2018 Volume 41 Issue 12 Pages 1748-1756
2018 Volume 41 Issue 12 Pages 1748-1756
Alternative medicines attract attention because stroke is rarely expected to make a full recovery with the most advanced medical technology. Angelica gigas (AG) is a well-known herbal medicine as a neuroprotective agent. The present study introduced mesenchymal stem cells (MSCs) to identify for the advanced treatment of the cerebrovascular disease. The objective of this research is validation of the enhanced effects of multiple combined treatment of AG extract with MSCs on stroke through angiogenesis. Our results confirmed that AG extract with MSCs improved the neovascularization increasing expression of angiogenesis-regulated molecules. The changes of brain and the behavioral ability showed the increased effects of AG extract with MSCs. As a result, AG extract and MSCs may synergistically increase the therapeutic potential by enhancing neovascularization. This mixed approach provides a new experimental protocol of herbal medicine therapy for the treatment of a variety of diseases including stroke, trauma, and spinal cord injury.
Worldwide, 6.2 million people have suffered from stroke, which was the second leading cause of death following heart disease in 2015.1) Despite rapid medical advances, there are still few therapeutic options available for the disease.2) Several studies have proposed that traditional Chinese medicines (TCMs) have beneficial effects on infarct brain by stroke.3,4) Unlike Western medicines focusing on diseases, TCMs focus on correcting imbalances in the body through herbs and acupuncture.5,6) Some herbal extracts may promote microcirculation in the brain,6–8) protect against ischemic reperfusion injury,6,8) present neuroprotective effects,6,7) and prevent apoptosis.8)
Angelica gigas (AG) is a typical herbal medicine. The dried root of AG, which is commonly known as Danggui in TCMs, shows curative properties in various circulatory disorders.9–11) In previous studies, AG extracts were confirmed to have anti-inflammatory, antioxidant, and neuroprotective effects.11–13) The therapeutic effects of AG extract have been established over a longer period of time. Specifically, the efficacy of AG extract has been studied in the brain following ischemic stroke. A previous study showed that AG extract decreased neuronal death, increased angiogenic factor expression and activated cell survival signaling pathways such as the phosphatidylinositol 3-kinase (PI3K)/Akt pathway.14) Although AG extract is known to have a neuroprotective effect on ischemic stroke in the brain, we would like to identify an enhanced treatment option for treatment of ischemic stroke.
Recently, the field of regenerative medicine using stem cells has emerged as an alternative therapy against various diseases. Transplantation of stem cells in stroke patients has become an attractive research theme for promising therapy.15) Overall, mesenchymal stem cells (MSCs) are a promising therapeutic option for stroke. Application of MSCs via intravenous injection has been shown to be a safe and effective manner in animal stroke models16–20) and clinical cases.21,22) Despite the limited and low migration of MSCs to the injured area and their poor survival rate,20,23) injection of MSCs can lead to recovery of brain function by paracrine mechanisms for treatment of ischemic stroke. MSCs exert strong immunomodulatory effects by attenuating cytokine and chemokine activation and promoting the production of growth factors, which help the brain function recovery of injured tissue.24) Furthermore, there are few side effects such as immune system rejection associated with MSCs. However, poor viability of the transplanted MSCs is a major challenge of stem cell-based therapy. In the injured region, impoverished blood supply, low oxygen pressure, and inflammation may generate oxidative stress, as well as cytotoxic radicals and proteins. Such a pro-apoptotic microenvironment may cause the death of transplanted cells through several different mechanisms, including apoptosis.25) Following transplantation, the survival rate of transplanted cells was very low in experimental models.26,27)
On this basis, we estimated that multiple combined treatment with AG extract and MSCs could provide a synergistic and curative effect. In the present study, the tube formation of human umbilical vein endothelial cells (HUVECs) and the release of angiogenic factors in the brain tissue of the middle cerebral artery occlusion (MCAo) rat model were confirmed in the group co-treated with AG extract and MSCs together in comparison with the groups treated with AG extract or MSCs alone. Behavioral changes were also investigated with two other tests. We found that administration of AG extract and MSCs together could enhance the therapeutic effects of MSCs, including neuroprotection and angiogenesis. Herein, a new and synergistic approach for application of herbal medicine through treatment with stem cells to alleviate the patient’s pain associated with stroke is proposed.
