2017 年 40 巻 9 号 p. 1389-1398
2017 年 40 巻 9 号 p. 1389-1398
In order to avoid adverse drug reactions (ADRs), pharmacists are reconstructing ADR-related information based on various types of data gathered from patients, and then providing this information to patients. Among the data provided to patients is the time-to-onset of ADRs after starting the medication (i.e., ADR onset timing information). However, a quantitative evaluation of the effect of onset timing information offered by pharmacists on the probability of ADRs occurring in patients receiving this information has not been reported to date. In this study, we extracted 40 ADR–drug combinations from the data in the Japanese Adverse Drug Event Report database. By applying Bayes’ theorem to these combinations, we quantitatively evaluated the usefulness of onset timing information as an ADR detection predictor. As a result, when information on days after taking medication was added, 54 ADR–drug combinations showed a likelihood ratio (LR) in excess of 2. In particular, when considering the ADR–drug combination of anaphylactic shock with levofloxacin or loxoprofen, the number of days elapsed between start of medication and the onset of the ADR was 0, which corresponded to increased likelihood ratios (LRs) of 138.7301 or 58.4516, respectively. When information from 1–7 d after starting medication was added to the combination of liver disorder and acetaminophen, the LR was 11.1775. The results of this study indicate the clinical usefulness of offering information on ADR onset timing.
One of the main tasks of a pharmacist who is engaged in dispensing drugs is to give instructions on the proper use of drugs to improve patient adherence. Article 25-2 of the Pharmacists Act requires pharmacists to provide necessary information and guidance based on pharmaceutical knowledge. Under this act, pharmacists have established a social standing as professional pharmaceutical advisers. We have recently reported the findings of an internet-based survey of 436 pharmacists and 562 patients on their opinion regarding the role of a pharmacist.1) We found that a role in “responsible monitoring of patients” was closely linked to the concept of pharmacists being “advisors on the use of pharmaceuticals.” Regarding the “responsible monitoring of patients,” there is a large gap between pharmacists and patients in terms of how they perceive the role of a pharmacist, indicating a role discrepancy. In addition, “responsible monitoring of patients” as a subordinate concept consists of “grasping the presence/absence of drug effectiveness,” “changes in health status by drugs,” and “consciousness to protect patients from adverse drug reaction (ADR).” The gap regarding role cognition between pharmacists and patients may disappear if adverse effects post administration of a medication and changes in patient health are found.
As one method to prevent the worsening of ADRs, when pharmacists are dispensing drugs they provide patients with information on the initial symptoms (ISs) of ADRs of the drug, so that patients can identify the onset of ADRs early and take the necessary action.2) A single ADR can often have multiple ISs, and among these ISs, there will be some that are more sensitive in predicting an ADR than others. In order to increase awareness regarding ADRs, we recently reported a quantitative evaluation method using Bayes’ theorem that can be used to evaluate the usefulness of different ISs of an ADR.3,4) In one example, we looked at abnormal hepatic function, which is an ADR of terbinafine. The developed method was used to calculate likelihood ratios (LRs) for different ISs of abnormal hepatic function, and the calculated LR values were 5.17 for fever, 2.44 for nausea, and 1.43 for a rash, indicating that fever has the highest information value and is the most useful predictor of abnormal hepatic function following treatment with terbinafine.
In addition, Ghajar et al. have reported a method that can be used to increase the diagnostic probability of whether a rash that develops while taking sulfonamide is a sulfonamide-induced ADR or not.5) In this study, the authors analyzed five factors for prior odds using Bayes’ theorem: (1) the patient’s allergy history, (2) skin conditions, (3) the onset of a rash after taking the medication, (4) recovery after discontinuation of the treatment, and (5) the response to drug re-administration. They then calculated the posterior odds by multiplying the prior odds by the LRs of each of the above five factors, and reported that these factors increase the probability of a correct diagnosis. For example, in the case of a skin rash, if the onset of skin rash caused by sulfonamide is restricted to the beginning of treatment, onset timing information can contribute to an increase in the probability of identifying an ADR.
Accordingly, in this study, the concepts of prior probability, posterior probability, and likelihood ratio used in Bayesian statistics were applied to the identification and prevention of ADRs. Thus, when a specified ADR of a certain drug has a characteristic onset timing, confirmation of onset timing information by a pharmacist (treatment side), or the offering of this information to patients, can be expected to heighten the probability of discovering the ADR, with a certain likelihood ratio. In a similar study, without the use of likelihood ratios, safe administration of chemotherapy was successfully achieved by confirmation of ADR onset timing between the medical personnel and the patients.6) With regard to the usefulness of likelihood ratios, Robert et al. state that “the information contributed by the test is summarized in one number corresponding to each level of test result. Additionally, likelihood ratios are particularly well suited for describing the overall odds of disease when a series of diagnostic tests is used.”7) Thus, the value of offering information can be quantitatively evaluated by employing likelihood ratios.3,4) When applying Bayes’ theorem to increase the ADR detection rate, the general onset probability of an ADR in the time elapsed since starting the medication is required as the prior probability, and the onset probability for a specific ADR–drug combination is required as the posterior probability. In order to obtain the general onset probability of an ADR, it is necessary to analyze many cases in order to guarantee the reliability of the analysis results. In this case, it is useful to analyze data from a large integrated ADR database or an adverse drug event reporting system database. Adverse event reporting system databases on the use of post-marketing drugs have already been established in the U.S.A., the U.K., the Netherlands, and Canada. In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has published data on reports of spontaneous adverse events (AEs) in Japan as the Japanese Adverse Drug Event Report database (JADER). A vast amount of information on more than 300000 AEs has been collected thus far in JADER. Because the JADER database contains time information, such as the medication start date, medication end date, and AE date, studies performing time analyses related to the onset of AEs have been reported.8–10)
In this study, we examined the relationship between ADR onset timing information and posterior probability. According to our literature survey, no other studies have been reported that quantitatively examine the value of information provided to patients by applying Bayes’ theorem to ADR onset timing information. This study quantitatively evaluated the usefulness of ADR onset timing information in an attempt to apply the concept of subjective probability to the work of community pharmacists.
In general, the probability of whether or not a subject has a disease before undergoing an examination is expressed using the ratio of patients with that disease in the population, and this is defined as the prior probability. In the case of the examination result being positive and the patient being diagnosed with the disease, the probability that they really have contracted the disease is defined as the posterior probability. The usefulness of the diagnostic examination can then be evaluated, with a high posterior probability and a high ratio of posterior probability to prior probability (i.e., a high LR) corresponding to a high usefulness. Bayes’ theorem is a method of obtaining the morbidity probability of a patient by multiplying the likelihood ratio of the examination result by the prior odds. In this study, we used the posterior probability and LR to evaluate the usefulness of ADR onset timing information (i.e., the time-to-onset of the ADR, referred to as Nday-onset-info), by considering the prior probability as the probability of an ADR occurring after taking a general medication, and the diagnostic examination as the ADR onset time information.
