Abstract
We reported a novel transport mechanism of curcumin, independent of improved solubility, which involved direct contact of amorphous solid particles with the cell membrane. This mechanism has potential as a novel systemic delivery system of poorly water-soluble drugs. In this study, the transport mechanism of furosemide (FUR), which is transported by the same novel mechanism, was examined. In vitro cell permeation studies under air-interface conditions (AICs) revealed that the permeation from powders sprayed on cell monolayers was significantly higher than that under liquid-covered conditions (LCCs) from their solutions. The permeation from amorphous solid particles was faster than that from crystals. Similar results were derived from in vitro studies using an artificial membrane, with which the permeation of FUR could be examined without water. These findings clearly indicated that the transport mechanism of FUR is the same as that of curcumin. For the application of this new transport mechanism, the in vivo absorption of FUR was examined after pulmonary insufflation, which allows the solid particles to make direct contact with the epithelial cells. Pulmonary absorption of FUR from the amorphous powder was almost complete and was faster than that after intragastric administration of the solution, suggesting that FUR was absorbed from the lung by the same mechanism as the in vitro study. This new transport mechanism, which is independent of water dissolution, could be exploited to develop a novel delivery system for poorly water-soluble drugs, using pulmonary powder inhalation.
Because the increase in the number of poorly water-soluble drugs is a major concern in recent drug development,1–4) many systems and techniques have been developed to enhance drug solubility.5,6) However, improved solubility has not always improved membrane transport or bioavailability (BA).7–11) This suggests a limitation of the conventional strategy focusing only on solubility. A new concept and strategy are necessary to overcome the problems of the conventional approach.
In our previous manuscript, a novel transport mechanism of curcumin (CUR), which is categorized as biopharmaceutical classification system (BCS) class 4, was described.9,12) The results suggested the direct involvement of solid particles, which was independent of water dissolution. Generally, in the case of poorly water-soluble drugs, such as CUR, the poor solubility must be improved to enhance BA, and many reports have focused solely on this, even with BCS class 4 drugs such as CUR.13–17) However, not only the solubility, but also the membrane permeability of these drugs should be considered. Therefore, this novel concept that increased drug permeation, which is independent of drug solubility, can improve the BA of poorly water-soluble drugs might be a breakthrough. In previous studies, the involvement of solid particles was not clarified. In these studies, it was very difficult to dismiss involvement of the dissolved drug, because the evaluation of the transport of CUR from the solution was impossible due to its extremely low solubility.9,18,19) To confirm and clarify the details of the new transport mechanism, other drugs or chemicals that utilize this transport should be studied. Because CUR is a supplement and not a medication, a clinically utilized pharmaceutical is preferable.
Furosemide (FUR) is a BCS class 4 diuretic.20,21) Its solubility in distilled water is very low (18.3 µg/mL). However, its solubility increases to more than 1 mg/mL at neutral pH, because FUR is a weakly acidic drug.21,22) The permeability of FUR through Caco-2 cell monolayers is very low.23,24) The oral BA is known to be variable and erratic.22,25) Although these BA findings are generally accepted to be caused by poor solubility in low gastric pH and low site-specific absorption, the reasons have not been justified. Similar to that of CUR, some reports have focused on the poor dissolution property of FUR to improve its BA.21,26) Both poor solubility and poor permeability should be increased to improve poor BA. Therefore, if the transport mechanism of FUR is the same as that observed with CUR, a simple and novel systemic drug delivery system could be developed, which would be an alternative to the conventional approach that considers only solubility.
The impact of the novel delivery system of CUR has been shown already in a previous report on its in vivo pulmonary absorption.12) Intrapulmonary administration has several advantages for drug absorption, such as high drug permeation due to the thin alveolar epithelium and rich vascular network, as well as the avoidance of hepatic first pass effects and low activity of proteases.27,28) The lung is an attractive site for the systemic delivery of drugs such as peptides and proteins.29–31) However, most inhaled dry powder formulations have been used as topical therapies to treat conditions such as asthma and influenza infection. Due to the many advantages of using the lung, developing pulmonary formulations for systemic drug delivery is worthwhile. To take full advantage of the novel transport mechanism, pulmonary administration appears to be an optimal route because alveolar epithelia are exposed externally with a small volume of fluid on their surface, enabling applied powder particles to contact directly with their cell membranes.
