2017 年 40 巻 9 号 p. 1515-1524
2017 年 40 巻 9 号 p. 1515-1524
Curcumin, a polyphenol derived from the rhizome of the naturally occurring plant Curcuma longa, has various pharmacological actions such as antioxidant and anti-inflammatory effects. In this paper, we evaluated the role of its internal metabolite, curcumin β-D-glucuronide (curcumin monoglucuronide, CMG), by investigating curcumin kinetics and metabolism in the blood. Firstly, we orally administered highly bioavailable curcumin to rats to elucidate its kinetics, and observed not only the free-form of curcumin, but also, curcumin in a conjugated form, within the portal vein. We confirmed that curcumin is conjugated when it passes through the intestinal wall. CMG, one of the metabolites, was then orally administered to rats. Despite its high aqueous solubility compared to free-form curcumin, it was not well absorbed. In addition, CMG was injected intravenously into rats in order to assess its metabolic behavior in the blood. Interestingly, high levels of free-form curcumin, thought to be sufficiently high to be pharmacologically active, were observed. The in vivo antitumor effects of CMG following intravenous injection were then evaluated in tumor-bearing mice with the HCT116 human colon cancer cell line. The tumor volume within the CMG group was significantly less than that of the control group. Moreover, there was no significant loss of body weight in the CMG group compared to the control group. These results suggest that CMG could be used as an anticancer agent without the serious side effects that most anticancer agents have.
Curcumin is a component of Curcuma longa, and its rhizome has long been used as a food, spice, colorant, and herbal medicine. Curcumin has anti-inflammatory,1,2) free radical scavenger-mediated antioxidant,3,4) anticancer,5,6) anti-allergy,7,8) anti-arthritis,9,10) anti-diabetes,11) hepatoprotective,12) neuroprotective,13) antidepressant,14) and muscle damage protective15) physiological effects. With regard to chronic inflammation that is particularly associated with lifestyle-related diseases, many recent studies have investigated the inhibitory effects of curcumin on activated nuclear factor-kappaB (NF-κB),16,17) and signal transducer and activator of transcription 3 (STAT3).17,18)
However, since curcumin is highly lipophilic, its solubility in water is very low at approximately 11 ng/mL.19) For the most part, curcumin is not absorbed into the body when it is ingested,20,21) which therefore poses a major problem for its clinical application. Theracurmin®, a formulation of curcumin that has high bioavailability, has been developed using fine-particulation and surface processing techniques22–24) to solve this problem; it shows a bioavailability that is 27 times higher than that of curcumin powder and is marketed as a commercial food additive suitable for oral intake in humans.22) Interestingly, in a rat model of post-myocardial infarction, left ventricular fractional shortening was restored by uptake of Theracurmin® at a dose of 1/100 compared to commercially available curcumin powder.25) Furthermore, in a randomized double-blind placebo-controlled prospective study of patients with osteoarthritis of the knee, visual analog scalescores for knee pain were significantly lower in the Theracurmin® group than in the placebo group, and Theracurmin® reduced celecoxib a nonsteroidal anti-inflammatory drug (NSAID)-dependence to a significantly greater extent than placebo.26) Furthermore, the promising efficacy of curcumin has been demonstrated across a variety of medical fields, in preclinical studies and in human clinical trials, using the highly bioavailable formulation of curcumin, Theracurmin®.27–36)
In general, once they are absorbed into the body, polyphenols are mainly conjugated with glucuronic acid or sulfuric acid in the liver, with some excreted into the duodenum with bile, and some released into the blood. It is thought that the metabolites excreted into the duodenum could be hydrolyzed back to the original compound by hydrolases including β-glucuronidase, and then reabsorbed through the intestinal tract, and returned to the liver through the portal vein via enterohepatic circulation.37–39) This effect has been seen with the flavonoid genistein, where twin plasma concentration peaks resulting from enterohepatic circulation were observed.40) However, the mechanisms of curcumin metabolism have not been fully elucidated due to its low bioavailability.
