2017 年 40 巻 8 号 p. 1183-1191
2017 年 40 巻 8 号 p. 1183-1191
Exosomes are derived from various sources, including primary and cultured cell lines and body fluids. It is now evident that they are important for communication between cells. They have, therefore, been proposed as potential carriers to deliver drugs to specific sites. In this study, we examined stability of exosomes derived from human saliva. Exosomes were stored at 4°C for up to 20 months and their membrane integrity assessed. Several exosomal markers, such as dipeptidyl peptidase IV (DPP IV; membrane marker) and programmed cell death 6-interacting protein (Alix, lumen marker), were retained intact after 20 months storage at 4°C. Moreover, intact exosomes could be isolated from whole saliva that had been stored at 4°C. Membrane disruption with detergents such as Triton X-100 and Nonidet P-40 caused partial solubilization of DPP IV and release of Alix into the supernatant. In contrast, sodium dodecyl sulfate treatment caused a complete disruption of the membrane. In addition, membrane stability was maintained after freezing and thawing. These results indicated that human saliva-derived exosomes are stable, maintaining their membrane integrity over a long storage period.
Exosomes are extracellular vesicles, with diameters of 30–100 nm, secreted from various types of cells. These include reticulocytes, lymphocytes, dendritic cells and intestinal epithelial cells.1,2) Exosomes contain various biomolecules, such as RNAs and proteins, and are released into the extracellular space upon fusion of multivesicular bodies with the plasma membrane. Thus, it is now recognized that exosomes are involved in intercellular communication via interacting with target cells and delivering their contents.3) Many RNAs, proteins and lipids are now listed in the database of exosomes, ExoCarta, as candidates for intracellular communication molecules.4) In fact, it was reported that exosomes contained both mRNA and microRNA (miRNA) that could be transferred to and be functional in target cells.5) In addition, it was also suggested that exosomes could be distinctive biomarkers for diseases such as cancer and Alzheimer’s disease.6–8)
Human whole saliva (WS) is an aqueous complex mixture of proteins and minerals.9) It contributes to maintaining oral cavity integrity through its lubricating, antibacterial, antiviral and buffering actions. Because exosomes were reportedly released into WS, it is important to elucidate their contributions to the pathophysiological roles of saliva. In our previous research, we isolated two types of extracellular vesicles from human WS and designated them as exosome I and exosome II.10,11) Exosome II had a size and morphology consistent with previous reports and contained abundant dipeptidyl peptidase IV (DPP IV). By proteomic and transcriptomic analyses, we identified many proteins and RNAs in the salivary exosomes, some of which might be involved in local immune defense in the oral cavity.11–13) The demonstration of exosome-associated biomaterials supported the concept that exosomes can participate in the pathophysiological action of saliva. It was reported that breast cancer exosome-like microvesicles altered the protein and mRNA compositions of exosome-like vesicles derived from salivary glands.14) In addition, in patients with oral lichen planus, a chronic inflammatory oral mucosal disease, miR-4484 levels in salivary exosomes were significantly increased.15)
Because exosomes have pathophysiological significance, it is important to elucidate their stability under various conditions in the oral cavity, digestive tract and plasma. Several studies described the stability of exosomes derived from urine,16) plasma17,18) and bovine milk.19) These studies demonstrated high stability of proteins or microRNA in exosomes under a variety of storage conditions. However, the characteristic features of salivary exosomes are still not known.
In our study, we examined the stability of exosomes (exosome II) prepared from human saliva for the first time, employing exosomal marker proteins and DPP IV activity as indicators of the salivary exosomes. We demonstrated that salivary exosomes were stable and retained their membrane integrity for up to 20 months when stored at 4°C and could also be stored in WS at 4°C for at least 28 d. Moreover, the membrane integrity of the salivary exosomes was partially resistant to nonionic detergents such as Nonidet P-40 (NP-40) and Triton X-100.