Likelihood Ratio Test Based on Bayes’ TheoremBayes’ theorem is given by Eq. 1:
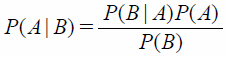 | (1) |
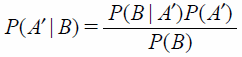 | (2) |
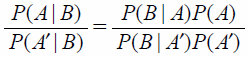 | (3) |
Equation 3 can be expressed as Eq. 4 where A is the event that the patient has a disease (denoted disease +) and B is the event of a positive test result (denoted test +).
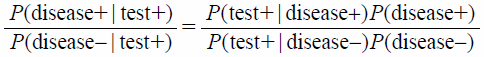 | (4) |
Equation 4 can be expressed as:
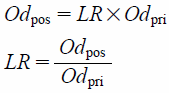 | (5) |
Further, the two-by-two contingency table of the survey data for obtaining Eq. 5 is shown in Table 1. From Eq. 5, the likelihood ratio is given by the equation below:
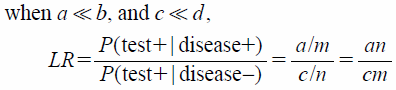 | (6) |
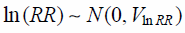 |
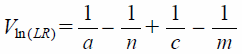 |
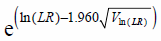 |
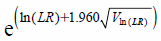 |
| Test-positive | Test-negative | Total | |
|---|---|---|---|
| Disease | a | b | m |
| Normal | c | d | n |
| Total | a+c | b+d | m+n |
The incidence of an ADR occurring in a predetermined, arbitrary period of time for all of the ADRs recorded in the JADER database was defined as the prior probability (Ppri). In addition, for a specific drug–ADR combination, the probability of an ADR occurring during the same period as the prior probability was calculated and defined as the posterior probability (Ppos).
Calculation of Likelihood RatiosFrom Eq. 5:
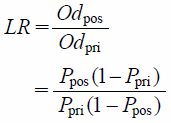 | (7) |
The JADER database contains four types of data: the case list table (“demo”), the drug information table (“drug”), the ADR table (“reac”), and the primary disease table (“hist”). Each table is linked to “case identification numbers.” The reason for drug administration registered in “drug,” AE name registered in “reac,” and primary disease name and complications registered in “hist” are based on the preferred terms (PTs) listed in ICH Medical Dictionary for Regulatory Activities/Japanese version (MedDRA/J). The version at the time of aggregation was unified in version 16.0. For the analysis in this study, “demo,” “drug” and “reac” from April 2004 to June 2015 were downloaded from the PMDA website (http://www.info.pmda.go.jp/fukusayoudb/CsvDownload.jsp). Each downloaded table was concatenated using identification numbers to create a data frame. The data structure of the JADER database is in compliance with international safety reporting guidance, ICH E2B.
Data CleaningBecause the data reported to the JADER database includes duplicate and missing data, we cleaned the data frame.13,14) First, “gender,” “age,” “weight,” and “height” in “demo”; “drug name (general name),” “medication start date,” and “medication end date” in “drug”; and “AE” and “AE date” in “reac” were all deleted as duplicate reports of the same cases. Next, we deleted reported cases where the “medication start date,” “medication end date,” or “AE onset date” data was missing. In this study, we wanted to estimate how much a pharmacist could improve the detection rate of ADRs when giving medication instructions in a community pharmacy. Hence, we extracted the cases in which the drugs involved were suspected of causing ADRs and were orally administered.
Targeted Combinations of ADRs and DrugsFor convenience, we extracted the top ten ADRs from the obtained cleaned dataset. In addition, we extracted the top 10 types of drug for each of the top ten ADRs (i.e., 100 types of ADR–drug combination in total). Among the 100 types of ADR–drug data extracted, those with a number of reported cases less than 100 were excluded from the calculation. Furthermore, in combinations where a specific drug had a high rate of concomitant use among the tested ADRs–drug, the effect of the concomitant drug on the ADR could not be ignored. For example, if there 100 reported cases of ADR-A occurring with drug-B, but in 50 of those cases drug-C was concomitantly used alongside drug-B, it can be inferred that drug-C has some effect on the onset of ADR-A. Therefore, for convenience, we excluded ADR–drug combinations involving specific drugs whose rate of concomitant use exceeded 20% from the calculation.
Time-to-Onset Analysis Utilizing LRsWe calculated the number of days to ADR onset from the “medication start date” and the “AE onset date” in “reac” using Eq. 8.
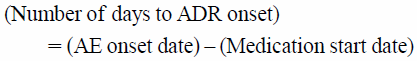 | (8) |
During data cleaning, the total number of reported cases decreased from 349375 to 115718 (number of drug–ADR combinations: 357497). Table 2 shows the 100 types of drug–ADR combinations extracted from the 115718 cases. The number of cases of these 100 combinations was 19493, which was equivalent to 16.85% (19493/115718) of the cleaned dataset. In Table 2, ★ represents a drug–ADR combination in which the rate of concomitant use of a certain drug exceeded 20%, and ※ represents combinations in which the number of reported cases was less than 100. There were 8598 cases (40 combinations) that were not marked with ★ and ※, accounting for 7.43% (8598/115718) of the cleaned dataset. These cases were taken forward for the subsequent calculations.