It has been reported that 99% of ingested curcumin exists as conjugates in the blood in mice41) and there are almost no free-form curcumin detected in humans.42) Furthermore, it was found that curcumin β-D-glucuronide (curcumin monoglucuronide, CMG) (Fig. 1) was the major metabolite among several conjugates, and there were negligible amounts of free-form curcumin in the blood when commercially available curcumin powder was fed to rats.43)

Once curcumin is taken into the blood, it is immediately metabolized to curcumin conjugated forms, mainly to CMG.43) On the other hand, the in vitro pharmacological activity of curcumin (for example, its anticancer effects) is associated with the free-form of the compound.43,44) Thus, the problem exists that the blood levels of the pharmacologically active form of curcumin, that is, free-form curcumin, are not sufficiently high to be clinically useful.
As there are still many questions that remain unanswered regarding curcumin and its conjugates in the blood, the purpose of this study was to reveal the role of the conjugated form of curcumin, especially CMG, by investigating the kinetics and metabolism in circulating blood.
We orally administered a highly absorbable form of curcumin, Theracurmin®, and measured plasma levels of free-form and conjugated curcumin in the portal vein and the abdominal portion of the vena cava to understand its basic kinetics. We then synthesized CMG and measured plasma levels of free-form and conjugated curcumin after oral or intravenous administration of CMG to clarify the mechanism of metabolism. In addition, we investigated the antitumor effects of CMG by its intravenous administration to tumor-bearing mice transplanted with HCT116, a human colon cancer cell line.
All animal experiments were approved by the Animal Experiment Committee of Chubu University, Musashino University, and Nissei Bilis Co., Ltd. The care and treatment of rats in Chubu University was in accordance with their guidelines (Permission No. 2610032 and 2710033).
Synthesis of CMGCMG was synthesized using a slight modification of a previous method.44)
Acetobromo-α-D-glucuronic acid methyl ester (1.0 g, 2.52 mmol) and vanillin (326.0 mg, 2.15 mmol) were dissolved in quinoline (8.7 mL), prior to the addition of silver oxide (522.0 mg) to the mixture at 0°C. After stirring for 30 min, the mixture was stirred at room temperature for a further 90 min. After the addition of acetic acid (26.0 mL), the mixture was poured into distilled water (260.0 mL), and passed through a celite pad. The filtrate layer obtained was extracted with ethyl acetate twice, and the organic layer was washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue obtained was purified by SiO2 flash column chromatography (AcOEt–n-hexane=30 : 70 then 80 : 20) to give β-D-glucopyranosideuronic acid, 4-formyl-2-methoxyphenyl, methyl ester, triacetate (1, 491.8 mg, 49%) as a white solid.
Synthesized 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-1,4-hexadiene (483.0 mg, 2.06 mmol) and B2O3 (214.9 mg, 3.08 mmol) were dissolved in ethyl acetate (5.0 mL), and stirred for 30 min at 85°C. Compound 1 (805.0 mg, 1.72 mmol), dissolved in ethyl acetate (10.0 mL), and (n-BuO)3B (0.82 mL, 3.05 mmol) were then added to the mixture, and stirred for 1 h at 85°C. The mixture was treated with piperidine (71 µL, 0.72 mmol), and stirred for an additional 30 min. Thereafter 0.5 N HCl (7.0 mL) was added, and after stirring for 1 h at 50°C, the mixture was cooled to room temperature, extracted with ethyl acetate twice, and the organic layer was washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue obtained was purified by SiO2 flash column chromatography (AcOEt–n-hexane=60 : 40) to give curcumin β-D-glucopyranosiduronic acid 2,3,4-tri-O-acetyl, methyl ester (2, 917.7 mg, 78%) as a yellow foam.