Glycine-proline-4-methyl-coumaryl-7-amide (Gly-Pro-MCA) was from Peptide Institute Inc. (Osaka, Japan). Sephacryl S-500 HR was from GE Healthcare UK Ltd. (Buckinghamshire, U.K.). For electron microscopic analysis, EM 25% Glutaraldehyde (G011/1) from TAAB Laboratories Equipment Ltd. (Berkshire, England) and collodion-coated 200 mesh copper grids (No. 6511, Nisshin EM Corporation, Tokyo, Japan) were used for sample preparation. Detergents used were sodium dodecyl sulfate (SDS), Sigma-Aldrich, St. Louis, MO, U.S.A.), Triton X-100 (Sigma-Aldrich) and NP-40 (Thermo Fisher Scientific, Waltham, MA, U.S.A.). Protease inhibitor cocktail (SIGMAFAST Protease Inhibitor Tablets) was from Sigma-Aldrich. All other reagents were of the highest quality available.
Isolation of Exosomes from Human WSEthical approval was obtained from the institutional review board of Teikyo Heisei University (approval number 26-088). Human WS was collected from healthy volunteers (22–49 years old) from our laboratory and written informed consent for this specific study was obtained. Exosomes were purified from WS as previously described, with a slight modification.11) Briefly, a volume of 30 mL WS was added to an equal volume of Tris-buffered saline (20 mM Tris–HCl, pH 7.4 and 150 mM NaCl). Cell debris and bacteria from the oral cavity were pelleted by centrifuging the WS sample at 8000×g for 15 min at room temperature. A portion of the supernatant was collected at 4°C for exosome isolation from WS. The supernatant was filtered through a 5.0 µm cellulose acetate filter and concentrated to approximately 1 mL in an Amicon Ultra-15 centrifugal filter device with a 100 kDa exclusion (Millipore Corporation, Bedford, MA, U.S.A.). The concentrated filtrate was purified by gel filtration chromatography on a Sephacryl S-500 column equilibrated with Tris-buffered saline (pH 7.4). Fractions were collected and analyzed for absorbance at 280 nm and DPP IV activity. Those fractions corresponding to a small peak of absorbance with high DPP IV activity were pooled and filtered through a 0.45 µm cellulose acetate filter. Samples were concentrated and exchanged into phosphate buffered saline (PBS) (pH 7.4) using Amicon Ultra-4 with a 100 kDa exclusion. Protein concentration was determined using the BCA reagent (Thermo Fisher Scientific). These concentrated solutions were used for further characterization analyses. Isolated exosome samples were stored at 4°C in PBS for up to 20 months.
DPP IV Activity AssayDPP IV activity was assayed as previously described.10) Briefly, the assay mixture contained 50 µL 0.4 mM Gly-Pro-MCA, 100 µL 100 mM Tris–HCl (pH 8.5) and 50 µL enzyme solution. After incubation for 20 min at 37°C, 2.8 mL 1 M sodium acetate (pH 4.2) was added to terminate the reaction. Fluorescence intensity, corresponding to liberated 7-amino-4-methyl-coumarin, was measured at 460 nm with excitation at 380 nm.
Transmission Electron Microscopic AnalysisTransmission electron microscopic analysis was performed as described previously, with a slight modification.11) The concentrated solution of exosome fractions, prepared as described, was mixed 9 : 1 with 25% glutaraldehyde in phosphate buffer (pH 7.2) and then applied to collodion-coated 200 mesh copper grids. The grids were stained with 2% uranyl acetate, pH 7, and embedded with 2% methylcellulose/0.4% uranyl acetate, pH 4. After drying, the grids were examined with a transmission electron microscope (TEM-1010; JEOL, Tokyo, Japan).
Dynamic Light Scattering (DLS) StudiesThe size distribution profile of salivary exosomes was determined by DLS, based on the laser diffraction method, using a Zetasizer Nano-ZS90 instrument (Malvern Instruments, Worcestershire, U.K.). Samples of 10 µg protein/mL were analyzed at constant temperature (25°C) and data were acquired and analyzed using proprietary Malvern software.