The dried root of AG is commonly known as Danggui in TCMs. Roots of AG were purchased from a medicinal materials company (Onggihanyakguk, Daegu, Republic of Korea). We only used roots that passed the sensory test based on the National Standard of Traditional Medicinal (Herbal and Botanical) Materials. AG extract was prepared by the following procedure. Roots (500 g) were boiled in X10 distilled water for 2 h, passed through a filter paper, and concentrated under vacuum. The final yield of the concentrated extract was 11.1% of the dried powder. AG extract was stored at 4°C and dissolved in saline prior to use.14)
Cell CultureMSCs were purified as previously described.28) Bone marrow from rat femurs and tibias was flushed with phosphate-buffered saline (PBS; HyClone, Logan, UT, U.S.A.) containing 10% fetal bovine serum (FBS; HyClone). Mononuclear cells were isolated by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) for 30 min at 16000 rpm, then suspended in fresh Dulbecco’s modified Eagle’s media (DMEM; HyClone) containing 10% FBS. Cells were then plated at a density of 7×105 cells/plate and incubated in a humidified atmosphere of 5% CO2 at 37°C. HUVECs were purchased from ATCC (Manassas, VA, U.S.A.) and maintained in culture with Endothelial Basal Medium-2 (Lonza, Walkersville, MD, U.S.A.) supplemented with growth factors (Lonza) containing 2% FBS, as detailed by the manufacturer.
Tube Formation AssayA total of 150 µL of growth factor reduced Matrigel (Corning Inc., Corning, NY, U.S.A.) was added to a 24-well plate and polymerized for 30 min at 37°C. After seeding the HUVECs (1×105 cells) on the Matrigel-precoated wells, AG extract was added for each concentration to confirm dose-dependent effect. Next, to compare the extent of tube formation, AG extract, MSCs (2×104 cells), and AG extract with MSCs were added to the upper chamber on the Matrigel-precoated wells with HUVECs. The number of tubules and branches was checked every 3 h. The tubules and branches were counted using a microscope, and each experiment was repeated three times.
Western BlottingBrain tissues were frozen in liquid nitrogen and lysed in buffer (Cell Signaling Technology, Beverly, MA, U.S.A.) containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM Na2-ethylenediaminetetraacetic acid (EDTA; Gibco, Grand Island, NY, U.S.A.), 1 mM ethyleneglycoltetraacetic acid (EGTA; Sigma-Aldrich, St. Louis, MO, U.S.A.), 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride. Protein concentrations were determined using a BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Bartlesville, OK, U.S.A.). Quantified proteins were separated in a 6–10% sodium dodecyl sulfate-polyacrylamide gel, and transferred to Immobilon®-P Transfer Membrane (Millipore, Temecula, CA, U.S.A.). After membrane blocking with Tris-buffered saline-Tween 20 (TBS-T, 0.1% Tween 20) containing 5% nonfat dried milk for 1 h at room temperature, the membranes were washed three times with TBS and incubated with primary antibody (concentration-dilution; 1/1000, Ang-1, Tie-2, vascular endothelial growth factor (VEGF)-A, ZO-1, Occludin, and PI3K; Santa Cruz Biotechnology, Dallas, TX, U.S.A.; p-Akt and Akt; Cell Signaling Technology) for 6 h at room temperature or overnight at 4°C. The membranes were washed three times with TBS-T for 5 min, and then incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (Thermo Fisher Scientific Inc.). After washing, the bands were detected by ECL reagent (Advansta Inc., Menlo Park, CA, U.S.A.). The band intensities were quantified using the ImageJ software version 1.40 g (NIH, http://www.nih.gov). Each experiment was repeated three times.
AnimalsMale Sprague Dawley (SD) rats weighing an average of 290±10 g (KOATECH, Gyeonggi-do, Republic of Korea) were used in the experiments. The animals were housed at an ambient temperature of 25±1°C, relative humidity of 50±10% and 12 h light/dark cycle with free access to food and water. All animals were handled according to the animal welfare guidelines issued by the Korean National Institute of Health and the Korean Academy of Medical Sciences for the care and use of laboratory animals.14)
Preparation of Ischemic Stroke Rat ModelThe ischemic stroke rat model was prepared by MCAo and reperfusion following a standard procedure.29) Briefly, rats were anesthetized with isoflurane (4% initial, 2% maintenance) using isoflurane in a mixture of 30% oxygen and 70% nitrous oxide. Under the operating microscope, the left common carotid artery (CCA) was exposed and the external carotid artery (ECA) and occipital artery were ligated together. A 3-0 nylon suture was inserted into the internal carotid artery (ICA) through the ECA and the intraluminal suture was secured with suture tied around the ECA. After 90 min of occlusion, the intraluminal suture was withdrawn for reperfusion. After withdrawal of the suture, the therapeutic agents were injected into groups II–V through the ECA. In the sham group, the ECA was surgically prepared for insertion of the suture, but it was not inserted.14)
All animals were randomly divided into five groups (n=16 per a group): group I, sham-operation (Sham); group II, MCAo/reperfusion-induced ischemic group with saline treatment (Control); group III, AG extract-treated group at a dose of 50 mg/kg (AGE); group IV, 1×106 MSC-treated group (MSCs); and group V, AG extract at a dose of 50 mg/kg with 1×106 MSC-treated group (AGE+MSCs). Before MCAo, group III and V were administered AG extract (200 mg/kg) orally once daily. All animals were euthanized after 7 d of stroke. The brain tissues were harvested and measured for related protein level (n=4 per group), the brain infarction volume (n=4 per group), water content (n=4 per group) and immunohistochemistry (n=4 per group).14)
Behavior TestWe conducted the forced swim test and cylinder test for the study to determine the exercise capacity of animals.30) In the forced swim test, rats were placed in an open cylinder (height, 60 cm; diameter, 20 cm) for 6 min. Water (depth, 30 cm; temperature, 25°C) was included into the cylinder. An immobile time was measured for the last 4 min. In the cylinder test, rats were placed in a transparent cylinder (height, 30 cm; diameter, 20 cm) for 6 min. We recorded performance of the left paralyzed forelimb for the last 4 min. The number of independent wall placements for the right and left forelimbs, as well as the placement of both forelimbs simultaneously, was recorded. Each experiment was repeated five times.