Compound 2 (875.1 mg, 1.29 mmol) was dissolved in methanol (24.5 mL), and 1 N NaOH solution (24.5 mL) was added dropwise at 0°C, and stirred for 1 h at 50°C. Using 50% formic acid the pH of the solution was adjusted to 3–4, and the yellow solid was filtered and collected. The solid obtained was purified by HPLC (PEGASIL ODS SP100, ϕ20×250 mm, eluent: CH3CN–H2O=45 : 55, 0.1% TFA) to give CMG (253.1 mg, 36%) as a yellow solid. All of the physico-chemical and spectral data of CMG were consistent with those given in the previous report.44)
Oral Administration of Curcumin in Rats for Blood Sampling from the Portal Vein and the Abdominal Portion of the Vena CavaSprague–Dawley (SD) rats (five male rats per group, seven weeks old, Japan SLC) were fasted for at least 12 h before each rat within a group was orally administered Theracurmin® (Theravalues Corporation, Japan) at doses equivalent to 1.5, 5, and 15 mg/kg of curcumin. After 30 min and 1 h, blood was sampled under isoflurane anesthesia from the portal vein and abdominal portion of the vena cava using a syringe containing EDTA2Na (final concentration 1 mg/mL, Dojindo Laboratories Co., Ltd., Kumamoto, Japan). The blood obtained was centrifuged (1600 rpm, for 15 min, at 4°C) to isolate plasma.
Oral Administration of CMG in RatsSD rats (four male rats, eight weeks old, mean weight 268 g, Charles River Laboratories Japan, Inc.) were fasted for at least 12 h prior to undergoing inhalation anesthesia using chloroform on the day of blood sampling. CMG was dissolved in distilled water, and administered orally to each of the four rats per group at doses of 1.48 mg/kg (equivalent to 1.0 mg/kg of curcumin), or 14.8 mg/kg (equivalent to 10 mg/kg of curcumin). After 30 min, 1, 2, and 4 h, approximately 0.5 mL of blood was sampled from the caudal vein. The blood obtained was centrifuged (3000 rpm, for 10 min, at 4°C) to isolate plasma.
Intravenous Administration of CMG in RatsSD rats (five male rats, nine weeks old, weight 330.2–358.8 g, Charles River Laboratories Japan, Inc.) were fasted for at least 12 h prior to blood sampling under isoflurane anesthesia. Here, catheters were placed in the femoral vein for curcumin administration, and in the femoral artery for blood sampling. Thereafter, CMG was dissolved in water for injection and intravenously injected via the catheter at a dose of 30 mg/kg (equivalent to 20 mg/kg of curcumin). After 1, 3, 5, 10, 15, 30 min, 1, 2, 4, and 8 h, approximately 0.6 mL of blood was sampled from the femoral vein. The blood obtained was centrifuged (3000 rpm, for 10 min, at 4°C) to isolate plasma.
Sample Preparation and Measurement of Curcumin Levels in Rat PlasmaThe preparation of the plasma samples and measurement were carried out based upon a previous report.22)
0.1 M sodium acetate buffer (pH 5.0) and β-glucuronidase from Helix pomatia (hydrolysis enzyme for glucuronides and sulfates) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) were added to the plasma, which was then incubated for 1 h at 37°C to hydrolyze the curcumin conjugates (for the measurement of free-form curcumin, distilled water was added instead of β-glucuronidase, and the solution was not incubated). Thereafter, an internal standard was added, and the solution was extracted twice with chloroform. After removing the solvent by centrifugal drying, 50% methanol was added to the residue. The solution was used for analysis of curcumin levels using LC-MS/MS (API 3200 LC-MS/MS System with Shimadzu Prominence HPLC). LC-MS/MS was performed under the following conditions: for HPLC, we used a Waters Atlantis T3 column, while the mobile phase for solution A consisted of water containing 0.1% formic acid, and solution B consisted of acetonitrile containing 0.1% formic acid. Gradient elution was performed with a sample injection volume of 2 µL and mobile phase starting from 45 to 95% (0–2 min), 95% (2–7.5 min), from 95 to 45% (7.5–7.51 min), and 45% (7.51–15 min) concentration of B. MS was operated under the positive ionization mode: Electron Spray thermo Ionization (ESI), and the measurement mode: Multiple Reaction Monitoring (MRM), with curcumin: 369.1/177.2 (m/z), and mepronil: 270/119 (m/z).
Plasma curcumin levels resulting from β-glucuronidase treatment were measured as the total plasma level of curcumin, and curcumin levels in plasma that was not treated with β-glucuronidase were measured as the plasma level of free-form curcumin. Furthermore, the level calculated by subtracting the plasma free-form curcumin level from the total plasma curcumin level was defined as the plasma level of conjugated curcumin. However, the conjugated form of curcumin observed here includes not only CMG but other conjugates as well.