SDS-Polyacrylamide Gel Electrophoresis (PAGE) and Western Blotting AnalysisTwo micrograms of proteins in the exosome fractions, prepared as described, were separated by SDS-PAGE on a SuperSep HG, 5–20% gradient gel (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and protein bands were visualized by silver staining (2D-Silver Stain Reagent II, Cosmo Bio, Tokyo, Japan). To detect specific proteins in the salivary exosomes, protein bands were transferred to polyvinylidene difluoride (PVDF) membranes using a wet transfer method (Bio-Rad Laboratories, Inc., Hercules, CA, U.S.A.). Nonspecific binding sites were blocked by incubating the membrane in 100 mM Tris–HCl (pH 7.4) and 150 mM NaCl with 5% skim milk and 1% Tween 20. Blots were incubated overnight at 4°C with goat anti-DPP IV antibody (R&D Systems, Inc., Minneapolis, MN, U.S.A.), goat anti-programmed cell death 6-interacting protein (Alix) polyclonal antibody (Santa Cruz Biotechnology, Inc., Dallas, TX, U.S.A.), mouse anti-tumor susceptibility gene 101 (Tsg101) polyclonal antibody (Abcam plc, Cambridge, U.K.) or rabbit anti-CD9 polyclonal antibody (System Biosciences, Mountain View, CA, U.S.A.), followed by horseradish peroxidase-labeled secondary antibodies. The protein bands were visualized using a LAS-4000 mini luminescent image analyzer (GE Healthcare Bio-Science Corp., Piscataway, NJ, U.S.A.) and the ECL prime Western blotting detection kit (GE Healthcare).
Salivary Exosome Stability in Human WSTo analyze stability of exosomes in WS, freshly obtained WS (supernatants prepared as described) was stored at 4°C until use for time course experiments. To compare elution patterns of freshly prepared and stored exosomes, freshly obtained WS supernatant was divided into three portions. One was immediately used for exosome isolation and others were stored at 4°C for up to 28 d prior to exosome isolation. DPP IV activity and protein concentration (Pierce™ BCA Protein Assay Kit, Thermo Fisher Scientific) were used to determine degradation index values for the salivary exosomes in each sample.
Detergent Treatment and Freezing and Thawing of Salivary ExosomesDetergent (NP-40, Triton X-100 or SDS) was added to exosome samples, each at a final concentration of 1%. The mixtures were allowed to stand for 30 min at room temperature. Samples were then ultracentrifuged at 100000×g for 2 h at 10°C and the supernatants collected. The pellets were suspended in PBS and analyzed by DLS or electron microscopic analyses. For DPP IV activity measurements, the pellets were washed twice (each time, suspended in 100 µL PBS and ultracentrifuged at 100000×g for 1 h at 10°C) because SDS inhibits DPP IV activity. Each pellet was then homogeneously suspended in 1% NP-40 in PBS. For Western blot analysis, protease inhibitor cocktail in PBS was added to the pellets and the samples incubated for 15 min at room temperature prior to addition of detergents. For freeze–thaw cycle treatment, salivary exosomes were stored at 4, −20 or −80°C overnight and then thawed on ice before analysis.
Exosomes were prepared from WS of a single volunteer by sequential chromatography on ultra-filtration and gel filtration columns and were stored at 4°C. Exosomes were eluted in a single peak just after the void volume and most of the contaminating proteins were removed (Fig. 1A). Similar elution patterns of exosomes were obtained when the WS of twelve other volunteers were used (data not shown). When the morphology of exosomes immediately after preparation was compared with that of exosomes stored for 20 months, by electron microscopy (Fig. 1B), both exosome preparations contained distinctive particles with average diameters of 70 nm and there was little morphological difference between them.

(A) Gel chromatography elution profiles of exosomes from fresh human saliva. Exosome fractions shown in the figure were prepared as described in Materials and Methods. The exosomes were analyzed on the day of preparation (Day 0) or at 20 months after storage at 4°C (B) Morphology of human saliva-derived exosomes without (Day 0) or with storage at 4°C, analyzed by electron microscopy. (C) Protein contents of human saliva-derived exosomes, with or without storage at 4°C. Two micrograms of proteins from each sample were electrophoresed on a single gel and transferred to a PVDF membrane followed by immunoblotting using anti-DPP IV, anti-CD9, anti-Alix, or anti-Tsg101 antibodies. Upper panel, silver-stained gel. Lower panel, Western blotting analyses of salivary exosomal marker proteins.