| ID | ADR | Drug | ADR–drug abbreviation | Case number |
|---|---|---|---|---|
| 1 | Interstitial lung disease | Gefitinib | Il-Ge | 783 |
| 2 | Interstitial lung disease | Erlotinib hydrochloride | Il-Er | 679 |
| 3 | Interstitial lung disease | Methotrexate | Il-Me | 545 |
| 4 | Interstitial lung disease | Tegafur ∙ Gimeracil ∙ Oteracil potassium | Il-Tg | 403 |
| 5 | Interstitial lung disease | Everolimus | Il-Ev | 348 |
| 6 | Interstitial lung disease | Amiodarone hydrochloride | Il-Am | 230 |
| ★ 7 | Interstitial lung disease | Ribavirin | 205 | |
| 8 | Interstitial lung disease | Loxoprofen sodium hydrate | Il-Lo | 110 |
| 9 | Interstitial lung disease | Imatinib mesylate | Il-Im | 113 |
| ★10 | Interstitial lung disease | Prednisolone | 84 | |
| 11 | Hepatic function abnormal | Sorafenib tosylate | Hf-So | 433 |
| 12 | Hepatic function abnormal | Terbinafine hydrochloride | Hf-Tb | 243 |
| 13 | Hepatic function abnormal | Ticlopidine hydrochloride | Hf-Ti | 191 |
| 14 | Hepatic function abnormal | Loxoprofen sodium hydrate | Hf-Lo | 180 |
| ★15 | Hepatic function abnormal | Tegafur ∙ Uracil | 159 | |
| 16 | Hepatic function abnormal | Gefitinib | Hf-Ge | 137 |
| 17 | Hepatic function abnormal | Atorvastatin calcium hydrate | Hf-At | 132 |
| 18 | Hepatic function abnormal | Carbamazepine | Hf-Ca | 130 |
| 19 | Hepatic function abnormal | Fluvastatin sodium | Hf-Fl | 117 |
| 20 | Hepatic function abnormal | Lansoprazole | Hf-La | 103 |
| ★21 | Platelet count decreased | Ribavirin | 486 | |
| 22 | Platelet count decreased | Sunitinib malate | Pc-Su | 531 |
| ★23 | Platelet count decreased | Simeprevir sodium | 354 | |
| ★24 | Platelet count decreased | Tegafur ∙ Gimeracil ∙ Oteracil potassium | 369 | |
| ★25 | Platelet count decreased | Lenalidomide hydrate | 252 | |
| 26 | Platelet count decreased | Dasatinib hydrate | Pc-Da | 215 |
| 27 | Platelet count decreased | Sorafenib tosylate | Pc-So | 166 |
| 28 | Platelet count decreased | Everolimus | Pc-Ev | 154 |
| ★29 | Platelet count decreased | Dexamethasone | 137 | |
| 30 | Platelet count decreased | Temozolomide | Pc-Tz | 122 |
| 31 | Anaphylactic shock | Levofloxacin hydrate | As-Le | 139 |
| 32 | Anaphylactic shock | Loxoprofen sodium hydrate | As-Lo | 100 |
| ※33 | Anaphylactic shock | Garenoxacin mesilate hydrate | 96 | |
| ※34 | Anaphylactic shock | Cefcapene pivoxil hydrochloride hydrate | 53 | |
| ※35 | Anaphylactic shock | Diclofenac sodium | 44 | |
| ※36 | Anaphylactic shock | Eperisone hydrochloride | 44 | |
| ※37 | Anaphylactic shock | Tosufloxacin tosylate hydrate | 40 | |
| ※38 | Anaphylactic shock | Moxifloxacin hydrochloride | 40 | |
| ※39 | Anaphylactic shock | Common cold remedy (over-the-counter drugs) | 34 | |
| ※40 | Anaphylactic shock | Analgesic anti-inflammatory drugs (over-the-counter drugs) | 32 | |
| ★41 | White blood cell count decreased | Ribavirin | 557 | |
| ★42 | White blood cell count decreased | Simeprevir sodium | 257 | |
| ★43 | White blood cell count decreased | Tegafur ∙ Gimeracil ∙ Oteracil potassium | 310 | |
| ★44 | White blood cell count decreased | Telaprevir | 268 | |
| 45 | White blood cell count decreased | Dasatinib hydrate | Wb-Da | 171 |
| 46 | White blood cell count decreased | Sunitinib malate | Wb-Su | 152 |
| 47 | White blood cell count decreased | Temozolomide | Wb-Tz | 111 |
| ※48 | White blood cell count decreased | Valganciclovir hydrochloride | 96 | |
| ※49 | White blood cell count decreased | Prednisolone | 79 | |
| ※50 | White blood cell count decreased | Procarbazine hydrochloride | 88 | |
| 51 | Pyrexia | Lamotrigine | Py-Lm | 282 |
| ★52 | Pyrexia | Ribavirin | 226 | |
| 53 | Pyrexia | Sorafenib tosylate | Py-So | 111 |
| 54 | Pyrexia | Carbamazepine | Py-Ca | 104 |
| ★55 | Pyrexia | Simeprevir sodium | 94 | |
| ※56 | Pyrexia | Tegafur ∙ Gimeracil ∙ Oteracil potassium | 86 | |
| ※57 | Pyrexia | Sunitinib malate | 94 | |
| ※58 | Pyrexia | Mesalazine | 89 | |
| ※59 | Pyrexia | Live Attenuated Human Rotavirus Vaccine, Oral | 82 | |
| ※60 | Pyrexia | Loxoprofen sodium hydrate | 61 | |
| ★61 | Neutrophil count decreased | Ribavirin | 561 | |
| ★62 | Neutrophil count decreased | Tegafur ∙ Gimeracil ∙ Oteracil potassium | 306 | |
| ★63 | Neutrophil count decreased | Lenalidomide hydrate | 259 | |
| ★64 | Neutrophil count decreased | Telaprevir | 194 | |
| ★65 | Neutrophil count decreased | Simeprevir sodium | 152 | |
| 66 | Neutrophil count decreased | Dasatinib hydrate | Nc-Da | 143 |
| ★67 | Neutrophil count decreased | Dexamethasone | 141 | |
| 68 | Neutrophil count decreased | Sunitinib malate | Nc-Su | 103 |
| ※69 | Neutrophil count decreased | Prednisolone | 72 | |
| ※70 | Neutrophil count decreased | Temozolomide | 72 | |
| 71 | Liver disorder | Terbinafine hydrochloride | Ld-Tb | 196 |
| 72 | Liver disorder | Ticlopidine hydrochloride | Ld-Ti | 194 |
| 73 | Liver disorder | Loxoprofen sodium hydrate | Ld-Lo | 136 |
| 74 | Liver disorder | Sorafenib tosylate | Ld-So | 100 |
| 75 | Liver disorder | Acetaminophen | Ld-Ac | 102 |
| ※76 | Liver disorder | Carbamazepine | 94 | |
| ※77 | Liver disorder | Levofloxacin hydrate | 89 | |
| ※78 | Liver disorder | Atorvastatin calcium hydrate | 83 | |
| ※79 | Liver disorder | Fluvastatin sodium | 87 | |
| ※80 | Liver disorder | Cefcapene pivoxil hydrochloride hydrate | 80 | |
| ★81 | Anemia | Ribavirin | 1321 | |
| ★82 | Anemia | Teraprevir | 722 | |
| ★83 | Anemia | Simeprevir sodium | 251 | |
| ★84 | Anemia | Lenalidomide hydrate | 186 | |
| 85 | Anemia | Dasatinib hydrate | An-Da | 167 |
| ★86 | Anemia | Tegafur ∙ Gimeracil ∙ Oteracil potassium | 147 | |
| 87 | Anemia | Everolimus | An-Ev | 122 |
| ★88 | Anemia | Dexamethasone | 113 | |
| ※89 | Anemia | Sorafenib tosylate | 83 | |
| ※90 | Anemia | Sunitinib malate | 87 | |
| ★91 | Pneumonia | Methotrexate | 262 | |
| ★92 | Pneumonia | Prednisolone | 182 | |
| ★93 | Pneumonia | Tacrolimus hydrate | 118 | |
| ★94 | Pneumonia | Lenalidomide hydrate | 135 | |
| ★95 | Pneumonia | Dexamethasone | 115 | |
| 96 | Pneumonia | Tegafur ∙ Gimeracil ∙ Oteracil potassium | Pn-Tg | 100 |
| ※97 | Pneumonia | Ciclosporin | 54 | |
| ※98 | Pneumonia | Everolimus | 54 | |
| ※99 | Pneumonia | Ribavirin | 55 | |
| ※100 | Pneumonia | Mycophenolate mofetil | 30 |
★: Combinations for which the rate of concomitant use of a certain drug exceeded 20%. ※: Combinations for which the number of reported cases was less than 100.