Safety Studies of CMG Following Intravenous Injection in MiceSlc: ICR mice (five male mice per group, six weeks old, weight 31.7–37.0 g at the time of administration) were administered CMG at doses of 125, 250, 500, or 1000 mg/kg, at a dosage volume of 10 mL/kg, as a single intravenous injection. Observation was performed immediately after administration, then at 30 min, 1, 2, 4, and 6 h after administration and once per day from the following day (in the 1000 mg/kg group, acute symptoms were apparent immediately after administration, and therefore observation was carried out frequently up to 1 h after administration). On day 14 following administration, after the mice had been fasted for at least 4 h, a laparotomy was performed under anesthesia induced by intraperitoneal injection of pentobarbital sodium, and 0.6 mL or more of blood was sampled from the abdominal aorta. The blood was treated with the anticoagulant heparin sodium, and using the plasma obtained by centrifugal separation (3000 rpm, for 10 min, at 4°C), transaminase levels (AST(GOT) and ALT(GPT)) were measured. After blood sampling, the mice were euthanized, and the skull, organs, and tissue in the thoracic and abdominal cavities were macroscopically observed.
Assessment of the Antitumor Effects of CMG Following Intravenous Injection in MiceIn BALB/c/AnNCr j-nu/nude mice (eight female mice per group, six weeks old, weight 14.9–18.5 g at the time of cancer cell transplantation, Charles River Laboratories Japan, Inc.), 4×106 HCT116 colorectal cancer cells (ATC C® CCL-247™, derived from the colon of human adult males) were subcutaneously transplanted. From day 7 following transplantation, 90 mg/kg of CMG dissolved in water for injection (equivalent to 61 mg/kg of curcumin) or water for injection (10 mL/kg) as a control, were intravenously administered 10 times at a frequency of three times a week for three weeks (days 0, 3, 5, 7, 10, 12, 14, 17, 19, 21). The tumor volume was assessed seven times (days 0, 4, 7, 11, 14, 18, 21), with the length, height and width of the tumor being measured using a pair of calipers. The tumor volume was calculated as length×width×height×0.5 (mm3), and the relative tumor volume was calculated as the tumor volume at each timepoint (mm3)/tumor volume on day 0 (mm3)×100. Body weight was measured on days 0, 7, 14 and 21 using electronic scales (UW4200S, Shimadzu Corporation).
Homogenization of Tumor Tissue Sample to Measure Curcumin LevelsThe tumor tissue was isolated from mice upon complete blood removal and frozen 2 h after intravenous administration on day 21. The physiological saline was added to the thawed tumor tissue and homogenized with a glass homogenizer to prepare tumor sample. All preparations were done under ice cooling. The preparation of the samples and measurement of curcumin levels were carried out according to the method in “Sample Preparation and Measurement of Curcumin Levels in Rat Plasma” described above.
As a result of oral administration of highly absorbable Theracurmin®, which equated to a dose of curcumin of 1.5 mg/kg, blood samples from the portal vein and abdominal portion of the vena cava showed that at 0.5 h after administration free-form curcumin levels were 5.1±2.3 and 2.1±0.9 ng/mL, respectively, while conjugated curcumin levels were 87.9±45.2 and 65.1±46.3 ng/mL, respectively. At 1 h, free-form curcumin levels were 0.8±0.6 and 2.0±2.8 ng/mL, respectively, and conjugated curcumin levels were 37.7±25.5 and 29.6±20.0 ng/mL, respectively (Fig. 2, Table 1). Free-form and conjugated curcumin levels resulting from doses of 5 and 15 mg/kg are also shown in Table 1. Therefore, as measured in the blood of the portal vein and abdominal portion of the vena cava of rats after oral administration of Theracurmin®, it was confirmed that curcumin is present in the blood in a dose-dependent manner.