We then compared the protein contents of both exosome preparations (Fig. 1C). In the stored preparation, compared with freshly prepared, there was a slight shift of the major bands to lower molecular weights of approximately 55, 70 and 120 kDa. This suggested that there was cleavage of some proteins in the sample during storage. On the other hand, when specific proteins known to be salivary exosomal markers,11) located either in the membrane fraction (DPP IV and CD9) or inner lumen (Alix and Tsg101), were analyzed, they primarily maintained their original molecular weights in the stored sample. These results suggested that the exosomes were morphologically stable even 20 months after preparation, having stringently maintained the membrane structure needed to protect proteins from unidentified contaminating proteases. Previously, by monitoring urinary exosome-specific proteins, such as Tsg101, stability of urinary exosomes was tested and their stable nature demonstrated.16)
Characterization of the Stability of Salivary Exosomes in Human WSWS samples from four volunteers were individually stored at 4°C for up to 28 d. As shown in Fig. 2A, the total protein content and DPP IV activity in WS were both maintained during a 28 d storage, suggesting that the exosomes were stable in WS. Based on these results, we compared the elution profiles of freshly prepared and stored WS samples, using DPP IV activity as a salivary exosomal marker. The elution profile of DPP IV activity from stored WS showed little difference from that from the freshly prepared WS (Fig. 2B). The amounts of eluted exosomal proteins obtained were 106, 139 and 89.4 µg and total DPP IV activities were 2.4, 2.8 and 2.4 nmol/min, respectively, in exosome fractions from WS samples stored for 0, 7, 28 d. These results suggest that the exosomes and DPP IV were maintained intact during storage.
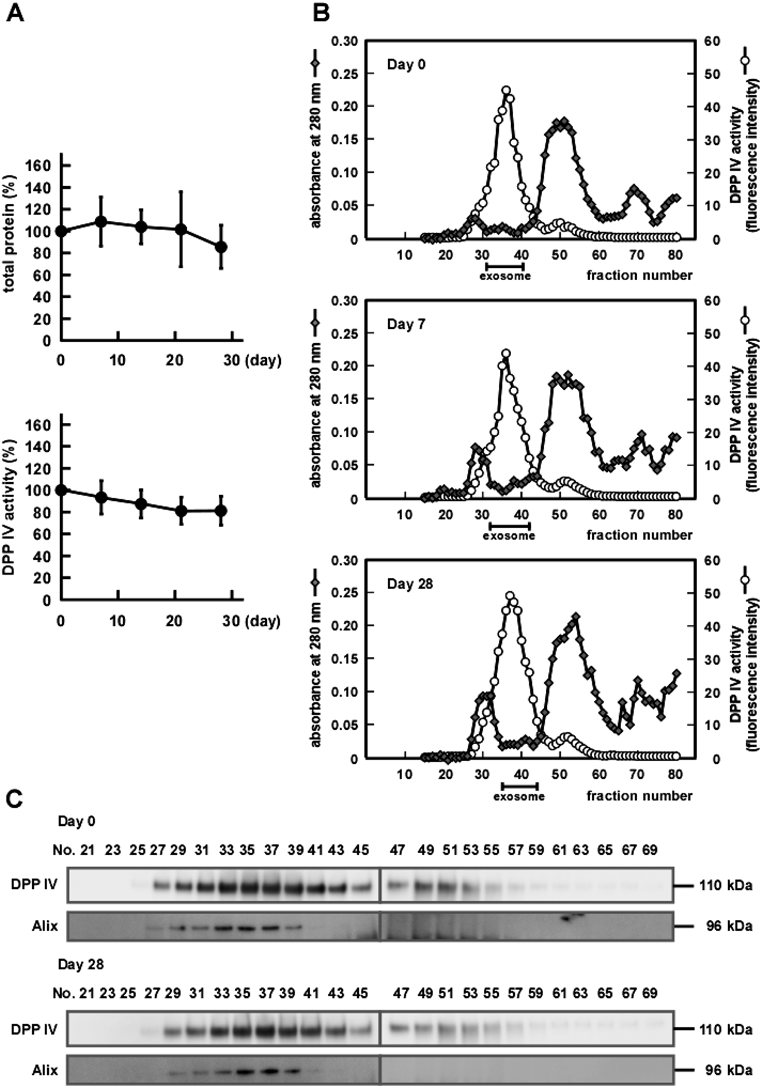
WS was collected from four volunteers and stored at 4°C for the indicated time periods. (A) Changes in amount of total protein (upper) and DPP IV activity (lower) of human whole saliva. Amounts of total proteins and DPP IV activities of fresh or stored WS were measured and represented as the mean±standard deviation (S.D.). (B) Gel chromatography elution profiles of human saliva-derived exosomes. Fresh WS and WS stored for 7 or 28 d were separated on ultra-filtration and gel filtration chromatography columns. (C) Western blotting analyses of salivary exosomal marker proteins eluted from gel filtration columns. Numbers correspond to the fraction numbers from the gel filtration column chromatography shown in panel B. Over all, 20 µL of each fraction was electrophoresed on SDS-PAGE and analyzed by Western blotting.