The first aim of this study was to clarify the transport mechanism of FUR, using Madin–Darby canine kidney (MDCK) cell monolayers. The second aim was to study in detail the mechanism of transport of FUR using an artificial membrane. Using this artificial membrane, the membrane transport could be evaluated without water, enabling quantitative assessment of the contribution of aqueously-dissolved FUR. Finally, the third aim was to evaluate the efficiency of the systemic delivery by pulmonary application using an in vivo animal study.
MATERIALS AND METHODS
MaterialsFUR was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Polyvinylpyrrolidone (Kollidon®12PF) was kindly supplied by BASF (Ludwigshafen, Germany). Dodecane and phosphatidylcholine were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Dulbecco’s phosphate-buffered saline (D-PBS), urethane, and bovine serum albumin (BSA) were obtained from Sigma-Aldrich, Inc. (St. Louis, MO, U.S.A.). MDCK cells were obtained from DS Pharma Biomedical Co., Ltd. (Osaka, Japan). α-Minimum essential medium (α-MEM), antibiotic–antimycotic solution (AA), and fetal bovine serum (FBS) were purchased from Nacalai Tesque, Inc. (Kyoto, Japan) and Thermo Scientific (Kanagawa, Japan), respectively. All other reagents used in this study were commercially available and analytical grade.
Preparation of Amorphous Solid Particles of FURAmorphous solid particles of FUR (FUR-K12) were prepared according to a modification of a previous method.32) Briefly, the mixture of FUR and Kollidon®12PF (4 : 1 by weight) was milled in an oscillatory ball mill (Mixer mill MM400, Retsch GmbH & Co., Hann, Germany) at a frequency of 30 Hz in 25 mL jars containing a 12 mm stainless steel ball. The total milling time was 90 min. The content of FUR in each lot of FUR-K12 was determined after preparation, which ranged from 72–76%. The same process was performed on FUR bulk powder (Milled FUR). The physical mixture (PM) was prepared by mixing FUR and Kollidon®12PF (4 : 1 by weight) for 5 min in the mill without the ball.
Physicochemical Properties of FUR FormulationsX-Ray Powder Diffraction (XRPD)XRPD was measured with a MiniFlex 600 (Rigaku, Tokyo, Japan). The sample was plated on a glass holder and exposed to CuKα radiation at a wavelength of 0.15406 nm, voltage of 40 kV, and current of 15 mA. The sample was scanned at 0.33°/min over a range of 3–50° (2θ) at a 0.02° step.
Differential Scanning Calorimetry (DSC)DSC thermograms were measured using a DSC 3500 (NETZSCH-Gerätebau GmbH, Germany). The sample (1 mg) was crimped in an aluminum pan, equilibrated at 30°C for 5 min, and then heated to 250°C at 10°C/min. Nitrogen was used as the purging gas at a flow rate of 30 mL/min.
Dynamic Light Scattering (DLS)The mean size of the FUR-K12, was determined by DLS using a Zetasizer ZSP (Malvern Instruments Inc., Worcestershire, U.K.) equipped with 633 nm He–Ne laser as a light source with a 173° detection angle. The sample was dispersed in distilled water and sonicated using Brasonic® (Branson Ultrasonics, Emerson Japan, Ltd., Kanagawa, Japan) prior to measurement.
Transmission Electron Microscopy (TEM)The morphology of Bulk FUR and FUR-K12 were investigated using transmission electron microscopy (TEM), JEM-2100 (JEOL Co., Ltd. Tokyo, Japan), with an accelerating voltage of 80 kV. The FUR or FUR-K12 suspensions were adsorbed on the mesh (collodion film). After drying at 25°C, the TEM image of the samples was taken.
Dissolution of FurosemideThe modified previous method9) was employed for dissolution study. The sample of Bulk FUR and FUR-K12 (10 mg) was added to 40 mL of Hanks’ balanced salt solution (HBSS, pH 7.4). Each suspension was stirred at 100 rpm at room temperature. The sample was taken at predetermined time intervals and centrifuged for 10 min to obtain the supernatant. The concentration of furosemide in the supernatant was determined by HPLC.