Measurement of Infarct VolumeAll animals were euthanized by decapitation after 7 d of stroke. The brain tissues were harvested and cut into 2 mm coronal slices starting 2 mm from the frontal pole. Each slice was stained with 2,3,5-triphenyltetrazolium chloride (TTC) for measurement of the infarction volume. The infarction volume was calculated as the infarct volume (mm3) per brain using the ImageJ software version 1.40. Infarction volumes were expressed as a percentage of the contralateral hemisphere volume using the formula.14) This experiment was repeated four times.
Measurement of the Water Content in BrainAfter 7 d of stroke, all animals were sacrificed and brains were collected. The pons and olfactory bulbs were removed and wet weight (ww) of the brain was measured. All brains were dried at 110°C for 24 h, and dry weight (dw) of the brain was measured. Whole water content in brains was calculated using the formula.31) This experiment was repeated four times.
ImmunohistochemistryAnimals were sacrificed after 7 d of stroke, and their brains were excised. The brains were perfusion-fixed with 10% (v/v) neutral buffered formaldehyde for 24 h, transversely sectioned into two comparably thick sections, and embedded in paraffin. Sections of 2 µm thickness were mounted on gelatin-coated glass slides to ensure different stains could be used on successive sections of tissue cut through the infarcted area. The sections were stained with mouse anti-CD31 (Abcam, Cambridge, MA, U.S.A.) and then Texas red-conjugated goat anti-mouse immunoglobulin G (IgG) (Abcam). Nuclei visualization was explored by 4′,6-diamidino-2-phenylindole (DAPI) counterstaining (blue). All images were produced using an excitation filter under reflected light fluorescence microscopy and a computer equipped with MetaMorph software version 4.6 (Universal Imaging Corp., http://www.universal-imaging.com).
Statistical AnalysisAll data are expressed as the means±standard error of the mean (S.E.M.). Comparisons between more than two groups were performed by one-way ANOVA, using Bonferroni’s correction. A p-value <0.05 was considered significant.
To investigate the actual effects of AG extract on HUVECs, a tube formation assay was performed. The development of tubular and branchlike structure was checked to determine if it was concentration- and time-dependent. Increasing concentrations of the treated extract promoted formation of branches and tubes up to 0.1 µg/mL of AG extract in PBS (Figs. 1B–E). After 9 h, the number of tubes and branches in 0.1 µg/mL of AG extract increased 3.4- and 2.2-fold relative to those of cell water treatment, respectively (Figs. 1B, D). In addition, tubular and branchlike constructs were cumulated from 3 h to 9 h under the same concentration. The number of tubes and branches in the group treated with 0.1 µg/mL of AG extract for 9 h was 3.0- and 1.6-fold higher than those in the group treated for 3 h, respectively (Figs. 1C, E). These results suggest that treatment with AG extract can affect enhanced development of tubular and branchlike structures of HUVECs in a concentration- and time-dependent manner in vitro.