To further check the integrity of exosomes, elution profiles of DPP IV and Alix from the gel filtration column were compared in freshly prepared and stored exosome samples. As shown in Fig. 2C, DPP IV and Alix co-eluted in both the freshly prepared sample and in that stored for 28 d, without a shift in fractionation. This suggested that stored exosomes still retained Alix inside and thus had maintained their membrane integrity.
Next, we further analyzed the stability of the protein components and the morphology of exosomes from WS stored up to 28 d. The left panel of Fig. 3A shows the protein profiles obtained from exosomes derived from either fresh or stored WSs. It was apparent that, compared with exosomes from fresh WS (Day 0), exosomes from stored WS (Days 7 and 28) had higher intensities in several protein bands, that is, at ca. 30 and ca. 150 kDa. This suggested that some protein degradation occurred during storage. However, the salivary exosomal marker proteins such as DPP IV, CD9, Alix and Tsg101 remained intact during storage when analyzed by Western blotting (upper right panel of Fig. 3A). Mucin 5B is abundant in exosomal fractions and, thus, is regarded as being associated with exosomes11) and our data suggested that mucin 5B was cleaved during storage (lower right panel of Fig. 3A). After 7 d storage, bands of >250 kDa were increased in intensity and, presumably, were derived from intact mucin 5B of higher molecular weight. After 28 d storage, further degradation appeared to have occurred, as indicated by even lower molecular weight mucin 5B-positive bands. These results suggested that, while degradation of some exosome-associated proteins occurred during storage of WS prior to exosome preparation, the exosome membranes retained their integrity enough to retain Alix. Indeed, morphological analyses by electron microscopy revealed that the exosomes retained their morphological integrity (Fig. 3B). In addition, particle size analyses by DLS (Fig. 3C) showed similar diameters (ca. 70 nm) irrespective of storage time, further supporting maintenance of the morphological integrity of the exosomes during storage. Thus, we concluded that salivary exosomes were relatively stable, even in WS, which contains numerous proteins such as lysozyme, kallikreins and amylase.20) It was reported the exosomes derived from the colon carcinoma cell line, LIM1863, were also stable in plasma over a period of 3 months.18)
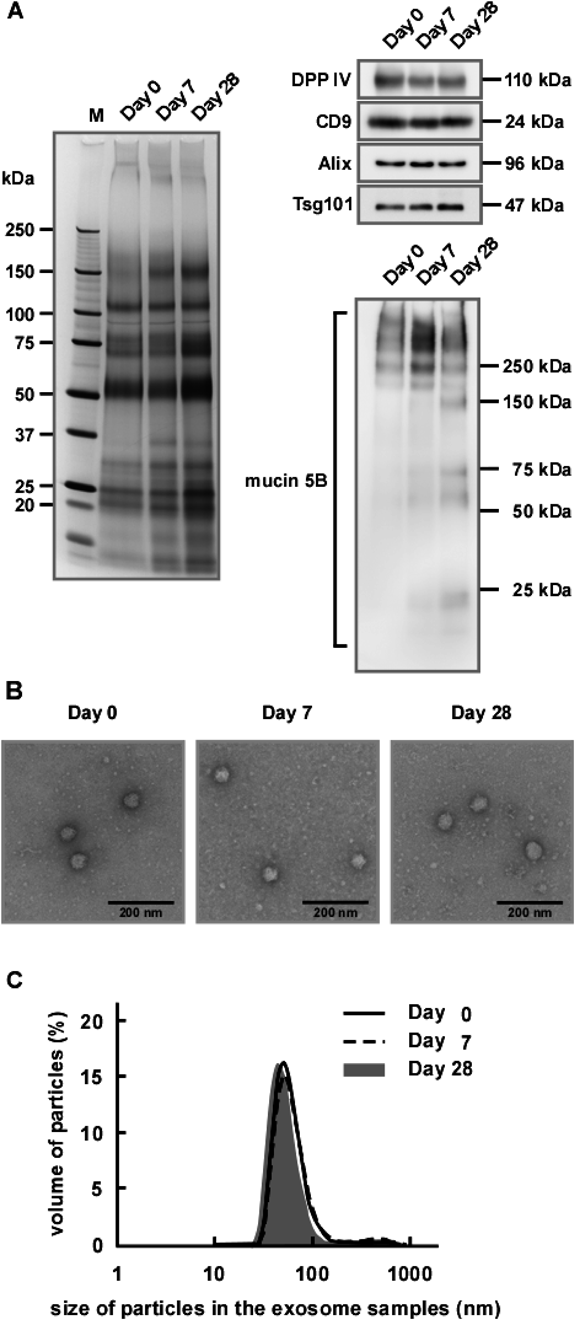
Exosomes fractionated in Fig. 2B were used. (A) Two micrograms of each exosome was electrophoresed on SDS-PAGE and visualized with silver staining (left panel) or analyzed by Western blotting using anti-DPP IV, anti-CD9, anti-Alix, and anti-Tsg101 antibodies (right upper panel). Right lower panel shows Western blot analysis using anti-mucin 5B. (B) Morphological analyses of exosomes derived from stored human saliva, under electron microscopy. (C) Particle sizes of exosomes derived from human saliva. Particle size was measured by a Zetasizer, as described in Materials and Methods.