Table 3 shows the calculation results of the 40 types of combinations targeted for calculation. The probability of an ADR being reported during each time period (Ppri) considering all of the ADR–drug combinations was Ppri0=0.0605 (21617/357497), Ppri1–7=0.1838 (65712/357497), Ppri8–14=0.1116 (39885/357497), Ppri15–30=0.1446 (51684/357497), Ppri31–60=0.1358 (48536/357497), Ppri61–90=0.0697 (24900/357497), Ppri91–180=0.1026 (36673/357497), Ppri181–365=0.0815 (29131/357497), and Ppri366+=0.1101 (39359/357497). LR was calculated from Ppri and Ppos in a preset period after taking the drug. As a result, it was clear that the onset timing of the same ADRs differs depending on the particular drug. For example, the timing of onset of interstitial lung disease was shown to be from 366 d onward after the start of treatment in the case of methotrexate and amiodarone hydrochloride, whereas in the case of gefitinib, erlotinib hydrochloride, tegafur/gimeracil/oteracil potassium, everolimus, loxoprofen sodium hydrate, and imatinib mesylate, interstitial lung disease occurs as an ADR within 90 d of starting treatment with the drug.
| ID | ADR–drug | Day | 0 | 1–7 | 8–14 | 15–30 | 31–60 | 61–90 | 91–180 | 181–365 | 366+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | N | 21617 | 65712 | 39885 | 51684 | 48536 | 24900 | 36673 | 29131 | 39359 | |
| Ppri | 0.0605 | 0.1838 | 0.1116 | 0.1446 | 0.1358 | 0.0697 | 0.1026 | 0.0815 | 0.1101 | ||
| 1 | Il-Ge | N | 3 | 109 | 125 | 209 | 141 | 85 | 51 | 30 | 30 |
| Ppos | 0.0038 | 0.1392 | 0.1596 | 0.2669 | 0.1801 | 0.1086 | 0.0651 | 0.0383 | 0.0383 | ||
| LR | 0.0598 | 0.7181 | 1.5128 | 2.1544* | 1.3981 | 1.6266 | 0.6095 | 0.4491 | 0.3220 | ||
| 95%CI | 0.0193 to 0.185 | 0.6032 to 0.8548 | 1.2879 to 1.7770 | 1.9178 to 2.4202 | 1.2037 to 1.6239 | 1.3303 to 1.9889 | 0.4674 to 0.7949 | 0.3161 to 0.6380 | 0.2267 to 0.4574 | ||
| 2 | Il-Er | N | 1 | 116 | 107 | 160 | 132 | 76 | 60 | 21 | 6 |
| Ppos | 0.0015 | 0.1708 | 0.1576 | 0.2356 | 0.1944 | 0.1119 | 0.0884 | 0.0309 | 0.0088 | ||
| LR | 0.0229 | 0.9149 | 1.4896 | 1.8241 | 1.5361 | 1.6835 | 0.8480 | 0.3597 | 0.0721 | ||
| 95%CI | 0.0032 to 0.1623 | 0.7702 to 1.0868 | 1.2711 to 1.7457 | 1.6291 to 2.0425 | 1.3259 to 1.7796 | 1.3794 to 2.0546 | 0.6512 to 1.1043 | 0.2535 to 0.5105 | 0.0508 to 0.1023 | ||
| 3 | Il-Me | N | 1 | 4 | 12 | 15 | 37 | 50 | 131 | 98 | 197 |
| Ppos | 0.0018 | 0.0073 | 0.0220 | 0.0275 | 0.0679 | 0.0917 | 0.2404 | 0.1798 | 0.3615 | ||
| LR | 0.0286 | 0.0328 | 0.1793 | 0.1675 | 0.4636 | 1.3492 | 2.7682* | 2.4713* | 4.5757* | ||
| 95%CI | 0.004 to 0.2027 | 0.0124 to 0.0871 | 0.1025 to 0.3138 | 0.1017 to 0.2759 | 0.3396 to 0.6328 | 1.0357 to 1.7576 | 2.3836 to 3.2148 | 2.0649 to 2.9576 | 4.091 to 5.1179 | ||
| 4 | Il-Tg | N | 0 | 20 | 24 | 57 | 111 | 79 | 50 | 34 | 28 |
| Ppos | 0.0000 | 0.0496 | 0.0596 | 0.1414 | 0.2754 | 0.1960 | 0.1241 | 0.0844 | 0.0695 | ||
| LR | 0.0000 | 0.2319 | 0.5043 | 0.9748 | 2.4198* | 3.2569* | 1.2391 | 1.0386 | 0.6035 | ||
| 95%CI | Inf. | 0.1513 to 0.3555 | 0.3421 to 0.7434 | 0.7663 to 1.2401 | 2.065 to 2.8356 | 2.6716 to 3.9704 | 0.9558 to 1.6064 | 0.7528 to 1.4329 | 0.4221 to 0.8628 | ||
| 5 | Il-Ev | N | 1 | 7 | 14 | 52 | 101 | 82 | 61 | 18 | 12 |
| Ppos | 0.0029 | 0.0201 | 0.0402 | 0.1494 | 0.2902 | 0.2356 | 0.1753 | 0.0517 | 0.0345 | ||
| LR | 0.0448 | 0.0912 | 0.3338 | 1.0395 | 2.6029* | 4.1177* | 1.8594 | 0.6148 | 0.2887 | ||
| 95%CI | 0.0063 to 0.3172 | 0.0438 to 0.1899 | 0.1998 to 0.5577 | 0.8089 to 1.3358 | 2.2081 to 3.0684 | 3.4065 to 4.9774 | 1.4802 to 2.3358 | 0.392 to 0.9642 | 0.1656 to 0.5034 | ||
| 6 | Il-Am | N | 2 | 11 | 10 | 21 | 28 | 17 | 23 | 45 | 73 |
| Ppos | 0.0087 | 0.0478 | 0.0435 | 0.0913 | 0.1217 | 0.0739 | 0.1000 | 0.1957 | 0.3174 | ||
| LR | 0.1363 | 0.2230 | 0.3620 | 0.5945 | 0.8824 | 1.0661 | 0.9720 | 2.7418* | 3.7583* | ||
| 95%CI | 0.0343 to 0.5418 | 0.1253 to 0.397 | 0.1974 to 0.6637 | 0.3954 to 0.8938 | 0.6235 to 1.2487 | 0.6746 to 1.6848 | 0.6595 to 1.4325 | 2.1093 to 3.564 | 3.1087 to 4.5436 | ||
| 8 | Il-Lo | N | 0 | 39 | 10 | 14 | 18 | 6 | 8 | 8 | 7 |
| Ppos | 0.0000 | 0.3545 | 0.0909 | 0.1273 | 0.1636 | 0.0545 | 0.0727 | 0.0727 | 0.0636 | ||