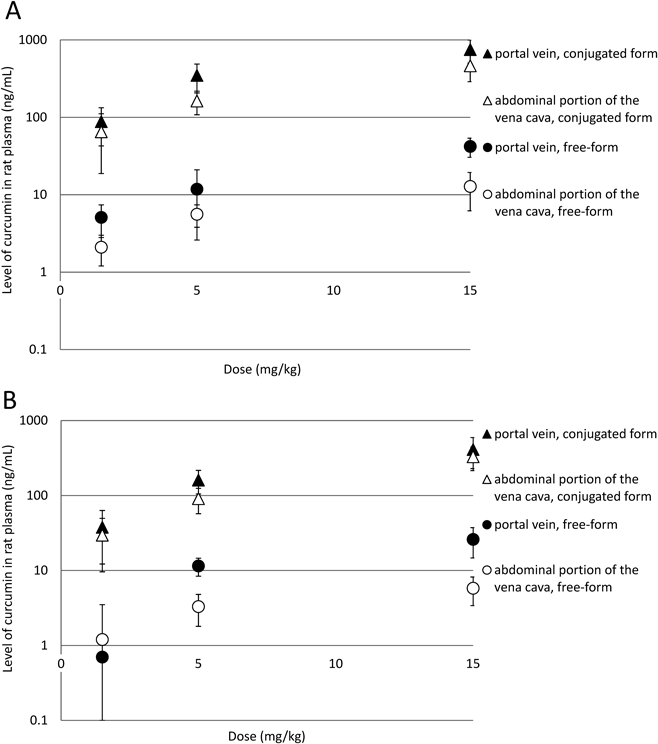
A: 0.5 h after administration. B: One hour after administration. ●, in portal vein, free-form curcumin. ▲, in portal vein, conjugated curcumin. ○, in abdominal portion of the vena cava, free-form curcumin. △, in abdominal portion of the vena cava, conjugated curcumin. Each point and bar represents the mean±standard deviation of the mean (S.D.M.). (n=5).
| Dose (mg/kg) | n | Time (h) | Free-form (ng/mL) | Conjugated form (ng/mL) | Free-form (%) | |
|---|---|---|---|---|---|---|
| 1.5 | 5 | Portal vein | 0 | 0.0 | 0.0 | — |
| 0.5 | 5.1±2.3 | 87.9±45.2 | 5.5 | |||
| 1 | 0.8±0.6 | 37.7±25.5 | 2.2 | |||
| Abdominal portion of the vena cava | 0 | 0.0 | 0.0 | — | ||
| 0.5 | 2.1±0.9 | 65.1±46.3 | 3.2 | |||
| 1 | 2.0±2.8 | 29.6±20.0 | 6.5 | |||
| 5 | 5 | Portal vein | 0 | 0.0 | 0.0 | — |
| 0.5 | 11.8±9.2 | 347.6±140.7 | 3.3 | |||
| 1 | 11.5±3.1 | 160.8±55.9 | 6.7 | |||
| Abdominal portion of the vena cava | 0 | 0.0 | 0.0 | — | ||
| 0.5 | 5.6±1.8 | 162.8±54.9 | 3.3 | |||
| 1 | 3.3±1.5 | 91.0±33.8 | 3.5 | |||
| 15 | 5 | Portal vein | 0 | 0.0 | 0.0 | — |
| 0.5 | 42.1±11.7 | 750.0±247.3 | 5.3 | |||
| 1 | 26.0±11.3 | 410.9±182.1 | 6.0 | |||
| Abdominal portion of the vena cava | 0 | 0.0 | 0.0 | — | ||
| 0.5 | 12.8±6.6 | 463.1±174.6 | 2.7 | |||
| 1 | 5.8±2.4 | 329.5±114.4 | 1.7 |
Each value represents the mean±S.D. (n=5).
To date, it has been generally accepted that the conjugation of drugs is mostly carried out in the liver; however, with respect to curcumin, its conjugated form was detected in portal vein blood prior to entering the liver, which demonstrates that some conjugation takes place when it is absorbed from the intestinal tract. Furthermore, the proportion of free-form curcumin to total curcumin was higher in the portal vein blood than in the abdominal portion of the vena cava, suggesting that free-form curcumin that was not conjugated in the intestinal tract was metabolized into a conjugated form in the liver then circulated to the whole body.