In Vitro Transepithelial Transport StudyThe in vitro transepithelial transport study was carried out as reported previously.9) Briefly, MDCK cells (passage numbers; 80–90) were seeded in 12-well inserts (BD Falcon) at a density of 2.0×105 cells/mL. Cells were cultured with 5% (v/v) CO2 at 37°C for at least 10 d. Monolayers with transepithelial electrical resistance (TEER) greater than 5 kΩ·cm2 were used for the transport experiments. Before the transport study, the monolayer was preincubated with HBSS for 15 min. For experiments using liquid-covered conditions (LCCs), 0.5 mL of HBSS containing 1 mg/mL FUR (500 µg FUR) and 1.5 mL of HBSS containing 5% (w/v) BSA were added to the apical and basal compartments, respectively. For experiments using air-interface conditions (AICs), 2 mL of HBSS containing 5% BSA was added only to the basal compartment. The solid particles (500 µg of original bulk FUR or 700 µg FUR-K12) were sprayed over the surface of the AIC cell monolayers using compressed air in a syringe. A device prepared from a syringe fitted with a 2 cm vinyl tube (SV45, Natsume Co., Ltd., Tokyo, Japan) and an 18G needle was used for the spraying. The powder was packed into the vinyl tube. Samples were collected from the basal compartment at predetermined time intervals over 180 min. The concentration of FUR was quantified by HPLC after deproteinization with an organic solvent.
In Vitro Permeation Study through an Artificial MembraneThe artificial membrane was prepared according to a slightly modified method of a previous report.33) Dodecane containing 20% (w/v) lecithin (5 µL) was dripped on the support membrane of the cell culture insert and was allowed to spread over the membrane completely. TEER was measured to verify complete filling of all membrane pores with the lipid solution. The permeation studies were performed under LCCs and AICs, similar to that used in the in vitro transepithelial permeation study in section above. The concentration of FUR in all samples was determined in the same manner as mentioned above.
In Vivo Animal StudyMale Wistar/ST rats (Shimizu Laboratory Co., Ltd., Kyoto, Japan), weighing 280–340 g (10 weeks old), were used in all experiments. All animal experiments were approved by the Experimental Animal Committee at Doshisha Women’s College of Liberal Arts and were performed in accordance with the institutional guidelines for animal experiments. All rats were fasted overnight but were allowed free access to water for 18 h before each experiment. The study was performed in the same manner as previously reported.12) For intragastric administration, 1 mL of 1 mg/mL solution of FUR was introduced into the stomach using a sonde (dose: 1 mg/rat). For intrapulmonary administration, 700 µg of bulk FUR or 1 mg FUR-K12 was sprayed through a trachea cannula using the same device that was used for the in vitro permeation studies. The dose of FUR for the pulmonary applications of the original bulk FUR and FUR-K12 was approximately 700 µg. Blood samples (0.2 mL) were collected from the jugular vein 15, 30, 60, 90, 120, 180, 240, and 360 min after administration. A continuous intravenous infusion was also performed to determine the absolute BA. FUR dissolved in 1 mL D-PBS at the concentration of 0.25 mg/mL was infused into the right jugular vein for 5 min (0.2 mL/min). Blood samples were taken at 2, 5, 10, 20, 30, 45, 60, 90, 120, 180, and 240 min after the start of infusion. All pharmacokinetic parameters were calculated based on a non-compartmental analysis using WinNonlin® software (Ver. 6.3, Pharsight Corporation, Mountain View, CA, U.S.A.).
Analysis of FURThe determination of FUR was performed by the modification of a previous method.34) FUR was fluorometrically determined with HPLC using a fluorescence detector (LC-20 A and RF-10A, respectively, Shimadzu Co., Kyoto, Japan). The system was equipped with an octadecylsilyl column (Cosmosil™ 5C18-MS-II, 4.6×150 mm, Nacalai Tesque, Inc., Kyoto, Japan). The mobile phase was composed of 35% acetonitrile and 65% 20 mM potassium phosphate buffer and was delivered at 1 mL/min. The excitation and emission wavelengths of the fluorescence detection were 360 nm and 413 nm, respectively
Statistical AnalysisTwo-sided t-tests were employed for the statistical analysis. p<0.05 was considered as significant difference.
