2018 年 41 巻 1 号 p. 80-85
2018 年 41 巻 1 号 p. 80-85
The protective effects of seleno-L-methionine (SeMet) on oxidative stress in pancreatic islets were investigated with a short-term nicotinamide (NA) and streptozotocin (STZ)-induced diabetic mouse model. ICR mice were intraperitoneally injected twice with 100 mg/kg STZ and 120 mg/kg NA at a 1-d interval and were then orally administered 158 µg Se/kg SeMet with free access to a selenium-deficient diet for 5 weeks. Administration of SeMet significantly improved the levels of glycated hemoglobin (HbA1c), non-fasting and oral glucose tolerance-tested (OGTT) blood glucose, plasma adiponectin and hepatic glycogen that deteriorated by NA/STZ treatment. However, supplementary SeMet did not restore non-fasting plasma insulin levels in NA/STZ treatment group and significantly suppressed OGTT plasma insulin levels in the control group. Although SeMet significantly suppressed 8-hydroxy-2′-deoxyguanosine density in pancreatic islets, SeMet did not restore insulin density. The hepatic and pancreatic mRNA levels of glutathione peroxidase 1 (GPX1) increased by NA/STZ treatment or SeMet administration. These results suggest that although a physiological level of SeMet improves glucose tolerance by exhibiting insulin-mimetic activity in a short-term induced diabetic mouse model under insufficient Se status, the suppression of pancreatic oxidative stress with the induction GPX1 by SeMet supplementation is unlikely to restore insulin storage and secretion.
Selenium (Se) is one of the essential micronutrients for human and animals and exerts as a key component of selenoproteins to regulate many physiological functions.1) The families of glutathione (GSH) peroxidase (GPX) and thioredoxin reductase (TR) are the well-known Se-dependent antioxidant enzymes.2,3) GPX1 is particularly important because the antioxidant enzyme has the higher affinity than catalase for hydrogen peroxide (H2O2)4) that is formed by superoxide dismutase from superoxide anions5) or directly generated by oxidative stress in cytoplasm. Therefore, Se deficiency induces some pathological conditions including cancer and cardiovascular disease.6) The patients with Se-deficiency syndrome, Keshan disease cause cardiomyopathy with the pancreatic atrophy and degeneration.7) The fragility for the oxidative stress in pancreas may be responsible for the pathogenesis.8)
The early stages of type 2 diabetes is characterized by postprandial hyperglycemia by insufficient secretion of insulin from pancreatic β-cells and by the poor response on the target organs.9,10) As the etiology of type 2 diabetes is linked to genetic and lifestyle factors,11,12) the onset is preventable through the improvements of dietary habits and lifestyle.13) However, deteriorations in dietary habits and lifestyle are involved with oxidative stress14) and thereby connected with the onset of diabetes.15) Increased generation of reactive oxygen species (ROS) promotes a functional disorder in pancreatic β-cells.16) Therefore, development of strategies to protect β-cells and insulin target organs from oxidative stress are useful for effective prevention of type 2 diabetes. Here, we demonstrated that the ICR mouse strain mildly induced by nicotinamide (NA) and streptozotocin (STZ) was an appropriate model of diabetes.17)
There are conflicting reports on the relevance between supplementary Se or the physiological status and the prevalence of type 2 diabetes. Previous studies demonstrate that Se status of diabetic patients are physiologically insufficient compared with healthy individuals.18–20) This attracts attention that supplementation with Se may be beneficial for preventing type 2 diabetes. On the contrary, several epidemiological studies demonstrate that the relatively high intake with dietary Se augments the incidence of type 2 diabetes.21–23) Therefore, although Se deficiency may exacerbate a pathological condition associated with diabetes by downregulating the expression of Se-dependent GPX or TR family members, it is disputable whether supplementary Se is profitable for prevention of type 2 diabetes. In a previous study, the effects of supplementary selenocompounds–sodium selenite, methylseleninic acid, and seleno-L-methionine (SeMet) on glucose tolerance were compared in an short-term NA/STZ-induced mild diabetic mouse model, and SeMet increased pancreatic GPX1 activity the most.24) The objective of this study was to clarify effects of supplementary SeMet on 8-hydroxy-2′-deoxyguanosine (8-OHdG) and insulin densities as a marker for oxidative stress and insulin storage index in pancreatic islets as well as blood glucose and insulin, using a diabetic mouse model with controlled dietary Se sources under insufficient Se level.