Bio-absorbability of CMG Following Oral Administration in RatsCMG was orally administered to SD rats at doses of 1.48 mg/kg (equivalent to 1 mg/kg of curcumin) and 14.8 mg/kg (equivalent to 10 mg/kg of curcumin), and the resulting plasma curcumin levels are shown in Fig. 3. The Cmax (maximal concentration) of the total plasma levels of curcumin was 2.0±2.3 and 91.9±86.9 ng/mL, respectively, and the area under the curve (AUC)0–4 h was 3.9±5.7 and 167.0±143.4 ng·h/mL, respectively.

●, Curcumin monoglucuronide 1.48 mg/kg, p.o. (curcumin 1.0 mg/kg). ○, Curcumin monoglucuronide 14.8 mg/kg, p.o. (curcumin 10 mg/kg). Each point and bar represents the mean±S.D. (n=4).
Curcumin has extremely low water solubility at 11 ng/mL,19) and when 50 mg/kg of raw curcumin powder was orally administered to rats in our previous study, the Cmax was 13.0±5.8 ng/mL while the AUC0–6 h was 51.1±25 ng·h/mL.22) Although CMG has extremely high water solubility (≥100 mg/mL), in the present study it was orally administered to rats at a dose of 10 mg/kg (curcumin equivalent), and the resulting Cmax and AUC0–4 h were as described above. These data indicate that although some portion of CMG was absorbed through the intestinal tract and transferred to the blood, blood levels of curcumin were not very high compared to those seen following administration of curcumin powder which is used as a commercial food additive.
Therefore, we found that CMG, a major metabolite of curcumin, is not absorbed to a sufficient extent through the intestinal tract to support the enterohepatic circulation hypothesis. Thus, the results suggest that enterohepatic circulation is not the main pathway of curcumin metabolism in rats.
Metabolic Behavior of CMG Following Intravenous Administration and the Plasma Levels of Free-Form and Conjugated Curcumin in RatsSD rats were intravenously administered CMG at a dose of 30 mg/kg, and the resulting plasma levels of conjugated curcumin (calculated by subtracting free-form curcumin from the total curcumin levels) were 160.5±19.2 µg/mL at 1 min after administration, 77.8±6.4 µg/mL at 3 min, 80.5±7.2 µg/mL at 5 min, 46.9±10.7 µg/mL at 10 min, 22.9±5.3 µg/mL at 15 min, 10.2±3.5 µg/mL at 30 min, 4.4±1.4 µg/mL at 1 h, 1.5±0.4 µg/mL at 2 h, 0.4±0.1 µg/mL at 4 h, and 0.1±0.04 µg/mL at 8 h, indicating a decrease over time (Fig. 4). The AUC0.02–8 h of conjugated curcumin was 28.4±5.1 µg·h/mL. On the other hand, plasma free-form curcumin levels were 10.1±3.8 µg/mL at 1 min, 7.6±2.9 µg/mL at 3 min, 5.7±2.5 µg/mL at 5 min, 3.6±1.2 µg/mL at 10 min, 2.8±0.8 µg/mL at 15 min, 1.6±0.6 µg/mL at 30 min, 0.6±0.2 µg/mL at 1 h, 0.1±0.04 µg/mL at 2 h, 0.02±0.01 µg/mL at 4 h, and 0.01±0.01 µg/mL at 8 h, with a decrease over time that took place at a similar rate to the transition observed with the curcumin conjugates. The AUC0.02–8 h of free-form curcumin was 2.8±1.0 µg·h/mL.

●, Curcumin monoglucuronide 30 mg/kg, i.v. (curcumin 20 mg/kg), conjugated form. ○, Curcumin monoglucuronide 30 mg/kg, i.v. (curcumin 20 mg/kg), free-form. Each point and bar represents the mean±S.D. (n=5).