RESULTS
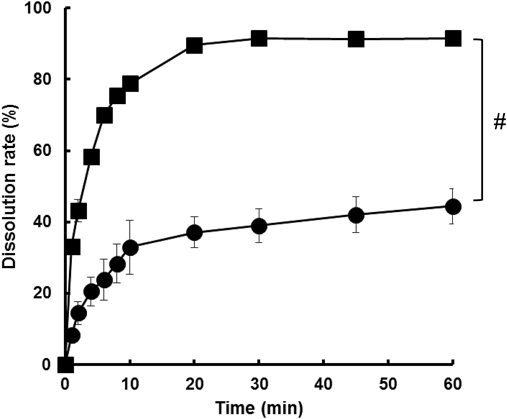
Physicochemical Properties of FUR FormulationsFigure 1 shows the XRPD profiles of the powder preparations. Figure 1A indicates that the original bulk FUR (Bulk FUR) has high crystallinity. The PM shows a diffraction pattern similar to Bulk FUR (Fig. 1C). On the other hands, the loss of crystallinity was confirmed in the two milled formulations (Figs. 1D, 1E). Figure 2 shows the DSC thermograms. The profile of Bulk FUR shows two endothermic peaks at approximately 135 and 220°C before heat decomposition (Fig. 2A). Two similar peaks were observed in the thermogram of PM (Fig. 2C). In contrast, the thermogram of FUR-K12 indicates an endothermic peak at ca. 65°C and an exothermic peak at ca. 120°C (Fig. 2E). Although a broad endothermic peak was confirmed at ca. 130°C in the FUR milled without K12 (Milled FUR), the thermogram may be similar to Bulk FUR. Figure 3 shows the particle size distribution of FUR-K12 determined with DLS. The particle size and the polydispersity index were 208.4±10.7 (nm) and 0.397, respectively. TEM images indicate that the shape of the particles is angular (Fig. 4A). The particle size of FUR-K12 was quite small compared with Bulk FUR (Fig. 4B). The dissolution profiles from Bulk FUR and FUR-K12 into HBSS are shown in Fig. 5. The dissolution from FUR-K12 was significantly faster and higher than that from Bulk FUR.
In Vitro Permeation StudyFigure 6 shows the cumulative MDCK transport profiles of FUR from solution and two powder formulations (Bulk FUR and FUR-K12). The AIC transport of FUR from each powder was much higher than the LCC transport from its solution. The cumulative transport amount from Bulk FUR and FUR-K12 at 180 min were 32.8±4.58 µg and 44.1±8.51 µg, respectively, while the result from the solution was 0.95±0.09 µg. In the powder formulations, FUR-K12 showed higher permeation than did Bulk FUR. From the result of the artificial membrane study (Fig. 7), the transport of FUR from each powder was similarly higher than from its solution. The cumulative transport amount from FUR-K12 (10.8±0.59 µg) was much greater than from the solution (0.13±0.03 µg).
In Vivo Pharmacokinetics StudyIn order to calculate the bioavailability (BA), the pharmacokinetic parameter after intravenous administration of FUR was examined. Figure 8 presents the profiles of the blood concentration after intravenous infusion and the obtained parameters are listed in Table 1. The area under the blood concentration vs. time curve (AUC) was 201±27.5 min·µg/mL. The total body clearance (CLtot) and the elimination half-life (t1/2) were 1.26±0.16 mL/min and 68.2±14.8 min, respectively (date not shown). Figure 9 shows the blood concentration–time profiles after intragastric administration of the solution (1 mg/rat). The AUC was 324±129 min·µg/mL, from which BA was calculated as 40.1%. Figure 9 also shows the blood concentration-time profiles after pulmonary administration of the powders. The AUC after application of Bulk FUR was 469±88.7 min·µg/mL. As listed in Table 1, the BA reached 89.5%, which is 2.2 times higher than that after intragastric administration (40.1%). The pulmonary absorption of FUR-K12 was superior to that of Bulk FUR. The AUC and BA were 591±83.0 min·µg/mL and 95.3%, respectively. As the mean resident time (MRT) indicates, the pulmonary absorption of FUR-K12 was fastest (87.7 min).