Permission for animal experiments was given from the Animal Experiment Room Administration Committee of Setsunan University, and experiments were performed according to the Animal Experiment Guidelines of the Faculty of Pharmaceutical Sciences, Setsunan University. Pathogen-free male 5 week-old ICR mice (about 25 g) were purchased from Japan SLC Inc. (Shizuoka, Japan). The animals were maintained at 23±1°C, approximately 40% relative humidity, a 12 h cycle of light and dark, and had ad libitum access to γ-ray-irradiated pelleted rodent chow (Type NMF, Oriental Yeast Co., Tokyo, Japan) and tap water. Mice were acclimated for 1 week prior to experiment.
NA/STZ treatment and SeMet supplementation were conducted as described previously.24) Briefly, ICR mice (10 mice/group) were fasted for 16 h and then intraperitoneally treated twice with 100 mg/kg STZ 15 min after an injection of 120 mg/kg NA at a 1-d interval. The next day, mice were orally administered 158 µg Se (2 µmol)/kg/d SeMet for 5 weeks with ad libitum access to sterilized water and a γ-ray-irradiated torula yeast-based diet (32.3±2.92 ng Se/g) as a Se-deficient diet.
Assays of Biochemical Parameters, Immunohistochemical Staining, and Selenoprotein mRNAsNon-fasting blood glucose, glycated hemoglobin (HbA1c), plasma insulin, free fatty acid (FFA), and adiponectin were measured with blood drawn from the tail vein at 10:00–12:00 after 5 weeks of NA/STZ treatment. Thereafter, the oral glucose tolerance test (OGTT) was performed by orally administering 2000 mg/kg glucose to overnight-fasted mice. The levels of glucose, HbA1c, and FFA were determined with Glucose Pilot (Aventir Biotech, LLC, West Carlsbad, CA, U.S.A.), DCA2000 (Siemens Healthcare K.K., Tokyo, Japan), and NEFA C-Test Wako (Wako Pure Chemical Industries, Ltd., Osaka, Japan), respectively. Plasma insulin and adiponectin levels were measured with enzyme-linked immunosorbent assay (ELISA) kits (Shibayagi Co., Ltd., Gunma, Japan; R&D Systems, Inc., Minneapolis, MN, U.S.A.). After OGTT, the liver was perfused with ice-cold saline through the portal vein under pentobarbital anesthesia, and then the liver and pancreas were removed. The pancreas was fixed in Bousin’s solution (picric acid : formalin : acetic acid at 15 : 5 : 1 v/v) overnight. The pancreatic specimen was embedded in paraffin and sectioned at 3 µm. The sections were placed on MAS-coated slide glass, dried overnight, deparaffinized with xylene and ethanol, and stained with hematoxylins and eosin (H&E) for morphological evaluation by light microscopy.
For analysis of 8-OHdG or insulin density in pancreatic islets, the avidin–biotin complex method with alkaline phosphatase25) was used with a slight modification. Briefly, the sections were washed with phosphate buffered saline (PBS) and sequentially treated with M.O.M™ Mouse immunoglobulin (Ig) Blocking Reagent (Vector Laboratories, CA, U.S.A.), anti-8-OHdG monoclonal antibody (1 mg/mL, clone N45.1; Japan Institute for the Control of Aging, Nikken SEIL Co., Ltd., Shizuoka, Japan), and M.O.M™ Biotinylated Anti-Mouse IgG antibody (Vector Laboratories). VECTSTAIN ABC-AP Kit and Vector Black Alkaline Phosphatase Substrate Kit (Vector Laboratories) were used for alkaline phosphatase detection. Immunostaining of insulin in pancreatic islets was performed with anti-insulin monoclonal antibody, Histofine® MOUSESTAIN, and 3,3′-diaminobenzidine substrate kits (Nichirei Biosciences Inc., Tokyo, Japan). Three pancreatic islets randomly selected in each mouse were used for evaluation. Densitometry was performed using NIH image J and used for quantitation of the immunohistochemical data on 8-OHdG or insulin with 5 appropriate locations prepared for each specimen, and the positive area was determined after setting a proper threshold. The following calculation was used: 8-OHdG or insulin density in pancreatic islets (%)=(8-OHdG or insulin-positive area in a pancreatic islet/total area in pancreatic islet)×100.