We next examined the solubility of exosome-associated proteins by treating salivary exosomes with detergents. Without detergents, DPP IV activity co-precipitated with exosomes, as expected (Fig. 4A). In the presence of a detergent such as NP-40 or Triton X-100, almost all DPP IV activity was detected in the supernatant fraction. However, as shown in Fig. 4B, precipitated fractions of NP-40 and Triton X-100-treated exosomes still contained some DPP IV protein, suggesting that the enzyme was not solubilized completely in these nonionic detergents. In contrast, CD9 was completely transferred to the supernatant fraction in the presence of these detergents. It was reported that DPP IV preferentially localized to lipid raft domains rich in sphingolipids and cholesterol and was resistant to solubilization with Triton X-100.21) We speculate that both proteins are integral membrane proteins, whereas DPP IV and CD9 might be localized in different membrane domains from each other. However, the precise distribution of DPP IV and CD9 in the membrane of exosomes remains unclear. We next treated the exosomes with SDS and found that this ionic detergent almost completely solubilized DPP IV. It seems likely that, while SDS completely destroyed membrane integrity, NP-40 and Triton X-100 did not.
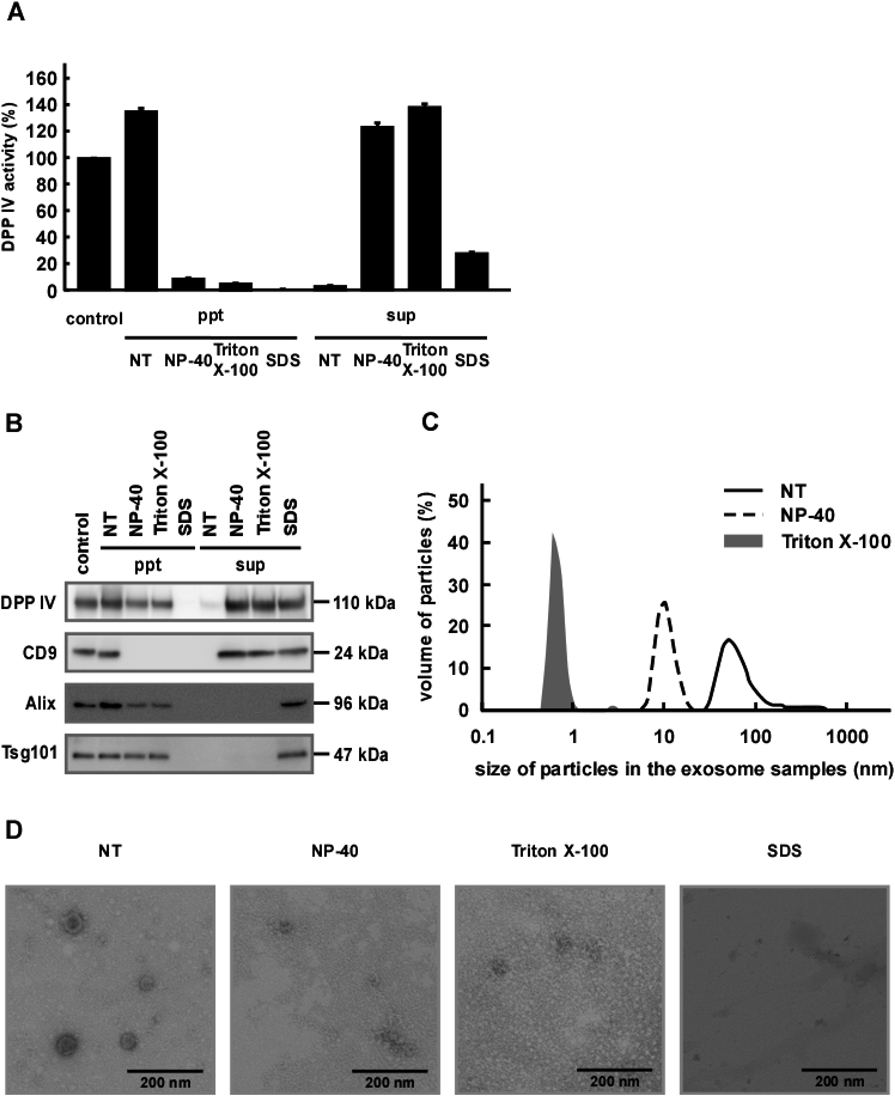
(A) Solubilization of DPP IV activity by detergents. Exosomes were treated with or without detergents and then centrifuged at 100000×g for 2 h. The pellets and supernatants were collected and DPP IV activities determined. The control sample was not treated with detergents and not centrifuged. NT was not treated with detergents but was centrifuged. (B) Western blotting analyses of salivary exosomal marker proteins in the pellets or supernatants. Two µg of proteins from a sample treated with detergent shown in panel A was subjected to Western blotting analyses. (C) Particle sizes of human saliva-derived exosomes, treated with or without detergents. Particle sizes were measured with a Zetasizer as described in Materials and Methods. (D) Morphological analyses of exosomes treated with or without detergents, by electron microscopy.
Alix is part of the endosomal sorting complex required for transport (ESCRT) complex inside exosomes and is used as an internal exosomal marker.22) In the absence of detergent, Alix was impermeable to the membrane and co-precipitated with the exosomes (Fig. 4B). Unexpectedly, we also did not detect Alix in the supernatants of NP-40 or TritonX-100 treated exosomes. This was consistent with the possibility that the exosomes still maintained their integrity, thus retaining Alix. However, the amount of Alix recovered by precipitation was clearly