mg2+ in the jejunum. Moreover, the preventive effect of MW on IMC-induced intestinal ulcers was similar whether the MW was administered 30 min before (pre-), at the same time as (co-), or 30 min after (post-) IMC administration (Fig. 2). In addition, we previously reported that the co-administration of Mg2+ does not affect the plasma IMC concentration.17) These results show that MW can suppress the onset of the IMC-induced intestinal ulcers.
It is important to clarify the mechanism of the preventive effect of Mg2+ against the IMC-induced intestinal ulcers in AA rats. It has been reported that excessive NO via iNOS plays a key pathogenic role in the ulcerogenic response (Whittle et al.10) and Konaka et al.11)). Tanaka et al.18) also reported that a selective inhibitor of iNOS (aminoguanidine) prevents the development of IMC-induced intestinal ulcerogenic lesions in AA rats. These reports suggest that the up-regulation of iNOS/NO contributes to NSAID-induced intestinal ulceration. Therefore, we measured the changes in iNOS mRNA and NO levels in IMC-induced intestinal ulcerogenic lesions treated with or without MW. The iNOS mRNA and NO levels in AA rats administered a single overdose of IMC were higher than those in untreated AA rats, and these enhanced iNOS mRNA and NO levels were also attenuated by the co-administration of MW (Fig. 4). Moreover, our previous report showed that Mg2+ prevents the expression in iNOS mRNA in the gastric mucosa of AA rats treated with 40 mg/kg IMC or 100 mg/kg loxoprofen.12,13) In addition, it was reported that Mg2+ deficiency leads to increased transcription of the iNOS gene by the enhancing nuclear factor-kappaB (NF-κB) activation in the rat macrophage,19) and we also reported that the Mg2+ related the excessive NO production via iNOS in the cultured human lens epithelial cell.20) Taking these findings together, we hypothesise that the administration of excessive IMC enhances the production of excessive NO via iNOS in the jejunal and ileal mucosa of AA rats, and that this enhancement of NO aggravates the formation of intestinal ulcerogenic lesions in IMC-administered AA rats. The co-administration of Mg2+ attenuates NO production via iNOS, resulting in the suppression of intestinal ulcerogenic lesions induced by IMC.
We also measured intestinal ulcerogenic lesions in AA rats treated with repetitive therapeutic doses of IMC (3 mg/kg). The repetitive administration of 3 mg/kg IMC induced intestinal ulcers in AA rats; however, the hemorrhagic ratio was lower than for those induced by the single overdose of IMC (40 mg/kg) IMC. The lesion area in the total small intestine of AA rats treated with repetitive therapeutic doses of IMC was approximately 0.35% (Fig. 5). Also, increases in the iNOS mRNA and NO levels in AA rats was observed following the administration of repetitive therapeutic doses of IMC (Fig. 6). These results support the notion that the mechanism for the induction of intestinal ulcerogenic lesions in the single overdose model is similar to that for repetitive therapeutic dose model, and both models are useful for studies on the suppression of IMC-induced intestinal ulcerogenic lesions in RA. Moreover, we demonstrated the preventive effect of Mg2+ on the induction of intestinal ulcerogenic lesions in AA rats treated with repetitive therapeutic doses of IMC. The co-administration of MW prevented the induction of lesions in AA rat treated with repetitive therapeutic doses of IMC (Fig. 5) as well as the increases in the levels of iNOS mRNA and NO in the jejunum and ileum (Fig. 6). In addition, the preventive effect by the administration of 200 mg/kg Mg2+ was higher than that in low dose (50 mg/kg) of Mg2+ (lesion area in total small intestine, 0.17±0.03%, n=6). There results showed that the ulcerogenic response was prevented by the administration of Mg2+. Further studies are needed to clarify the precise mechanisms of the therapeutic effects of the combination of Mg2+ and IMC. We previously reported that the excessive expression of interleukin (IL)-18 is related to the induction of iNOS, NO and interferon-γ in the intestinal mucosa of AA rats administered IMC.21) Therefore, we are now planning to investigate the relationship between Mg2+ and IL-18 expression in the small intestinal mucosa of IMC-administered AA rats.
In conclusion, we have found the co-administration of Mg2+ with IMC suppresses the ulcerogenic response in the jejunal and ileal mucosa of AA rats, probably by preventing the enhancement of iNOS and NO levels. Our previous reports showed that the oral administration of Mg2+ to AA rats prevents the development of inflammation, and the combination of IMC and Mg2+ provide an effective therapy of arthritic edema.17) Therefore, these findings provide significant information that can be used to design further studies to develop potent NSAIDs that do not cause gastrointestinal toxicity.
The authors declare no conflict of interest.