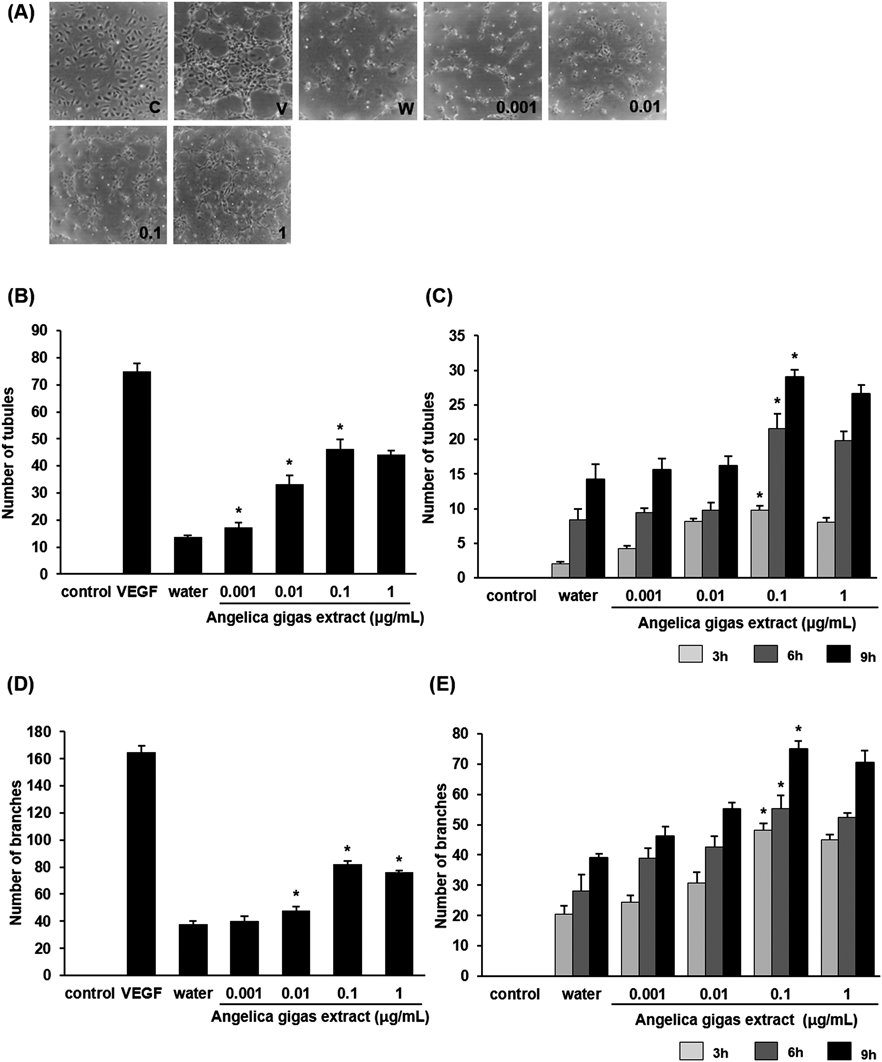
(A) Representative tube formation of HUVECs cultured in AG extract at different doses for 24 h: 0.001, 0.01, 0.1, 1, 10, 100 µg/mL. (B, D) Statistical analysis demonstrated that HUVECs cultured in AG extract induced tube formation in a dose-dependent manner. The most effective dose of AG extract is 0.1 µg/mL in formation of tubes and branches. (C, E) Formation of tubes and branches of HUVECs cultured in AG extract was enhanced in a time-dependent manner. C, control group; V, VEGF treatment group; W, cell water treatment group. Columns, mean; bars, standard error (S.E.) (* p<0.05).
After verification of the influence of AG extract for neovascularization, we compared the efficacy of treatment with AG extract only, MSCs only, and AG extract and MSCs together. Combined treatment with AG extract and MSCs showed the greatest potential for angiogenesis.32) Nam et al. demonstrated that transplantation of MSCs might assist in promoting angiogenesis and leading to neurological recovery.32) We assumed that MSCs are able to enhance angiogenesis by co-treatment with AG extract and increase the angiogenic capacity. The results indicated that combined treatment with AG extract and MSCs had better effects on neovascularization than treatment with AG extract or MSCs alone. Nine hours later, the number of tubes in the group co-treated with AG extract and MSCs was 4.1- and 1.8-fold higher than in the group treated with AG extract and MSCs alone, respectively (Figs. 2B, C). The number of branches showed a patter similar to that of tube formation, increasing 4.0- and 1.5-fold in the mixed treatment group. Taken together, these findings indicate that co-treatment of AG extract with MSCs could increase the capacity for new blood vessel formation following ischemic brain stroke considerably.
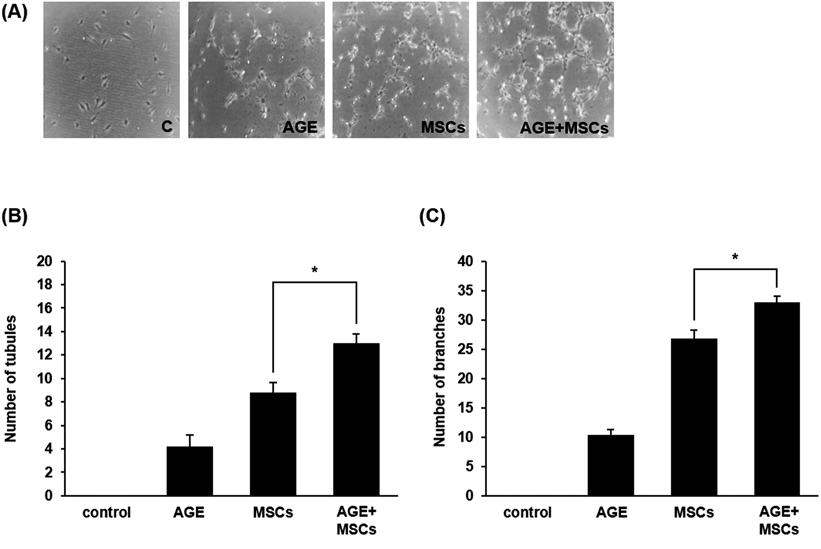
(A) Representative tube formation of HUVECs cultured in different conditions. (B, C) Statistical analysis demonstrated that multiple combined treatment of AG extract with MSCs showed increase of tube and branch formation compared to those treated with AG extract and MSCs alone. AGE, AG extract-treated group. Columns, mean; bars, S.E. (* p<0.05).