decreased in exosomes after detergent treatments. It was also possible that Alix in the supernatant was absorbed to the sample tube during the experimental manipulations. To address this, we treated the exosomes with SDS to prevent protein adsorption to the tubes. As expected, SDS treatment caused a complete transfer of Alix from the precipitated fraction to the supernatant (Fig. 4B). Nearly equivalent results were obtained when another soluble exosomal marker, Tsg101, was monitored instead. The particle size analyses shown in Fig. 4C indicated that detergents indeed destroyed the membrane structure, causing a dramatic decrease in the apparent average particle size of the exosomes. However, by electron microscopy, although membrane structure was not visible, the exosomes appeared to retain a vesicle-like morphology after treatment with NP-40 and Triton X-100 (Fig. 4D). After SDS treatment, the membrane structure appeared to be almost completely destroyed, with only a condensed area visible. It is possible that treatment with either NP-40 or Triton X-100 increased permeability of and damaged the exosomal membrane without a total loss of the proteins inside the exosomes. Damaged membranes might form debris, derived from intact membranes, and be easily degraded into smaller pieces during particle size measurements. Nonionic detergents are known to destroy lipid–lipid and lipid–protein interactions rather than protein–protein interactions.23) Depending on the location and/or composition of the membrane areas, nonionic detergent sensitivities might differ. In contrast, SDS treatment which is extremely effective for the solubilization of membrane proteins,23) caused complete destruction of the membrane structure, allowing release of the internal proteins. Recently, Aizaki et al. showed that the replication complex of hepatitis C virus (HCV) composed of HCV RNA and HCV nonstructural proteins was associated with lipid rafts and was resistant to NP-40 and Triton X-100.24) Since exosomes have a similar structure and biogenesis to viruses,25) core structures consisting of proteins and RNAs in exosomes may be rigid and resistant to NP-40 or Triton X-100. Moreover, Tsg101 and Alix were reported to be necessary for the formation of viruses and were associated with viral proteins such as Gag p6.26) We speculate that Tsg101 and Alix in exosomes may interact with various components in lipid raft domains or core structures which allows them to be resistant to NP-40 or Triton X-100. Several other studies employing detergents reported on the stability of exosomes.27–29) It would be interesting to compare the effects of detergents on the membrane integrity of exosomes prepared from various sources.