| LR | 0.0000 | 2.4391* | 0.7963 | 0.8629 | 1.2454 | 0.7706 | 0.6861 | 0.8841 | 0.5493 | ||
| 95%CI | Inf. | 1.8953 to 3.1389 | 0.441 to 1.438 | 0.5289 to 1.4077 | 0.8162 to 1.9003 | 0.3539 to 1.6779 | 0.352 to 1.3373 | 0.4536 to 1.7232 | 0.2682 to 1.125 | ||
| 9 | Il-Im | N | 0 | 2 | 3 | 5 | 20 | 18 | 25 | 18 | 22 |
| Ppos | 0.0000 | 0.0177 | 0.0265 | 0.0442 | 0.1770 | 0.1593 | 0.2212 | 0.1593 | 0.1947 | ||
| LR | 0.0000 | 0.0800 | 0.2172 | 0.2739 | 1.3689 | 2.5309* | 2.4853* | 2.1358* | 1.9541 | ||
| 95%CI | Inf. | 0.0203 to 0.316 | 0.0711 to 0.6634 | 0.1163 to 0.6453 | 0.9197 to 2.0374 | 1.6567 to 3.8664 | 1.7583 to 3.513 | 1.3981 to 3.2628 | 1.3429 to 2.8435 | ||
| 11 | Hf-So | N | 1 | 95 | 36 | 77 | 137 | 31 | 37 | 13 | 6 |
| Ppos | 0.0023 | 0.2194 | 0.0831 | 0.1778 | 0.3164 | 0.0716 | 0.0855 | 0.0300 | 0.0139 | ||
| LR | 0.0360 | 1.2480 | 0.7221 | 1.2798 | 2.9462* | 1.0300 | 0.8174 | 0.3489 | 0.1136 | ||
| 95%CI | 0.0051 to 0.255 | 1.0447 to 1.4908 | 0.5281 to 0.9874 | 1.045 to 1.5674 | 2.5646 to 3.3845 | 0.7336 to 1.4462 | 0.6005 to 1.1126 | 0.2042 to 0.596 | 0.0513 to 0.2515 | ||
| 12 | Hf - Tb | N | 2 | 11 | 9 | 79 | 115 | 17 | 6 | 3 | 1 |
| Ppos | 0.00825 | 0.0453 | 0.0370 | 0.3251 | 0.4733 | 0.0700 | 0.0247 | 0.0123 | 0.0041 | ||
| LR | 0.1289 | 0.2105 | 0.3063 | 2.8503* | 5.7191* | 1.0048 | 0.2215 | 0.1409 | 0.0334 | ||
| 95%CI | 0.0324 to 0.5125 | 0.1182 to 0.375 | 0.1613 to 0.5816 | 2.3776 to 3.417 | 5.0073 to 6.532 | 0.6352 to 1.5894 | 0.1005 to 0.4882 | 0.0458 to 0.4339 | 0.0047 to 0.2362 | ||
| 13 | Hf-Ti | N | 0 | 17 | 24 | 70 | 66 | 9 | 2 | 1 | 2 |
| Ppos | 0.0000 | 0.0890 | 0.1257 | 0.3665 | 0.3455 | 0.0471 | 0.0105 | 0.0052 | 0.0105 | ||
| LR | 0.0000 | 0.4338 | 1.1444 | 3.4230* | 3.3610* | 0.6605 | 0.0926 | 0.0593 | 0.0855 | ||
| 95%CI | Inf | 0.2756 to 0.6829 | 0.7871 to 1.6638 | 2.8402 to 4.1253 | 2.7646 to 4.0861 | 0.349 to 1.25 | 0.0233 to 0.3676 | 0.0084 to 0.4188 | 0.0215 to 0.3394 | ||
| 14 | Hf-Lo | N | 6 | 102 | 28 | 20 | 14 | 3 | 3 | 2 | 2 |
| Ppos | 0.0333 | 0.5667 | 0.1556 | 0.1111 | 0.0778 | 0.0167 | 0.0167 | 0.0111 | 0.0111 | ||
| LR | 0.5358 | 5.8066* | 1.4669 | 0.7396 | 0.5369 | 0.2264 | 0.1483 | 0.1267 | 0.0908 | ||
| 95%CI | 0.2439 to 1.1768 | 5.1093 to 6.5991 | 1.0436 to 2.062 | 0.4892 to 1.1181 | 0.3246 to 0.888 | 0.0737 to 0.6954 | 0.0483 to 0.4555 | 0.0319 to 0.5027 | 0.0229 to 0.3603 | ||
| 16 | Hf-Ge | N | 0 | 7 | 5 | 24 | 69 | 9 | 10 | 9 | 4 |
| Ppos | 0.0000 | 0.0511 | 0.0365 | 0.1752 | 0.5036 | 0.0657 | 0.0730 | 0.0657 | 0.0292 | ||
| LR | 0.0000 | 0.2391 | 0.3016 | 1.2567 | 6.4592* | 0.9392 | 0.6888 | 0.7926 | 0.2431 | ||
| 95%CI | Inf | 0.1162 to 0.492 | 0.1276 to 0.713 | 0.8738 to 1.8075 | 5.4688 to 7.6289 | 0.4994 to 1.7663 | 0.3792 to 1.2511 | 0.4215 to 1.4906 | 0.0926 to 0.6385 | ||
| 17 | Hf-At | N | 0 | 6 | 8 | 30 | 36 | 9 | 12 | 14 | 17 |
| Ppos | 0.0000 | 0.0455 | 0.0606 | 0.2273 | 0.2727 | 0.0682 | 0.0909 | 0.1061 | 0.1288 | ||
| LR | 0.0000 | 0.2114 | 0.5138 | 1.7403 | 2.3871* | 0.9774 | 0.8748 | 1.3374 | 1.1949 | ||
| 95%CI | Inf | 0.0967 to 0.462 | 0.2625 to 1.0058 | 1.2705 to 2.3839 | 1.8065 to 3.1544 | 0.5201 to 1.8366 | 0.51 to 1.5005 | 0.8149 to 2.1949 | 0.7666 to 1.8624 | ||
| 18 | Hf-Ca | N | 0 | 17 | 15 | 45 | 43 | 4 | 3 | 1 | 2 |
| Ppos | 0.0000 | 0.1308 | 0.1154 | 0.3462 | 0.3308 | 0.0308 | 0.0231 | 0.0077 | 0.0154 | ||
| LR | 0.0000 | 0.6680 | 1.0387 | 3.1325* | 3.1462* | 0.4240 | 0.2067 | 0.0874 | 0.1263 | ||
| 95%CI | Inf | 0.4288 to 1.0406 | 0.6453 to 1.672 | 2.473 to 3.9679 | 2.4634 to 4.0183 | 0.1616 to 1.1128 | 0.0675 to 0.6326 | 0.0124 to 0.6158 | 0.0319 to 0.4997 | ||
| 19 | Hf-Fl | N | 6 | 8 | 7 | 32 | 46 | 10 | 4 | 1 | 3 |
| Ppos | 0.0513 | 0.0684 | 0.2735 | 0.3932 | 0.0855 | 0.0342 | 0.0085 | 0.0256 | 1.0000 | ||
| LR | 0.8399 | 0.3259 | 0.5067 | 2.2276* | 4.1242* | 1.2483 | 0.3097 | 0.0972 | 0.2127 | ||
| 95%CI | 0.3852 to 1.8313 | 0.167 to 0.6362 | 0.247 to 1.0393 | 1.6578 to 2.9932 | 3.2923 to 5.1662 | 0.69 to 2.2583 | 0.1182 to 0.8114 | 0.0138 to 0.6843 | 0.0696 to 0.65 | ||
| 20 | Hf-La | N | 5 | 31 | 19 | 14 | 15 | 8 | 9 | 2 | 0 |
| Ppos | 0.0485 | 0.3010 | 0.1845 | 0.1359 | 0.1456 | 0.0777 | 0.0874 | 0.0194 | 0.0000 | ||