To identify the mechanism by which angiogenesis was promoted in response to treatment with multiple combined treatments of AG extract with MSCs, we examined the related protein expression level of the angiogenic factors using Western blot, including Ang-1, Tie-2, VEGF-A, ZO-1, Occludin, PI3K, and p-Akt/Akt. As shown in Fig. 3A, essential angiogenic factors such as Ang-1, Tie-2 and VEGF-A were expressed at levels 1.2-, 1.7- and 1.3-fold higher in response to co-treatment with AG extract and MSCs than in response to treatment with MSCs alone. In addition, the expression of the tight junction molecules ZO-1 and Occludin increased in the group treated with AG extract and MSCs together relative to that of the group treated with MSCs alone. The expression level of both molecules was 1.3- and 2.1-fold higher in the group treated with AG extract and MSCs together (Fig. 3B). The manner of multiple combined treatment also significantly enhanced phosphorylation of Akt and PIK3 expression, improving cell growth and proliferation, survival, growth factor production, and neovascularization by 1.4- and 2.2-fold relative to samples treated with MSCs alone (Fig. 3C).
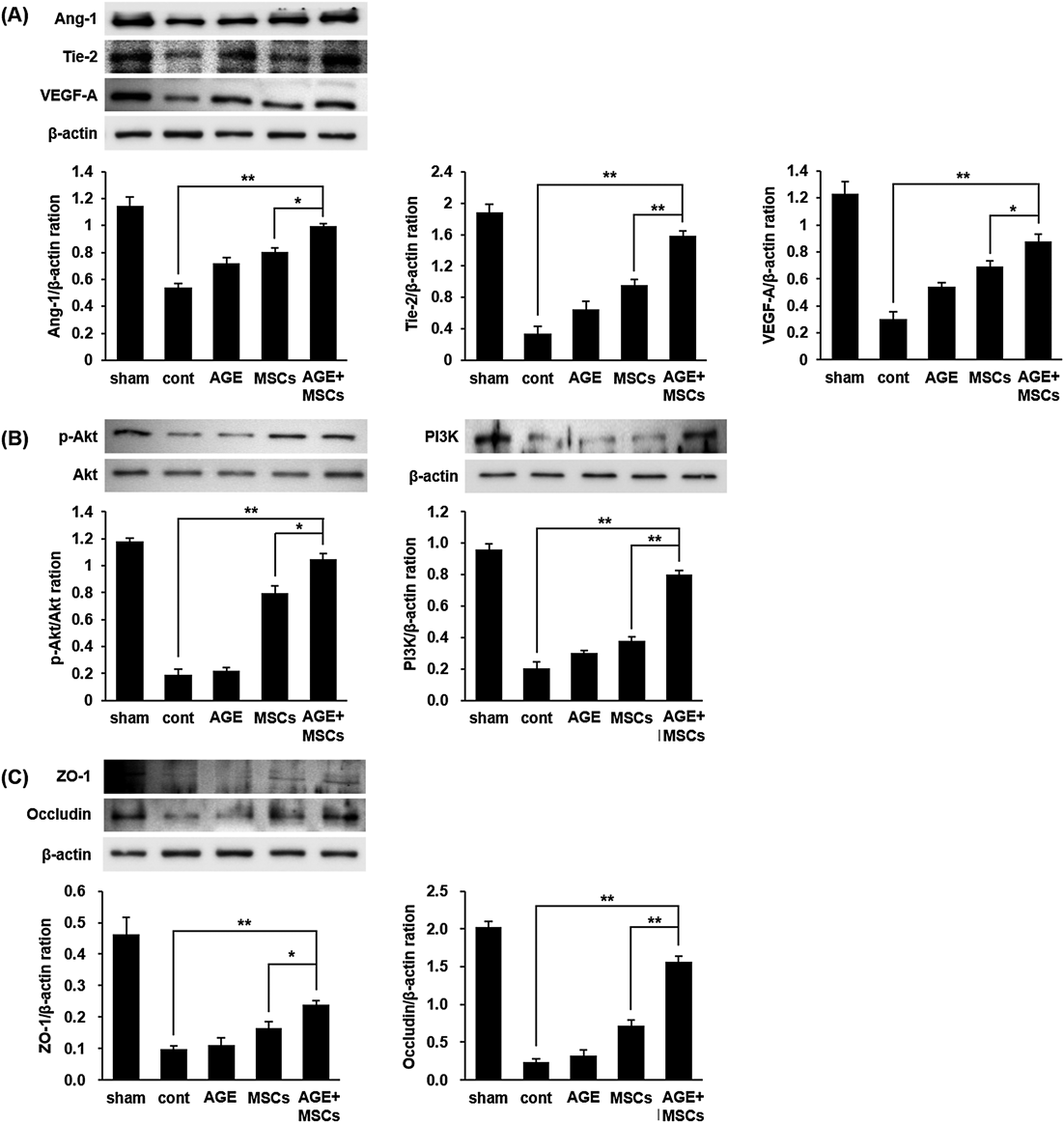
(A, B) Following MCAo/reperfusion, relative folds of Ang-1, Tie-2 and VEGF-A, and PI3K and p-Akt were calculated by normalization to β-actin and Akt, respectively. (C) The expression of ZO-1 and Occludin was also detected in the MCAo-induced rat ischemic brain tissues. The expression of all angiogenesis-regulated molecules was increased in multiple combined treatment of AG extract with MSCs compared to treatment of AG extract and MSCs alone. AGE, AG extract-treated group. Data in the histogram are expressed as means±S.E.M. of three independent experiments. n=4 per group. (** p<0.01 and * p<0.05).