Effects of Freezing and Thawing on Salivary Exosome StabilityFinally, we examined effects of storage temperature and/or freezing and thawing (i.e., 4, −20 and −80°C) on exosome morphology. The DPP IV activities of different preparations of exosomes were comparable (Fig. 5A), indicating that freeze–thaw performed only once had little effect on the enzyme activity. Similar results were obtained using exosomes from two other volunteers (data not shown). After storage at different temperatures, exosomal markers remained intact, indicating that the membrane structure retained integrity irrespective of the storage temperature (Fig. 5B). However, repeated freeze–thaw cycles appeared to cause release of DPP IV activity into the supernatant fraction (Fig. 5A). Thus, we assumed that storage at 4°C was the most favorable condition for retaining integrity of the exosomal membrane structure for relatively long periods. In addition, we observed that, by electron microscopy, exosomes had comparable diameters, irrespective of storage temperature, further supporting the conclusion that they retained membrane integrity during storage (Fig. 5C).

(A) Effects of storage temperature on DPP IV activity of exosomes. Exosomes were stored at the indicated temperatures for 1 d and then subjected to thawing. DPP IV activity was measured as an indicator of exosome stability. (B) Effects of storage temperature on the integrity of exosomes. Two µg of protein from stored exosomes was subjected to immunoblotting using antibodies for several salivary exosomal markers which were employed as indicators of membrane integrity of the exosomes. (C) Effects of storage temperature on the morphology of exosomes. Morphological changes of exosomes stored at different temperatures were detected by electron microscopy. (D) Particle sizes of human saliva-derived exosomes stored at different temperatures. Particle size was measured with a Zetasizer as described in Materials and Methods.
Based on particle size analyses, freezing and thawing had little effect on particle size (Fig. 5D), further suggesting that the human salivary exosomes were stable and retained integrity during long term storage. However, it is worth noting that our results were different from those reported by Zhou et al.,16) who observed a significant loss of exosomal marker proteins with storage at −20°C, compared with at 4°C. In contrast, at −80°C, they observed almost complete retention of those proteins after freezing and thawing. For this reason, they concluded that storage at −80°C was better than at −20°C for preserving proteins such as albumin, transferrin and globulin in exosomes. More recently, Pieters et al. reported that bovine milk-derived extracellular vesicles/exosomes were more stable after freeze–thaw cycles than those of cellular origin, from murine RAW 264.7 macrophages.19) It is possible that exosomes derived from different sources vary in their stabilities to freezing and thawing. Further work to compare the stabilities of exosomes derived from various sources is needed.
Exosomes can be derived from various sources, including primary and cultured cell lines and body fluids. We previously performed comprehensive studies on the contents of human saliva-derived exosomes.10–13) Exosomes are now widely investigated as potential biomarkers for cancer, neurodegenerative diseases, prion disease, and virus infection.30) Thus, it is important to elucidate the stability of exosomes for retention of small molecules. In our study, we examined the stability of human salivary exosomes for the first time. We found that the exosomes were stable after long storage periods, with or without freeze–thaw treatments. The exosomes retained DPP IV enzymatic activity after storage and Alix remained inside the exosomes, indicating that integrity of their membrane structure was maintained, keeping it impermeable to the inside protein(s). Thus, salivary exosomes might be a reliable source for biomarker discovery because they are stable under various conditions as shown in this study. Indeed, many specific microRNAs were reported to be contained in salivary exosomes isolated from normal and Sjögren’s syndrome subjects.31) Because saliva flows naturally into the gastrointestinal tract, further experiments would be important to elucidate the stability of salivary exosomes in the digestive tract are important to investigate their potential pathophysiological roles.
We thank Ms. Sachie Matsubara and Ms. Junri Sekiguchi (Laboratory for Electron Microscopy, Kyorin University School of Medicine) for technical assistance. This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant number 25460172.
The authors declare no conflict of interest.