| LR | 0.7927 | 1.9118 | 1.8012 | 0.9308 | 1.0850 | 1.1248 | 0.8376 | 0.2232 | 0.0000 | ||
| 95%CI | 0.3371 to 1.8641 | 1.4242 to 2.5663 | 1.1999 to 2.7037 | 0.5719 to 1.5148 | 0.6796 to 1.7323 | 0.5781 to 2.1885 | 0.4487 to 1.5636 | 0.0566 to 0.8805 | Inf | ||
| 22 | Pc-Su | N | 0 | 25 | 179 | 214 | 23 | 32 | 31 | 15 | 12 |
| Ppos | 0.0000 | 0.0471 | 0.3371 | 0.4030 | 0.0433 | 0.0603 | 0.0584 | 0.0282 | 0.0226 | ||
| LR | 0.0000 | 0.2194 | 4.0495* | 3.9944* | 0.2882 | 0.8566 | 0.5424 | 0.3277 | 0.1869 | ||
| 95%CI | Inf | 0.1496 to 0.3217 | 3.5929 to 4.5641 | 3.6005 to 4.4314 | 0.1932 to 0.4299 | 0.6121 to 1.1988 | 0.3854 to 0.7634 | 0.199 to 0.5397 | 0.1068 to 0.327 | ||
| 26 | Pc-Da | N | 4 | 72 | 24 | 53 | 23 | 7 | 18 | 8 | 6 |
| Ppos | 0.0186 | 0.3349 | 0.1116 | 0.2465 | 0.1070 | 0.0326 | 0.0837 | 0.0372 | 0.0279 | ||
| LR | 0.2946 | 2.2357* | 1.0006 | 1.9358 | 0.7625 | 0.4495 | 0.7993 | 0.4356 | 0.2320 | ||
| 95%CI | 0.1116 to 0.7779 | 1.8516 to 2.6995 | 0.6862 to 1.4591 | 1.5322 to 2.4458 | 0.5182 to 1.122 | 0.2169 to 0.9316 | 0.5136 to 1.244 | 0.2207 to 0.8599 | 0.1054 to 0.5107 | ||
| 27 | Pc-So | N | 1 | 28 | 41 | 64 | 10 | 5 | 9 | 6 | 2 |
| Ppos | 0.0060 | 0.1687 | 0.2470 | 0.3855 | 0.0602 | 0.0301 | 0.0542 | 0.0361 | 0.0120 | ||
| LR | 0.0942 | 0.9009 | 2.6119* | 3.7126* | 0.4081 | 0.4148 | 0.5015 | 0.4227 | 0.0986 | ||
| 95%CI | 0.0133 to 0.6648 | 0.6426 to 1.2629 | 2.0023 to 3.4071 | 3.0634 to 4.4994 | 0.2238 to 0.7443 | 0.1749 to 0.9835 | 0.2656 to 0.9468 | 0.1927 to 0.9273 | 0.0249 to 0.391 | ||
| 28 | Pc-Ev | N | 0 | 23 | 84 | 26 | 15 | 2 | 1 | 1 | 2 |
| Ppos | 0.0000 | 0.1494 | 0.5455 | 0.1688 | 0.0974 | 0.0130 | 0.0065 | 0.0065 | 0.0130 | ||
| LR | 0.0000 | 0.7796 | 9.5558* | 1.2019 | 0.6869 | 0.1758 | 0.0572 | 0.0737 | 0.1064 | ||
| 95%CI | Inf | 0.5347 to 1.1366 | 8.2703 to 11.0411 | 0.8465 to 1.7065 | 0.4247 to 1.111 | 0.0444 to 0.6967 | 0.0081 to 0.4035 | 0.0104 to 0.5199 | 0.0269 to 0.4216 | ||
| 30 | Pc-Tz | N | 1 | 6 | 6 | 19 | 23 | 10 | 16 | 18 | 23 |
| Ppos | 0.0082 | 0.0492 | 0.0492 | 0.1557 | 0.1885 | 0.0820 | 0.1311 | 0.1475 | 0.1885 | ||
| LR | 0.1284 | 0.2297 | 0.4119 | 1.0915 | 1.4789 | 1.1926 | 1.3205 | 1.9509 | 1.8779 | ||
| 95%CI | 0.0182 to 0.9043 | 0.1053 to 0.5012 | 0.1888 to 0.8988 | 0.722 to 1.65 | 1.0233 to 2.1373 | 0.6585 to 2.16 | 0.8362 to 2.0852 | 1.2733 to 2.9891 | 1.2994 to 2.714 | ||
| 31 | As-Le | N | 125 | 12 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Ppos | 0.8993 | 0.0863 | 0.0000 | 0.0072 | 0.0000 | 0.0000 | 0.0000 | 0.0072 | 0.0000 | ||
| LR | 138.7301** | 0.4196 | 0.0000 | 0.0429 | 0.0000 | 0.0000 | 0.0000 | 0.0817 | 0.0000 | ||
| 95%CI | 131.0271 to 146.8859 | 0.2443 to 0.7207 | Inf | 0.0061 to 0.3024 | Inf | Inf | Inf | 0.0116 to 0.576 | Inf | ||
| 32 | As-Lo | N | 79 | 11 | 2 | 6 | 0 | 0 | 0 | 1 | 1 |
| Ppos | 0.7900 | 0.1100 | 0.0200 | 0.0600 | 0.0000 | 0.0000 | 0.0000 | 0.0100 | 0.0100 | ||
| LR | 58.4516** | 0.5488 | 0.1625 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.1139 | 0.0816 | ||
| 95%CI | 52.7899 to 64.7205 | 0.3142 to 0.9584 | 0.0412 to 0.6408 | Inf | Inf | Inf | Inf | 0.0162 to 0.8007 | 0.0116 to 0.5737 | ||
| 45 | Wb-Da | N | 3 | 57 | 18 | 32 | 17 | 14 | 13 | 10 | 7 |
| Ppos | 0.0175 | 0.3333 | 0.1053 | 0.1871 | 0.0994 | 0.0819 | 0.0760 | 0.0585 | 0.0409 | ||
| LR | 0.2775 | 2.2202* | 0.9368 | 1.3622 | 0.7027 | 1.1911 | 0.7198 | 0.7001 | 0.3450 | ||
| 95%CI | 0.0904 to 0.852 | 1.7959 to 2.7447 | 0.6051 to 1.4503 | 0.9966 to 1.8619 | 0.4475 to 1.1034 | 0.7209 to 1.9679 | 0.4268 to 1.2139 | 0.3836 to 1.2776 | 0.167 to 0.7127 | ||
| 46 | Wb-Su | N | 0 | 8 | 29 | 79 | 4 | 8 | 15 | 7 | 2 |
| Ppos | 0.0000 | 0.0526 | 0.1908 | 0.5197 | 0.0263 | 0.0526 | 0.0987 | 0.0461 | 0.0132 | ||
| LR | 0.0000 | 0.2467 | 1.8775 | 6.4033* | 0.1720 | 0.7241 | 0.9578 | 0.5442 | 0.1078 | ||
| 95%CI | Inf | 0.1257 to 0.4843 | 1.3531 to 2.6051 | 5.4947 to 7.4621 | 0.0654 to 0.4524 | 0.3688 to 1.4216 | 0.5923 to 1.5487 | 0.2639 to 1.1221 | 0.0272 to 0.4271 | ||
| 47 | Wb-Tz | N | 2 | 12 | 6 | 18 | 23 | 7 | 20 | 12 | 11 |
| Ppos | 0.0180 | 0.1081 | 0.0541 | 0.1622 | 0.2072 | 0.0631 | 0.1802 | 0.1081 | 0.0991 | ||
| LR | 0.2851 | 0.5382 | 0.4550 | 1.1452 | 1.6637 | 0.8990 | 1.9227 | 1.3663 | 0.8891 | ||