In the present study, following intravenous administration of 30 mg/kg of CMG, it was found that inactive CMG, which is essentially known as a curcumin metabolite, was converted into free-form curcumin, the active form of the compound. Incubation of mouse serum with CMG at 37°C for 1 h did not result in the formation of free-form curcumin (data not shown). It was therefore suggested that blood cell components, endothelial cells and the liver are involved in the deconjugation reaction. When Theracurmin® was orally administered to give a dose of 15 mg/kg of curcumin, the free-form curcumin Cmax was 0.01 µg/mL, and when CMG was intravenously administered at a dose of 30 mg/kg (equivalent to 20 mg/kg of curcumin) the free-form curcumin Cmax was 10 µg/mL. Therefore, the amount of free-form curcumin obtained after intravenous administration of CMG corresponded to approximately 1000 times the plasma level of free-form curcumin seen following oral administration of the compound. Furthermore, it was found that high levels of free-form curcumin could be maintained in the blood, which could not be attained with oral administration of curcumin. We summarized the findings of these tests in Fig. 5.

Levels of free-form and conjugated curcumin following oral administration of curcumin and intravenous administration of CMG in rats.
As observed above, we found that high blood levels of curcumin could be achieved with selective chemical modification and a specific administration method. We also found that as well as being a metabolite, CMG is the precursor of free-form curcumin, which is the active form of curcumin. Therefore, when CMG is intravenously administered, it functions as a curcumin prodrug, and it should thereby facilitate the optimum pharmacological activity of curcumin that requires high blood levels of curcumin. Next, we evaluated the safety and efficacy of CMG.
Safety Study of CMG Following Intravenous Injection in MiceWe examined the single-dose toxicity of CMG intravenous administration in mice, with the entire group that had been administered 125 mg/kg showing no abnormalities during the observation period. In the group administered 250 mg/kg, one male exhibited piloerection. In the group administered 500 mg/kg, all mice showed reduced spontaneous motility, with loose bowel movements, and three males exhibited piloerection; however, all subjects had recovered by 6 h after administration. In the group administered 1000 mg/kg, all mice exhibited prone position and reduced spontaneous motility immediately after administration, and two males demonstrated respiratory distress, and three males exhibited bradypnea and paralytic gait, with all subjects dying within 1 h of administration.
With regards to body weight, on day 1, one male in the group administered 250 mg/kg, and three males in the group administered 500 mg/kg, exhibited weight loss; however, thereafter all mice showed steady weight gain. Blood biochemistry testing showed AST and ALT levels to be within the normal range, as shown in Table 2. Autopsy revealed that all mice including fatal cases exhibited no abnormalities. Under the present test conditions, the LD50 value with intravenous administration of CMG was between 500–1000 mg/kg, and the probit method calculated the LD50 value as 707 mg/kg.
| Dose (mg/kg) | n | AST (IU/L) | ALT (IU/L) |
|---|---|---|---|
| 125 | 5 | 37±4 | 24±7 |
| 250 | 5 | 34±3 | 25±5 |
| 500 | 5 | 37±3 | 34±6 |
Each value represents the mean±S.D. (n=5).
We verified the antitumor effects of intravenous administration of CMG (90 mg/kg) in tumor-bearing mice with colorectal cancer, with the tumor volume of the control group increasing over time from 17.6±2.9 mm3 on day 0 to 505.6±100.4 mm3 on day 21, with a relative tumor volume of 2828.8±332.8% on day 21 compared to day 0 (Fig. 6A). The tumor volume of the group administered CMG increased from 17.4±3.2 mm3 on day 0 to 190.2±70.1 mm3 on day 21, with a relative tumor volume of 994.6±313.3% on day 21. Compared to the control group, the tumor volume and the relative tumor volume of the group administered CMG were lower on day 21, indicating significant tumor growth suppression (Student’s t-test, p=0.02, 0.001, respectively).

A: The relative tumor volume (% vs. day 0). This was calculated as the tumor volume at each timepoint (mm3)/tumor volume on day 0 (mm3)×100. B: Change in mouse body weight. CMG was administered intravenously three times a week (days 0 to 21). ●, Control. ○, Curcumin monoglucuronide 90 mg/kg, i.v. (curcumin 61 mg/kg). Each point and bar represents the mean±standard error (S.E.). (n=8). * p<0.05 (Student’s t-test). C: Levels of free form and conjugated curcumin in mice tumor tissue after intravenous administration of CMG on day 21. ■, Curcumin monoglucuronide 90 mg/kg, i.v. (curcumin 61 mg/kg), conjugated form. □, Curcumin monoglucuronide 90 mg/kg, i.v. (curcumin 61 mg/kg), free-form. Each bar represents the mean±S.D. (n=8).