Table 1. Pharmacokinetic Parameters Obtained from
in Vivo Rat Studies
| Route | Form | Cmax (µg/mL) | Tmax (min) | AUC (min·µg/mL) | MRT (min) | BA (%) |
|---|
| Intravenous | Solution | — | — | 201±27.5 | 50.0±11.3 | — |
| Intragastric | Solution | 1.50±0.88 | 55.0±39.9 | 324±129 | 265±138 | 40.1 |
| Intrapulmonary | Bulk FUR powder | 3.73±0.69 | 47.2±16.0 | 469±88.7 | 112±29.0 | 89.5 |
| Intrapulmonary | FUR-K12 powder | 7.11±0.75 | 30.0±0.00 | 591±83.0 | 87.7±7.22 | 95.3 |
DISCUSSION
It has already been reported that several polymorphisms are confirmed for FUR.35,36) Bulk FUR is likely form I. In the DSC thermogram, two endothermic peaks are observed (Fig. 2A). The former is due to a phase transition to a higher temperature-stable form, and the latter is due to the melting endotherm.35) These characteristic peaks were also observed in the PM and the Milled FUR, indicating that FUR in the two preparations is still crystal. On the other hands, different peaks are found in the DSC thermogram of FUR-K12, an endothermic peak at ca. 65°C and an exothermic peak at ca. 120°C. These two peaks suggest a glass transition and recrystallization due to the amorphization by milling with the polymer K12. Although a small crystalline peak was also observed in the XRPD pattern, the result of DSC reveals that the FUR in FUR-K12 is partially amorphous. In addition, the particle size of FUR-K12 was slightly heterogeneous but nano-sized. However, the particle size of Milled FUR was not small as that of FUR-K12 (date not shown). Therefore, it was confirmed that only FUR-K12 was amorphous and nano-sized. All in vitro and in vivo experiments were carried out using Bulk FUR and FUR-K12.
The rank order of the transport across the monolayer of MDCK cells (solution<Bulk FUR<FUR-K12) agrees with the results of CUR from our previous study.9) According to our previous study, CUR showed very low AIC permeation as a crystalline powder. The apparent permeability coefficient of FUR (Papp) was 1.0±0.1×10−7 cm/s, which is similar to the data derived from Caco-2 cell monolayers (1.2±0.1×10−7 cm/s).37) In our previous studies, it was not clear if dissolved CUR was involved in the improved permeation because of the extremely low solubility of CUR. However, as Fig. 6 shows, the permeation of FUR was much lower from solution than from the powder formulations. Therefore, the improved permeation of each powder is clearly not due to dissolved drug. The AIC transport of FUR from FUR-K12 was 50 times larger than that from solution. A few manuscripts have reported the improved permeability of FUR by various methods such as a self-microemulsifying system,38) mesoporous silicon microparticles,39) and common excipients.40) Among these methods, a 4.6-fold increase in Papp is the best improvement compared with that of the control solution.39) In addition to the poor dissolution, the involvement of efflux transporters is discussed as one of the reasons for the low permeability. Inhibiting the transporter may be effective to improve the permeability in the reports. However, even in the manuscript describing the best improvement, the authors conclude that improving the dissolution property of the drug is the best method to enhance permeation. In the case of FUR, not only its solubility but also its permeability should be enhanced to improve BA. These reports might suggest a limitation of the conventional strategy that focuses only on the solubility. The strategy we propose is not only simple but also reasonable and much better than the conventional approach.
The novel finding in our previous studies was that the dissolved CUR is unlikely involved in its transepithelial transport. However, because of a small volume of water present on the surface of the cultured cell, the possibility could not be excluded that the drug is dissolved on the cell surface at high concentration. The high concentration of the drug around the membrane is a driving force for the high permeation from powder formulations. Therefore, an artificial membrane was used to exclude the possibility of the contribution of this surface water. The parallel artificial membrane permeation assay (PAMPA) has been recognized as a useful tool to evaluate the permeability of drug candidates as an alternative to cell monolayers in the drug development.41–44) The current system is often used for high-throughput screening that is designed to handle a large number of drug candidates at the same time. Some modifications were made to this system to adjust to the requirements of our study. A sufficient membrane area was important to spray the powder formulation. The area of the membrane utilized in this research (1.0 cm2) was approximately 3 times larger than that of the commercial product (0.3 cm2). The artificial membrane showed twice the TEER (larger than 10 kΩ·cm2) than did the MDCK cell monolayer used for the in vitro transepithelial permeation study. The lager TEER value suggests that all the pores of the membrane were filled with the dodecane solution of the lipid. Preliminary experiments showed no permeation of highly hydrophilic drugs through the prepared membrane (data not shown). Consequently, the artificial membrane used in this study was deemed suitable for the purpose