RNA isolation was performed with the acid guanidinium thiocyanate–phenol–chloroform method.26) The concentration and purity of extracted RNA were determined with A260/A280 measured by a NanoDrop 1000 spectrometer (Thermo Fisher Scientific, MA, U.S.A.). Synthesis of cDNA was conducted using PrimeScript RT reagent Kit (Perfect Real Time), and SmartCycler® II System (TaKaRa Bio Inc., Shiga, Japan) was used for real-time quantitative PCR analysis. The primer numbers used in PCR (TaKaRa Bio Inc., Shiga, Japan) were as follows: GPX1 (MA025680); GPX4 (MA055143); TR1 (MA050412); β-actin (MA050368). Cycling conditions were used as suggested in the SYBR Green Kit instructions. The content of GSH was determined by the HPLC method using 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate as a fluorescent reagent.27) The standard curve was linear over the range of 2 to 1000 nmol/mL.
Statistical AnalysisResults were statistically analyzed with a one-way ANOVA followed by a Bonferroni’s multiple comparison test using LightStone Origin Pro (Tokyo, Japan). Probability values <0.05 were considered significant. The data represent as the mean±standard deviation (S.D.)
To investigate the effects of administering SeMet on glucose tolerance and insulin sensitivity-related parameters in NA/STZ-induced diabetic mice under insufficient Se status, non-fasting blood glucose, HbA1c, and plasma insulin levels 5 weeks after NA/STZ treatment as well as blood glucose and plasma insulin levels during OGTT after the 5 weeks were monitored. The control and NA/STZ treatment groups exhibit insufficient Se status. As shown in Figs. 1 and 2, there was no significant effect of supplementary SeMet on non-fasting glucose, HbA1c, and plasma insulin levels in NA/STZ-untreated mice, as well as the glucose level during OGTT. However, SeMet significantly suppressed the non-fasting blood glucose and HbA1c levels that were increased by NA/STZ treatment (Fig. 1). Although the blood glucose level during OGTT increased in NA/STZ-treated mice, administration of SeMet significantly lowered glucose levels 90–120 min after glucose loading (Fig. 2A). The plasma insulin level was partly restored by SeMet in NA/STZ-treated mice, whereas SeMet suppressed the level in NA/STZ-untreated mice (Fig. 2B). The increase in plasma FFA level, and decrease in plasma adiponectin level and hepatic glycogen content were observed in NA/STZ-treated mice (Fig. 3). Mice with SeMet supplementation partially recovered from the suppression of insulin sensitivity.
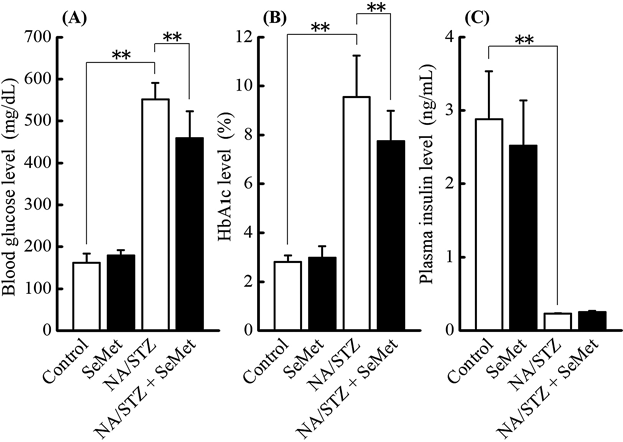
The values shown are the mean±S.D. (n=10). Significantly different from the control or NA/STZ treatment group at ** p<0.01.