To confirm the neuroprotective effects of AG extract and MSCs on stroke, we measured the infarction volumes in MCAo rat models by TTC staining. When compared to the control group (34.0%), AG extract at 50 mg/kg, 1×106 MSCs, and AG extract at doses of 50 mg/kg combined with 1×106 MSCs decreased the infarction volumes by 22.8, 16.9, and 13.2%, respectively (Figs. 4A, B). Additionally, 1×106 MSCs and AG extract at doses of 50 mg/kg combined with 1×106 MSCs significantly decreased the infarction volume (p<0.01 and p<0.001, respectively) compared with that of the control group. We next measured the water content in the brains of MCAo rats by the wet-dry method. The brain water content significantly increased (p<0.01) in the control group by 81.3% relative to the sham group (Fig. 4C). AG extract at doses of 50 mg/kg, 1×106 MSCs, and AG extract at doses of 50 mg/kg combined with 1×106 MSCs significantly decreased water content by 81.0, 79.7, and 79.5%, respectively, compared with that of the control group.
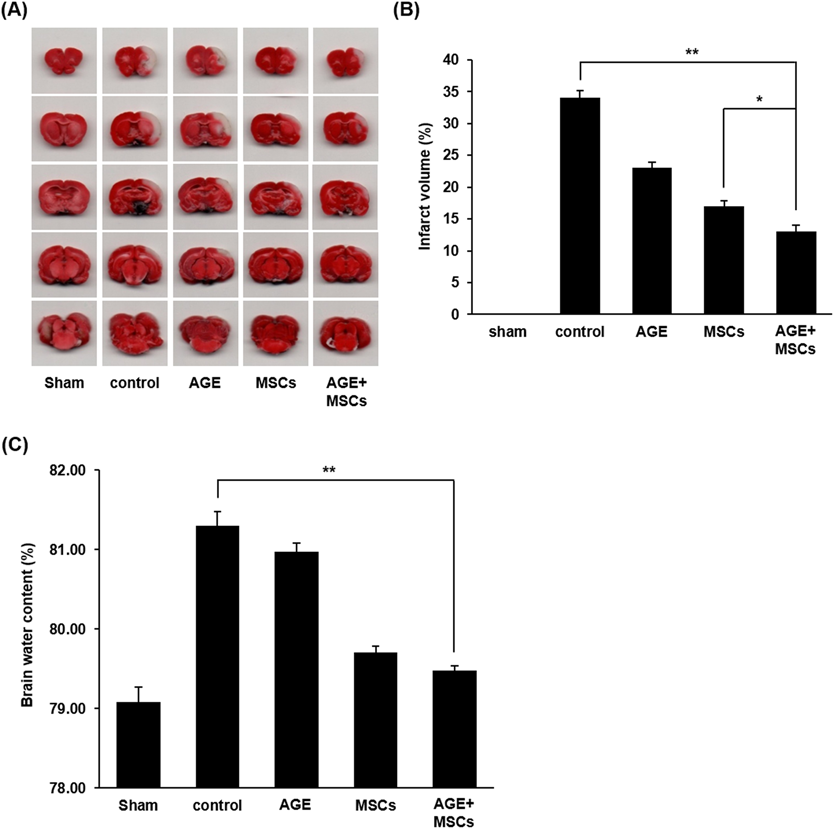
(A) Representative infarct brain tissues stained with TTC in the sham, control, AG extract, MSCs and multiple combined treatment of AG extract with MSCs groups 24 h after MCAo/reperfusion. (B, C) Quantification of infarct size and water content from each group 24 h after MCAo/reperfusion. These results indicated that administration of multiple combined treatment of AG extract with MSCs was the most highly significant effect on reduction of infarct size and mitigation of brain edema after stroke. AGE, AG extract-treated group. n=4 for each group. (** p<0.01 and * p<0.05). (Color figure can be accessed in the online version.)