Average mouse body weight in the control group was 19.4±0.5 g on day 0, 19.3±0.5 g on day 7, 19.9±0.6 g on day 14, and 20.3±0.7 g on day 21, while in the CMG group, average body weight was 19.8±0.5 g on day 0, 19.6±0.5 g on day 7, 20.7±0.4 g on day 14, and 21.1±0.6 g on day 21, with no statistically significant differences observed between the groups (Fig. 6B). Moreover, the levels of conjugated curcumin and free-curcumin in the isolated tumor tissue were 605.4±465.5 and 1464.5±840.9 ng/g, respectively (Fig. 6C). These results suggest that conjugated curcumin was deconjugated by β-glucuronidase to free-form curcumin at the tumor site and it was incorporated into cancer cells.
Therefore, in nude mice with subcutaneous transplantation of HCT116 human colorectal cancer cells, repeated intravenous injection of 90 mg/kg of CMG resulted in a significant effect on tumor volume growth, compared to the control group. In addition, from day zero to day 21, there was no significant difference in body weight between the groups, and there was no observable phenomenon that would indicate a problem with respect to the safety of CMG.
Further studies are planned to investigate the effects of intravenous administration of CMG on other types of cancer, as several reports have indicated that curcumin has an antitumor effect on various tumors including refractory pancreatic cancer,45–47) and lung cancer.48–50) In addition, further investigation on tumor suppressive effects by other curcumin metabolites are currently being undertaken.
In this study, after measuring curcumin levels in the portal vein and venous blood following oral administration of curcumin in rats, we observed the conjugated form of curcumin in portal vein blood prior to entering the liver. This confirmed that curcumin is conjugated not only in the liver but also by UDP-glucuronosyltransferase when passing through the intestinal wall. Furthermore, we synthesized CMG, a curcumin conjugate, and found that its oral absorption was poor. It was therefore speculated that CMG is excreted via the intestinal tract without hydrolysis, and it was proposed that enterohepatic circulation is not the main metabolic pathway for curcumin in rats.
We measured plasma levels of curcumin after intravenous administration of CMG (30 mg/kg) in rats, and interestingly, we found that free-form curcumin was present in the blood at a constant level (approximately 4–16% of the total curcumin level), irrespective of the fact that only CMG was administered intravenously. This suggests that CMG, essentially known as a curcumin metabolite with little pharmacological activity, was converted into free-form curcumin, which is the active form of the compound.
Based on these results, we conducted a preliminary safety evaluation of CMG using mice. In the groups administered 125–500 mg/kg all of the mice survived, whereas in the group administered 1000 mg/kg all mice died within 1 h of administration. Therefore, the LD50 value associated with intravenous administration of CMG is between 500–1000 mg/kg, and the LD50 value was calculated to be 707 mg/kg using the probit method.
In the evaluation of the antitumor effects of CMG following intravenous injection, on day 21 the tumor volume of the group administered CMG was less than that of the control group, indicating significant tumor growth suppression. Furthermore, from day zero to day 21, there was no significant difference observed in body weight between the groups, and there was no observable phenomenon that would indicate a problem with respect to the safety of CMG upon repeated intravenous administration at a dose of 90 mg/kg.
This is the first report that the intravenous administration of CMG results in high blood levels of free-form curcumin; that is, the active form of the compound, which cannot be attained with the oral administration of curcumin. Furthermore, we confirmed a preliminary demonstration of the safety and antitumor effects of intravenous CMG and revealed that CMG is applicable as a prodrug controlled by metabolism.
For the application of CMG injections, future pharmacology and pharmaceutical safety trials are planned, as well as research into potential clinical applications in humans as a cancer treatment that maximizes the pharmacological activity of curcumin without serious side effects.
This work was supported in part by the research Grants of the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant-in-Aid for Scientific Research on Innovative Area) and the Ministry of Education, Culture, Sports, Science and Technology of Japan.
M.K. and H.K. own equity in and scientific consultants for TheraBioPharma Inc.