of our research. Figure 7 shows the cumulative transport of FUR. The results agree with those from our in vitro cellular permeation study. The results suggest that the dissolved drug is not transported across the lipid membrane. On the contrary, the cumulative AIC transport from Bulk FUR and FUR-K12 was expectedly much higher than that from the solution. The amorphous formulation (FUR-K12) showed the highest transport, which was 80 times higher than that of the solution. This finding suggests that the permeation mechanism of FUR from the solution across the artificial membrane was different from that from the powder. It is generally believed that drug permeation follows the pH-partition theory. Because FUR is an acidic drug with pKa of 3.9,22) more than 99.9% of the dissolved FUR is ionized in the phosphate buffer at pH 7.0. This is the main reason why the transport is low from solution. It may be difficult to reasonably explain the mechanism of high permeation from Bulk FUR and FUR-K12 based on the pH partition theory. There exists no water into which FUR can dissolve on the apical side of the artificial membrane under AICs. Because the permeability of the dissolved FUR is low, it is reasonable to assume that the enhanced permeation from powder formulations is due not to the conventional permeation of dissolved FUR, but to the direct involvement of the solid particles. Our hypothesis assumes that FUR can be directly dissolved into the lipid membrane from the solid particle through the direct contact with the lipid. FUR can be dissolved not only in water but also in the lipid. It is generally and widely accepted that the solid drug is initially dissolved into water and then permeates through the membrane. The study describing usefulness of dry powder formulations for improving the pulmonary absorption using the AIC monolayer of Calu-3 cells has been already published.45) The conclusion of the manuscript is that the higher local concentration of drugs on the cell layer led the improved permeation of the drug. Consequently, solubility is a very important factor that determines the membrane permeation. However, as the results regarding CUR and FUR show, this concept is not always correct. The direct dissolution from solid particles into the lipid membrane is superior to that from the solution. If dissolved drugs are involved, there will be a clear discrepancy with regard to the solubility required for an improved cumulative transport amount. As shown in Fig. 7, the study using the artificial membrane has clarified that the transport from Bulk FUR was 24 times higher than that from the solution (concentration; 1 mg/mL). Assuming the passive diffusion, the solubility should be at least 24 mg/mL, which is much larger than the solubility of FUR (2.7 mg/mL at pH 6.8). In addition, as shown in Fig. 5, the dissolution of FUR from Bulk powder was significantly slow in comparison with that from FUR-K12. Therefore, the new drug transport mechanism that has been proposed is independent of the water-dissolved drug. The amorphization is advantageous to improve the permeability of FUR, which agrees with our previous study using CUR. In vitro permeation studies across MDCK monolayers and artificial membranes showed that the cumulative transport for 60 min was significantly higher from the amorphous formulation than that from the crystal. This finding is due to the better and faster dissolution from the amorphous solid than that from the crystal.
The artificial membrane under AIC is similar to the inside of the lungs.46,47) Alveolar epithelial cells are in direct contact with air, and are covered with phospholipid-rich pulmonary surfactant with little water on their surface. The lung is the more suitable drug application site than the gastrointestinal tract to take advantage of this new transport mechanism. We already revealed in our previous manuscript that the pulmonary administration of amorphous CUR powder resulted in much better BA of CUR.12)
The BA after intragastric administration of the solution was not as low as would be estimated from the low in vitro permeability and the initial increase in the blood concentration was rapid. Better and rapid absorption might be due to site-specific absorption in the stomach. As shown in the in vitro permeation studies, the permeability of the dissolved FUR is very low. At low gastric pH, the permeation should be increased, because the un-ionized fraction of FUR is increased. However, according to the previous report, no significant difference was found between pH 5.5 and 7.4 in the permeability across an artificial membrane.44) Other than the change in ionization, FUR could be precipitated in the stomach, due to the decreased solubility at low gastric pH. According to our novel transport mechanism, the particles precipitated in the stomach would be involved in the gastric absorption. According to our preliminary study in which the legation of the duodenum just below the stomach prevented the intragastric FUR from transferring to the small intestine, the plasma concentration levels are as high as those after normal intragastric administration of FUR solution, suggesting that FUR can be absorbed not only from the small intestine but also from the stomach.