The cylinder test was used to assess forelimb use and asymmetries in postural weight support during exploratory activity.33) MCAo rats exhibited increased asymmetry, indicating preferential use of the less-affected forelimb during upright support compared with Sham. There was no significant difference between groups that received AGE vs. control. However, our results showed that there was improved performance after MSCs transplantation. Furthermore, the AG extract with MSCs treated group showed much more behavioral recovery than other treatments (Fig. 5A). Stroke produced a decrease in cumulative activity (i.e., composed of swimming, climbing and immobility times).34) In the forced swim test, PBS treated MCAo rats displayed an obvious increase in immobility time during the FST. On the contrary, the immobility time was reduced in response to AG extract, MSCs, and AG extract with MSCs. The AG extract and MSCs co-treatment led to a sufficient improvement in exercise capacity (Fig. 5B).
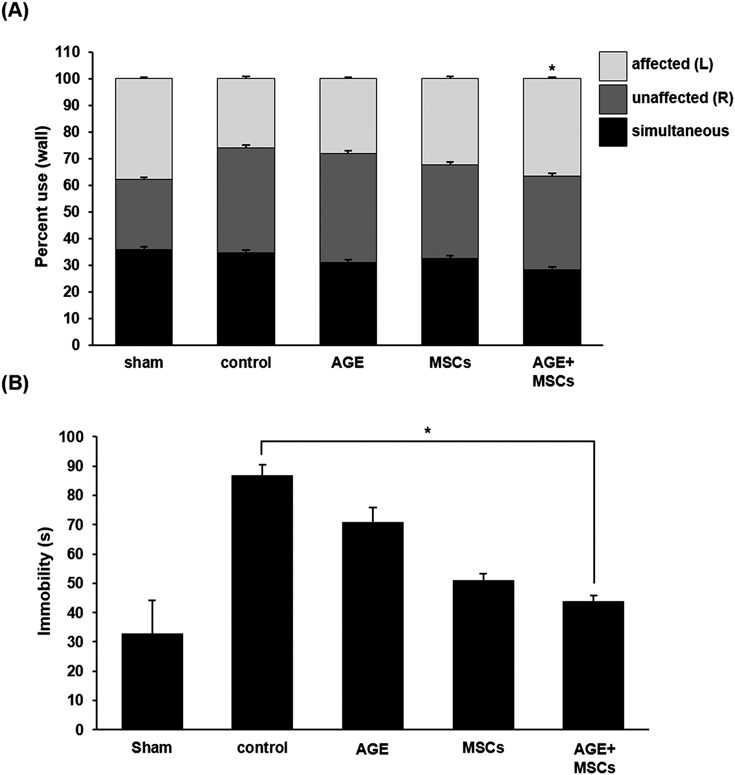
(A) Percent use of affected (left), unaffected (right), and simultaneous (both) use of the forelimbs during exploratory activity on the wall of the cylinder after MCAo. (B) Total amount of immobility time during the last 4 min of the test. AGE, AG extract-treated group. Data are expressed as the mean±S.E.M. n=10–12 for each group. (* p<0.05).
To examine the effects of co-treatment with AG extract and MSCs on angiogenesis after ischemic stroke, brain tissues were labeled with CD31, an endothelial cell marker. The marked cell number in the ischemic boundary zone was significantly higher in the group co-treated with AG extract and MSCs compared with the group treated with MSCs alone and with the PBS-treated group (both p<0.05, Figs. 6A, B). Thus, AG extract combined with MSCs treatment can increase angiogenesis in damaged tissues.
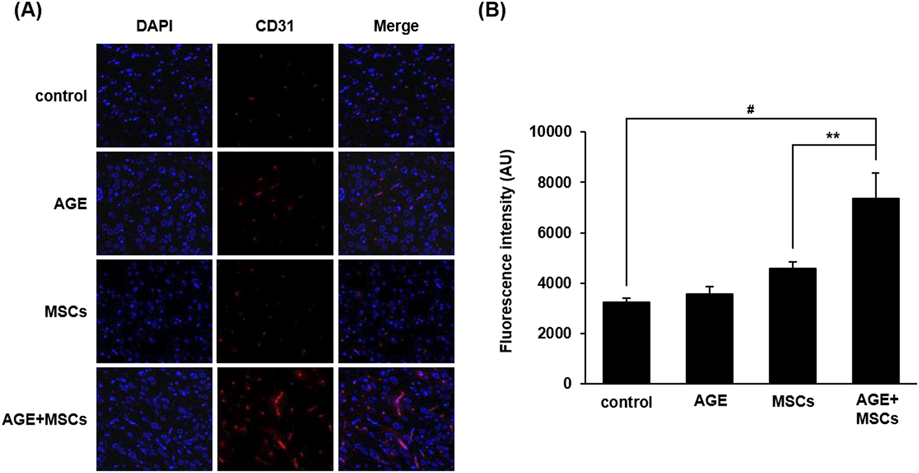
(A) Representative expression of DAPI and CD31 labeled endothelial cells in ischemic boundary zone from each administration. (B) Statistical analysis revealed a significant increase in the number of endothelial cells labeled with CD31 in treated with combined AG extract and MSCs relative to those treated with AG extract or MSCs alone. AGE, AG extract-treated group. Scale bar, 200 µm. Columns, mean; bars, S.E. (# p<0.001 and ** p<0.01). (Color figure can be accessed in the online version.)