| 95%CI | 0.0722 to 1.1258 | 0.3154 to 0.9184 | 0.2089 to 0.9909 | 0.7502 to 1.7481 | 1.1561 to 2.3942 | 0.4388 to 1.8417 | 1.2928 to 2.8596 | 0.8006 to 2.3316 | 0.5074 to 1.5581 | ||
| 51 | Py-Lm | N | 2 | 21 | 107 | 78 | 62 | 3 | 4 | 3 | 2 |
| Ppos | 0.0071 | 0.0745 | 0.3794 | 0.2766 | 0.2199 | 0.0106 | 0.0142 | 0.0106 | 0.0071 | ||
| LR | 0.1110 | 0.3573 | 4.8689* | 2.2624* | 1.7939 | 0.1436 | 0.1259 | 0.1212 | 0.0577 | ||
| 95%CI | 0.0279 to 0.4417 | 0.2368 to 0.5392 | 4.1926 to 5.6543 | 1.8729 to 2.7329 | 1.4396 to 2.2354 | 0.0466 to 0.4426 | 0.0476 to 0.3331 | 0.0393 to 0.3736 | 0.0145 to 0.2296 | ||
| 53 | Py-So | N | 0 | 28 | 41 | 19 | 11 | 4 | 4 | 3 | 1 |
| Ppos | 0.0000 | 0.2523 | 0.3694 | 0.1712 | 0.0991 | 0.0360 | 0.0360 | 0.0270 | 0.0090 | ||
| LR | 0.0000 | 1.4980 | 4.6642* | 1.2220 | 0.7002 | 0.4993 | 0.3270 | 0.3131 | 0.0735 | ||
| 95%CI | Inf | 1.0874 to 2.0637 | 3.6571 to 5.9487 | 0.8114 to 1.8403 | 0.3996 to 1.227 | 0.1907 to 1.307 | 0.1249 to 0.8559 | 0.1025 to 0.956 | 0.0104 to 0.5172 | ||
| 54 | Py-Ca | N | 0 | 13 | 36 | 27 | 18 | 2 | 3 | 1 | 4 |
| Ppos | 0.0000 | 0.1250 | 0.3462 | 0.2596 | 0.1731 | 0.0192 | 0.0288 | 0.0096 | 0.0385 | ||
| LR | 0.0000 | 0.6343 | 4.2158* | 2.0748* | 1.3323 | 0.2619 | 0.2598 | 0.1094 | 0.3233 | ||
| 95%CI | Inf | 0.3814 to 1.0548 | 3.2366 to 5.4912 | 1.4996 to 2.8706 | 0.8752 to 2.0281 | 0.0664 to 1.0333 | 0.0852 to 0.7924 | 0.0156 to 0.7694 | 0.1237 to 0.8452 | ||
| 66 | Nc-Da | N | 3 | 46 | 14 | 28 | 19 | 10 | 12 | 9 | 2 |
| Ppos | 0.0210 | 0.3217 | 0.0979 | 0.1958 | 0.1329 | 0.0699 | 0.0839 | 0.0629 | 0.0140 | ||
| LR | 0.3330 | 2.1057* | 0.8642 | 1.4407 | 0.9754 | 1.0043 | 0.8014 | 0.7571 | 0.1147 | ||
| 95%CI | 0.1085 to 1.022 | 1.6468 to 2.6924 | 0.5235 to 1.4267 | 1.0277 to 2.0197 | 0.6388 to 1.4893 | 0.5506 to 1.8317 | 0.4647 to 1.3822 | 0.401 to 1.4293 | 0.0289 to 0.4548 | ||
| 68 | Nc-Su | N | 0 | 1 | 11 | 44 | 15 | 6 | 18 | 6 | 2 |
| Ppos | 0.0000 | 0.0097 | 0.1068 | 0.4272 | 0.1456 | 0.05825 | 0.1748 | 0.0583 | 0.0194 | ||
| LR | 0.0000 | 0.0435 | 0.9521 | 4.4127* | 1.0850 | 0.8262 | 1.8526 | 0.6972 | 0.1601 | ||
| 95%CI | Inf | 0.0062 to 0.3059 | 0.5446 to 1.6645 | 3.5279 to 5.5194 | 0.6796 to 1.7323 | 0.38 to 1.7962 | 1.2175 to 2.819 | 0.3207 to 1.5157 | 0.0406 to 0.6316 | ||
| 71 | Ld-Tb | N | 1 | 4 | 11 | 65 | 92 | 8 | 10 | 2 | 3 |
| Ppos | 0.051 | 0.0204 | 0.0561 | 0.3316 | 0.4694 | 0.0408 | 0.0510 | 0.0102 | 0.0153 | ||
| LR | 0.0797 | 0.0925 | 0.4735 | 2.9359* | 5.6311* | 0.5684 | 0.4703 | 0.1162 | 0.1256 | ||
| 95%CI | 0.0113 to 0.563 | 0.0351 to 0.244 | 0.2667 to 0.8408 | 2.4063 to 3.582 | 4.8512 to 6.5364 | 0.2883 to 1.1206 | 0.2571 to 0.8603 | 0.0293 to 0.4614 | 0.0409 to 0.3861 | ||
| 72 | Ld-Ti | N | 1 | 13 | 11 | 70 | 82 | 6 | 7 | 1 | 3 |
| Ppos | 0.0052 | 0.0670 | 0.0567 | 0.3608 | 0.4227 | 0.0309 | 0.0361 | 0.0052 | 0.0155 | ||
| LR | 0.0805 | 0.3189 | 0.4787 | 3.3402* | 4.6605* | 0.4263 | 0.3275 | 0.0584 | 0.1270 | ||
| 95%CI | 0.0114 to 0.5686 | 0.1886 to 0.5392 | 0.2696 to 0.8499 | 2.7692 to 4.0289 | 3.9529 to 5.4947 | 0.1939 to 0.9372 | 0.1582 to 0.6778 | 0.0083 to 0.4125 | 0.0413 to 0.3904 | ||
| 73 | Ld-Lo | N | 3 | 63 | 21 | 20 | 15 | 2 | 6 | 0 | 6 |
| Ppos | 0.0221 | 0.4632 | 0.1471 | 0.1103 | 0.0147 | 0.0441 | 0.0000 | 0.0441 | 1.0000 | ||
| LR | 0.3505 | 3.8321* | 1.4541 | 1.0202 | 0.7891 | 0.1994 | 0.4038 | 0.0000 | 0.3731 | ||
| 95%CI | 0.1145 to 1.0733 | 3.1975 to 4.5927 | 0.9812 to 2.155 | 0.6806 to 1.5293 | 0.4895 to 1.272 | 0.0504 to 0.7892 | 0.1847 to 0.883 | Inf | 0.1706 to 0.8158 | ||
| 74 | Ld-So | N | 1 | 22 | 15 | 19 | 24 | 5 | 8 | 5 | 1 |
| Ppos | 0.0100 | 0.2200 | 0.1500 | 0.1900 | 0.2400 | 0.0500 | 0.0800 | 0.0500 | 0.0100 | ||
| LR | 0.1569 | 1.2524 | 1.4053 | 1.3879 | 2.0102* | 0.7030 | 0.7607 | 0.5933 | 0.0816 | ||
| 95%CI | 0.0223 to 1.103 | 0.8658 to 1.8115 | 0.8812 to 2.241 | 0.9259 to 2.0804 | 1.4181 to 2.8494 | 0.2991 to 1.6521 | 0.3913 to 1.4788 | 0.2525 to 1.3943 | 0.0116 to 0.5737 | ||
| 75 | Ld-Ac | N | 6 | 73 | 13 | 3 | 3 | 1 | 1 | 1 | 1 |
| Ppos | 0.0588 | 0.7157 | 0.1275 | 0.0294 | 0.0294 | 0.0098 | 0.0098 | 0.0098 | 0.0098 | ||