As can be seen from Fig. 9, the variabilities in blood concentrations after intrapulmonary administration are smaller than those after intragastric administration. The pulmonary absorption of FUR-K12 was superior to that of Bulk FUR. Surprisingly, the absorption was complete. Similarly to the finding on CUR, the improvement in the pulmonary BA was better from the amorphous formulation. The pulmonary absorption of Milled FUR was similar with that of Bulk FUR (data not shown). The absorption from PM was also comparable (data not shown). Consequently, the pulmonary absorption of FUR was not affected by the particle size and the excipient (K12). However, the effects of the type and mixing ratio of excipients are not clear. There are additional factors to be considered for the design of inhaled dry powder formulations.48)
The surface of alveolar epithelia is covered with a phospholipid-rich surfactant. The results from the artificial membrane study suggest that the interaction of solid particles with phospholipids enhances the transport of FUR based on our novel drug transport mechanism. The combination of the pulmonary surfactant with current drug delivery systems, such as polymeric carriers and dendrimers,47,49,50) has been proposed. However, this study revealed an advantage of amorphous solid particles for the systemic drug delivery of FUR based on a new drug transport mechanism. This novel mechanism might be involved in the improved bioavailability using pulmonary powder inhalation.
CONCLUSION
In vitro transport studies using cell monolayers and artificial membranes clarified that the transport of FUR from the powder under AICs was higher than that from the solution, and that the amorphous powder show better transport than the crystalline powder did, indicating a direct involvement of solid particles in the membrane transport of FUR. These findings further support the novel transport mechanism observed with CUR. The results derived from the in vivo animal study were similar to those from the in vitro transport studies. The solid particles, in particular the amorphous formulation, provided better pulmonary absorption than the solution did. For the systemic delivery of poorly water-soluble drugs such as FUR and CUR, the lung is the best application site to take full advantage of this novel transport mechanism. Using pulmonary inhalation of powder formulations, an efficient systemic delivery system of such a drug can be realized.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Wenlock MC, Austin RP, Barton P, Davis AM, Leeson PD. A comparison of physiochemical property profiles of development and marketed oral drugs. J. Med. Chem., 46, 1250–1256 (2003).
- 2) Stegemann S, Leveiller F, Franchi D, De Jong H, Linden H. When poor solubility becomes an issue: from early stage to proof of concept. Eur. J. Pharm. Sci., 31, 249–261 (2007).
- 3) Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev., 46, 3–26 (2001).
- 4) Shah DA, Murdande SB, Dave RH. A review: Pharmaceutical and pharmacokinetic aspect of nanocrystalline suspensions. J. Pharm. Sci., 105, 10–24 (2016).
- 5) Wanning S, Suverkrup R, Lamprecht A. Pharmaceutical spray freeze drying. Int. J. Pharm., 488, 136–153 (2015).
- 6) Fong SY, Brandl M, Bauer-Brandl A. Phospholipid-based solid drug formulations for oral bioavailability enhancement: A meta-analysis. Eur. J. Pharm. Sci., 80, 89–110 (2015).
- 7) Frank KJ, Rosenblatt KM, Westedt U, Holig P, Rosenberg J, Magerlein M, Fricker G, Brandl M. Amorphous solid dispersion enhances permeation of poorly soluble ABT-102: true supersaturation vs. apparent solubility enhancement. Int. J. Pharm., 437, 288–293 (2012).
- 8) Ueda K, Higashi K, Limwikrant W, Sekine S, Horie T, Yamamoto K, Moribe K. Mechanistic differences in permeation behavior of supersaturated and solubilized solutions of carbamazepine revealed by nuclear magnetic resonance measurements. Mol. Pharm., 9, 3023–3033 (2012).
- 9) Kimura S, Kasatani S, Tanaka M, Araki K, Enomura M, Moriyama K, Inoue D, Furubayashi T, Tanaka A, Kusamori K, Katsumi H, Sakane T, Yamamoto A. Importance of the direct contact of amorphous solid particles with the surface of monolayers for the transepithelial permeation of curcumin. Mol. Pharm., 13, 493–499 (2016).
- 10) Miller JM, Beig A, Krieg BJ, Carr RA, Borchardt TB, Amidon GE, Amidon GL, Dahan A. The solubility-permeability interplay: mechanistic modeling and predictive application of the impact of micellar solubilization on intestinal permeation. Mol. Pharm., 8, 1848–1856 (2011).
- 11) Poelma FG, Breas R, Tukker JJ. Intestinal absorption of drugs. III. The influence of taurocholate on the disappearance kinetics of hydrophilic and lipophilic drugs from the small intestine of the rat. Pharm. Res., 7, 392–397 (1990).