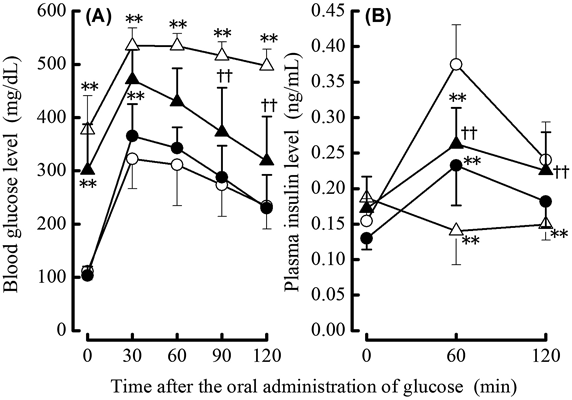
Control (○), SeMet supplementation (●), NA/STZ treatment (△), and NA/STZ treatment+SeMet supplementation (▲). The values shown are the mean±S.D. (n=10) and the bars represent as fine lines for ○, △ and bold lines for ●, ▲. Significantly different from the control group at ** p<0.01 and from the NA/STZ treatment group at †† p<0.01.
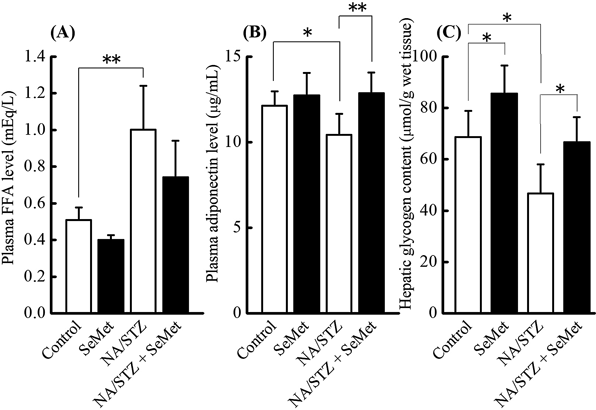
The values shown are the mean±S.D. (n=10). Significantly different from the control or NA/STZ treatment group at * p<0.05; ** p<0.01.
The effects of supplementary SeMet on oxidative stress and insulin production in pancreatic islets of NA/STZ-induced diabetic mice under insufficient Se status were investigated. H&E staining (data not shown) and immunostaining (Figs. 4A, 5A) showed that NA-STZ treatment induced atrophy in pancreatic islets. The 8-OHdG density in pancreatic islets was significantly increased by NA/STZ treatment and significantly decreased by SeMet supplementation (Fig. 4B). However, decreased insulin density from NA/STZ treatment was not restored by SeMet ingestion (Fig. 5B).
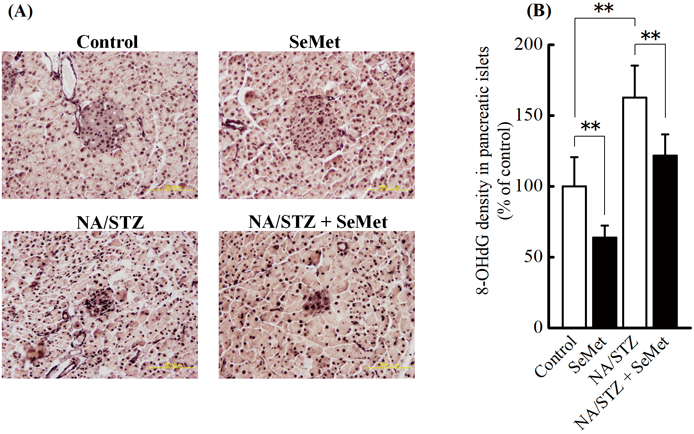
The values shown are the mean±S.D. (n=5). Significantly different from the control group at ** p<0.01.