| LR | 0.9711 | 11.1775** | 1.1632 | 0.1793 | 0.1929 | 0.1323 | 0.0866 | 0.1116 | 0.0800 | ||
| 95%CI | 0.4468 to 2.1108 | 9.8887 to 12.6343 | 0.7 to 1.9329 | 0.0588 to 0.5467 | 0.0633 to 0.5882 | 0.0188 to 0.9303 | 0.0123 to 0.6089 | 0.0159 to 0.7847 | 0.0114 to 0.5625 | ||
| 85 | An-Da | N | 2 | 57 | 17 | 34 | 18 | 10 | 13 | 8 | 8 |
| Ppos | 0.0120 | 0.3413 | 0.1018 | 0.2036 | 0.1078 | 0.0599 | 0.0778 | 0.0479 | 0.0479 | ||
| LR | 0.1883 | 2.3009* | 0.9025 | 1.5126 | 0.7690 | 0.8508 | 0.7385 | 0.5671 | 0.4067 | ||
| 95%CI | 0.0475 to 0.7467 | 1.8636 to 2.8409 | 0.5751 to 1.4163 | 1.1205 to 2.042 | 0.497 to 1.1898 | 0.4664 to 1.5519 | 0.4381 to 1.2448 | 0.2884 to 1.1152 | 0.2068 to 0.7998 | ||
| 87 | An-Ev | N | 4 | 6 | 15 | 22 | 27 | 23 | 16 | 7 | 2 |
| Ppos | 0.0328 | 0.0492 | 0.1230 | 0.1803 | 0.2213 | 0.1885 | 0.1311 | 0.0574 | 0.0164 | ||
| LR | 0.5267 | 0.2297 | 1.1163 | 1.3017 | 1.8092 | 3.1032* | 1.3205 | 0.6861 | 0.1347 | ||
| 95%CI | 0.2009 to 1.3809 | 0.1053 to 0.5012 | 0.6949 to 1.7933 | 0.8916 to 1.9004 | 1.2968 to 2.524 | 2.147 to 4.4852 | 0.8362 to 2.0852 | 0.3342 to 1.4086 | 0.0341 to 0.5325 | ||
| 96 | Pn-Tg | N | 1 | 2 | 11 | 22 | 13 | 18 | 19 | 7 | 7 |
| Ppos | 0.0100 | 0.0200 | 0.1100 | 0.2200 | 0.1300 | 0.1800 | 0.1900 | 0.0700 | 0.0700 | ||
| LR | 0.1569 | 0.0906 | 0.9842 | 1.6689 | 0.9512 | 2.9321* | 2.0521* | 0.8484 | 0.6084 | ||
| 95%CI | 0.0223 to 1.103 | 0.023 to 0.3573 | 0.5635 to 1.7189 | 1.1538 to 2.4141 | 0.5728 to 1.5795 | 1.9294 to 4.4559 | 1.369 to 3.0761 | 0.4103 to 1.7128 | 0.2978 to 1.243 |
CI: Confidence interval, Inf: Infinite, **: 10<LR, *: 2≤LR≤10
Three ADR–drug–onset timing (A–D–O) combinations showed an LR of more than 10. In these cases, onset timing information resulted in meaningful changes in the probability of identifying the occurrence of the ADR, which is considered to have considerable clinical value.7,16) In all, there were 51 A–D–O combinations with an LR greater than 2 but less than 10. In these cases, ADR onset timing information could well have certain clinical value in terms of patient awareness of ADRs.7,16) There were 68 A–D–O combinations with an LR of 1–2. Onset timing information for these A–D–O combinations is not likely to be clinically important.7,16)
There were 54 A–D–O combinations with an LR greater than 2. In the case of these 54 combinations, onset timing information could be clinically useful. When we included the starting day of medication (i.e., 0 d) as Nday-onset-info for the combination of anaphylactic shock with levofloxacin (Le) (ID 31 in Table 3) or loxoprofen (Lo) (ID 32 in Table 3), the LR was 138.7301 or 58.4516. Similarly, when we included the Nday-onset-info (i.e., 1–7 d) for the combination of liver disorder with acetaminophen (Ac) (ID 75 in Table 3), the LR was 11.1775. This indicates that for these three combinations, onset timing information is clinically very useful.16)
Using Bayes’ theorem, we quantitatively evaluated the extent to which information on the days to onset of ADRs after taking drugs is clinically useful to patients in identifying ADRs. In the case of anaphylactic shock due to levofloxacin or loxoprofen administration, the results suggested that information on the number of days of using the drugs is extremely useful for patient awareness of the ADR. We also showed that for the combination of liver disorder and acetaminophen, the addition of onset timing information showed a high likelihood ratio. From the results of this study, the offering of ADR onset timing information to patients by pharmacists to raise patient awareness of ADRs, together with the recognition by pharmacists of characteristic onset timing information for ADRs with specific drugs, are clinically useful because patient monitoring of changes in their physical condition while taking medication can prevent ill effects associated with ADRs from becoming more serious.
Study LimitationsThe data analyzed in this study are held in a spontaneously reported adverse event database system. They are therefore subject to bias by under-reporting, and prone to the effects of the release of safety information by the regulatory authorities, and to market trends.
The authors declare no conflict of interest.