Ischemic stroke begins with a sudden interrupting supply of blood flow, oxygen, glucose and energy in the lesion area, followed by ischemic cascades such as glutamate-induced exitotoxicity, calcium influx, inflammation response, blood–brain barrier (BBB) breakdown, edema and cell death.35,36) Despite many researchers’ efforts to control the disease, the current therapeutic strategy has poor clinical outcome.35)
In the present situation, traditional Chinese medicines (TCMs) are a promising source to overcome the limitation. Therapeutic approaches using herbal medicines provide an alternative and promising strategy for stroke.37) Because the alternative remedies can improve the recovery of neurological functions, some are considered to be effective for treatment of stroke patients.35) These medicinal herbs also have unique values for seeking drug candidates.35) In this study, AG was chosen because of its angiogenic effect on transient focal cerebral ischemia in rats.14) We confirmed the components of AG extract such as umbelliferone (163.03931 m/z) and nodakenin (409.14896 m/z), the decursinol angelate/decursin (327.12386 m/z), decursinol/marmesin (245.08208 m/z) using ultra-high-performance liquid chromatography hybrid quadrupole time-of-flight mass spectrometry (UHPLC/Q-TOF-MS) method (Figs. S1 and Table S1). Furthermore, we want to increase the curative effect of AG extract using stem cells. MSCs in regenerative therapy for patients that suffered from stroke were used as the target cell source in numerous preclinical studies.38) Therefore, we investigated the promotion of formation of new blood vessels in response to co-treatment of AG extract with MSCs in MCAo rats.
AG extract treatment increased tube formation in a dose- and time-dependent manner (Fig. 1). The most effective concentration of AG extract was selected and treated in subsequent experiments. Treatment of AG extract with MSCs increased tubular and branchlike form relative to treatment with AG extract or MSCs alone in vitro (Fig. 2). Based on this, we assumed that treated AG extract-induced VEGF-A increased the neovascularization with VEGF-A secreted from MSCs in vitro tube formation (Fig. 2). MSCs are a potent cell source for secretion of VEGF-A, which improves regional blood flow during wound healing and survival of stem cells during stem cell-based therapy in injured tissue.39,40) These findings indicated that treatment of MCAo with AG extract and MSCs was feasible and effective based on a MCAo rat model.
Expressions of Ang-1, Tie-2 and VEGF-A were stimulated slightly in response to AG extract or MSCs, but increased greatly in response to AG extract and MSCs together in the brain tissue of rat MCAo (Fig. 3). Ang-1, a ligand of Tie-2, promote vascular formation with other angiogenic factors.41,42) Tie-2 exert its functions in larger stages of vascular development during vascular remodeling and maturation with new blood vessel formation depending on the availability of VEGF-A.14) The results showed that treatment of brain tissues injured by ischemic stroke with AG extract and MSCs increased Ang-1, Tie-2 and VEGF-A expression in comparison with other groups. In addition, AG extract promotes the expression of tight junction molecules such as ZO-1 and Occludin in ischemic brain tissues. Hyper-stimulation of VEGF-A expression increased microvessel density with the maintenance of ZO-1 protein expression.43) The results indicated that combined treatment with AG extract and MSCs could induce angiogenesis after ischemic damage to the brain by strong expression of Ang-1, Tie-2 and VEGF-A, as well as tight junction molecules.
In addition, combined treatment with AG extract and MSCs promoted the expression of phosphorylation of Akt and PI3K, while treatment with either of these alone had lesser effects on the expression of these factors (Fig. 3). Activation of the PI3K/Akt signaling cascade promotes cell growth and proliferation, survival, growth factor production, and angiogenesis.44)
To investigate the therapeutic effects of combined treatment with AG extract and MSCs on the ischemic brain, a mixture of AG extract and MSCs was transplanted into MCAo rat models. Administration of the mixture into MCAo rats significantly reduced the infarction volume and brain water content (Fig. 4). Infarction volume is regarded as an important indicator for detecting the therapeutic effects of treatment in stroke models.45,46) Furthermore, more CD31 labeled cells were detected in the ischemic boundary zone following combined treatment relative to treatment with AG extract or MSCs alone (Fig. 6). It is already known that CD31 showed significant increases in expression in the boundary zone of the ischemic brain following treatment of AG extract with MSCs into the MCAo rat model.40,47) Overall, these results demonstrated that combined AG extract and MSCs led to a synergistic improvement in herbal medicine and stem cells by increasing angiogenesis for ischemic stroke treatment.
AG extract and MSCs may lead to a synergistic increase in therapeutic potential by enhancing neovascularization. This mixed approach provides a new experimental protocol of herbal medicine therapy for the treatment of a variety of diseases, including stroke, trauma, and spinal cord injury.
This study was supported by the Traditional Korean Medicine R&D program funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (KHIDI) (HI15C0163) and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (NRF-2017M3A9G7072568).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.