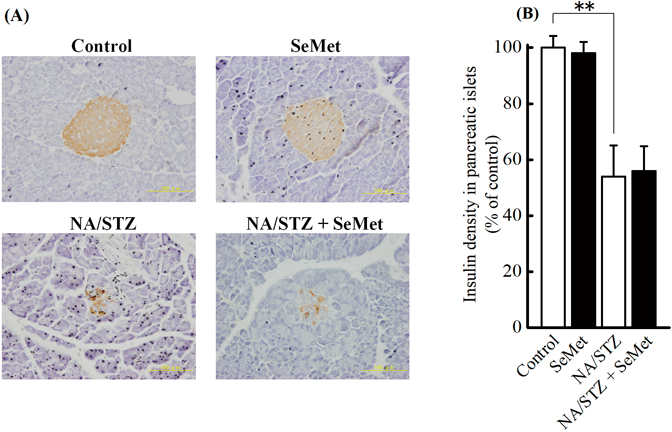
The values shown are the mean±S.D. (n=5). Significantly different from the control group at ** p<0.01.
The effect of SeMet on GSH content and mRNA expression of selenoenzymes, which are oxidative stress elimination-related indices in the liver and pancreas, was investigated. Although hepatic GSH content was much higher than pancreatic GSH content, there was no significant difference among control, SeMet, and NA/STZ treatment groups (Fig. 6). Hepatic and pancreatic GPX1 mRNA levels significantly increased by NA/STZ and/or SeMet (Fig. 7). Although hepatic GPX4 and TR1 mRNA levels were significantly elevated by NA/STZ treatment, there was no significantly consistent change in the mRNA levels by SeMet supplementation.
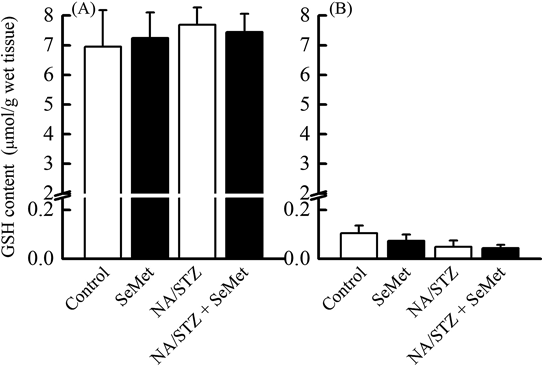
The values shown are the mean±S.D. (n=5).
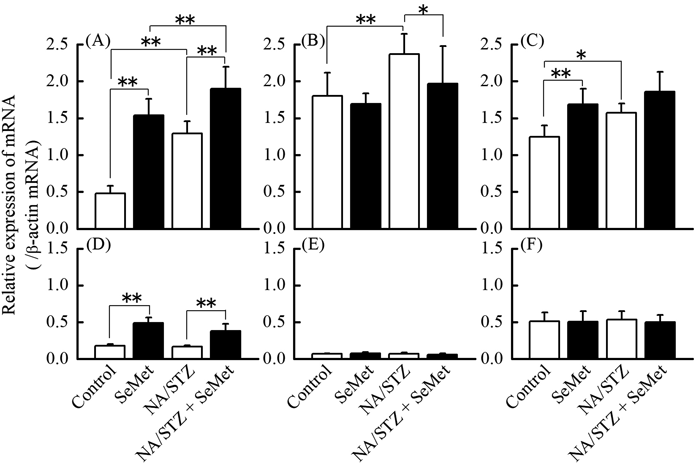
The values shown are the mean±S.D. (n=5). Significantly different from the control or NA/STZ treatment group at * p<0.05; ** p<0.01.
Type 2 diabetes that is characterized by postprandial hyperglycemia is deteriorated by insulin resistance, which causes insulin deficiency from pancreatic β-cell dysfunction in response to oxidative stress and inflammation of the endoplasmic reticulum.28) STZ is an nitric oxide donor, DNA-methylating agent, and is frequently used to induce an experimental animal model of diabetes.29) This agent produces free radicals in oxidative stress-fragile β-cells and causes the fragmentation of DNA chains, which leads to a large amount of intracellular nicotinamide adenine dinucleotide (NAD) consumption to repair damaged DNA by poly(ADP-ribose)-polymerase activation.29) The intake of NA effectively supplements NAD and protects β-cells.30) The ICR mouse strain with experimental diabetes induced by NA/STZ-treatment is more sensitive to hyperglycemia under diabetic conditions than the C57BL/6J strain.17) Using the NA/STZ-induced diabetic mouse model, the objective was to elucidate the protective effects of supplementary SeMet on oxidative stress in pancreatic islets under insufficient and physiological Se levels because this selenocompound improves pancreatic GPX1 activity the most in mice with insufficient dietary Se status.24) SeMet ameliorated HbA1c, non-fasting and OGTT blood glucose, plasma FFA and adiponectin, and hepatic glycogen deteriorated by NA/STZ treatment as shown in Figs. 1–3. This indicates that supplementary SeMet has a hypoglycemic effect under insufficient Se states. However, non-fasting plasma insulin levels were not improved by SeMet treatment (Fig. 1C). The plasma insulin level during OGTT was inconsistent because the level in the NA/STZ-untreated group was decreased by SeMet ingestion. This suggests that SeMet may have insulin-mimetic activity as reported previously about supplementation with other selenocompounds.31,32)
Immunochemical staining of 8-OHdG was used as a marker of oxidative stress in pancreatic islets because increased positive staining correlates with reduced β-cell volume density.33) We previously reported that SeMet exhibited the highest bioavailability of the 3 selenocompounds supplemented under insufficient Se status, particularly in the pancreatic Se content and GPX1 activity.24) However, as shown in Figs. 4 and 5, insulin density was not restored by SeMet ingestion, whereas 8-OHdG density in pancreatic islets significantly decreased with supplementation. This suggests that partial suppression of oxidative stress in pancreatic islets by SeMet does not restore insulin storage. In the patients with impaired glucose tolerance, the insulinogenic index, which indicates pancreatic β-cell function, more closely correlates with changes in serum thioredoxin levels than 8-OHdG concentration.34) As shown in Figs. 6 and 7, low GSH content and poor mRNA expression of antioxidant selenoenzymes in the pancreas may be responsible for elevated vulnerability to oxidative stress. GPX1 mRNA levels increased by NA/STZ and/or SeMet in both the liver and pancreas. The induction of GPX1 protein expression may suppress oxidative stress. However, in this study, SeMet may not restore GPX1 expressions in these organs to the normal levels because it is hard to recover Se status in the deficient diet-fed mice to that in the normal diet-fed mice by Se ingestion.35) A reversible oxidation and reduction of protein tyrosine phosphatases by ROS production and expressions of oxidative stress-elimination enzymes in insulin target tissues affect the insulin signaling.36) This may contribute to the hypoglycemic effect of supplementary SeMet under insufficient Se status. On the other hand, GPX1-deficient mice exhibit defective insulin secretion, but are protected from hepatic steatosis.37) Increased H2O2 in GPX1-deficient mice enhances hepatic insulin signaling and improves glucose metabolism.38) Taken together, hepatic or pancreatic GPX1 expression enhanced by sufficient or relatively high Se status may be related to the onset of insulin resistance by the suppression of ROS production.
In conclusion, supplementary SeMet improves markers for insulin function and the resistance, such as glucose tolerance, plasma adiponectin, and hepatic glycogen content by exhibiting insulin-mimetic activity in an NA/STZ-induced diabetic mouse model with insufficient Se status. However, the suppression of pancreatic oxidative stress by the induction GPX1 from SeMet supplementation is unlikely to restore insulin storage and secretion. Further studies are needed to clarify the function of selenoproteins, including GPX1, for the onset of hyperglycemia and insulin resistance.
This work was supported in part by Grant-in-Aid for Scientific Research (KAKENHI 20590128) from the Japan Society for the Promotion of Science.
The authors declare no conflict of interest.