- 12) Kimura S, Kiriyama A, Araki K, Yoshizumi M, Enomura M, Inoue D, Furubayashi T, Yutani R, Teraoka R, Tanaka A, Kusamori K, Katsumi H, Yamamoto A, Iga K, Sakane T. Novel strategy for improving the bioavailability of curcumin based on a new membrane transport mechanism that directly involves solid particles. Eur. J. Pharm. Biopharm., 122, 1–5 (2018).
- 13) Wang Y, Wang C, Zhao J, Ding Y, Li L. A cost-effective method to prepare curcumin nanosuspensions with enhanced oral bioavailability. J. Colloid Interface Sci., 485, 91–98 (2017).
- 14) Anwar M, Ahmad I, Warsi MH, Mohapatra S, Ahmad N, Akhter S, Ali A, Ahmad FJ. Experimental investigation and oral bioavailability enhancement of nano-sized curcumin by using supercritical anti-solvent process. Eur. J. Pharm. Biopharm., 96, 162–172 (2015).
- 15) Sadeghi F, Ashofteh M, Homayouni A, Abbaspour M, Nokhodchi A, Garekani HA. Antisolvent precipitation technique: A very promising approach to crystallize curcumin in presence of polyvinyl pyrrolidon for solubility and dissolution enhancement. Colloids Surf. B Biointerfaces, 147, 258–264 (2016).
- 16) Paradkar A, Ambike AA, Jadhav BK, Mahadik KR. Characterization of curcumin-PVP solid dispersion obtained by spray drying. Int. J. Pharm., 271, 281–286 (2004).
- 17) Onoue S, Takahashi H, Kawabata Y, Seto Y, Hatanaka J, Timmermann B, Yamada S. Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J. Pharm. Sci., 99, 1871–1881 (2010).
- 18) Tønnesen HH, Masson M, Loftsson T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int. J. Pharm., 244, 127–135 (2002).
- 19) Kaminaga Y, Nagatsu A, Akiyama T, Sugimoto N, Yamazaki T, Maitani T, Mizukami H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett., 555, 311–316 (2003).
- 20) Fasinu P, Pillay V, Ndesendo VM, du Toit LC, Choonara YE. Diverse approaches for the enhancement of oral drug bioavailability. Biopharm. Drug Dispos., 32, 185–209 (2011).
- 21) Nkansah P, Antipas A, Lu Y, Varma M, Rotter C, Rago B, El-Kattan A, Taylor G, Rubio M, Litchfield J. Development and evaluation of novel solid nanodispersion system for oral delivery of poorly water-soluble drugs. J. Control. Release, 169, 150–161 (2013).
- 22) Rabbie SC, Flanagan T, Martin PD, Basit AW. Inter-subject variability in intestinal drug solubility. Int. J. Pharm., 485, 229–234 (2015).
- 23) Corti G, Maestrelli F, Cirri M, Zerrouk N, Mura P. Development and evaluation of an in vitro method for prediction of human drug absorption: II. Demonstration of the method suitability. Eur. J. Pharm. Sci., 27, 354–362 (2006).
- 24) Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, Tokuda H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur. J. Pharm. Sci., 10, 195–204 (2000).
- 25) Granero GE, Longhi MR, Mora MJ, Junginger HE, Midha KK, Shah VP, Stavchansky S, Dressman JB, Barends DM. Biowaiver monographs for immediate release solid oral dosage forms: furosemide. J. Pharm. Sci., 99, 2544–2556 (2010).
- 26) Radwan SE, Sokar MS, Abdelmonsif DA, El-Kamel AH. Mucopenetrating nanoparticles for enhancement of oral bioavailability of furosemide: In vitro and in vivo evaluation/sub-acute toxicity study. Int. J. Pharm., 526, 366–379 (2017).
- 27) Chen L, Okuda T, Lu XY, Chan HK. Amorphous powders for inhalation drug delivery. Adv. Drug Deliv. Rev., 100, 102–115 (2016).
- 28) Patton JS. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev., 19, 3–36 (1996).
- 29) Amancha KP, Hussain A. Effect of protease inhibitors on pulmonary bioavailability of therapeutic proteins and peptides in the rat. Eur. J. Pharm. Sci., 68, 1–10 (2015).
- 30) Hussain A, Majumder QH, Ahsan F. Inhaled insulin is better absorbed when administered as a dry powder compared to solution in the presence or absence of alkylglycosides. Pharm. Res., 23, 138–147 (2006).
- 31) Santos Cavaiola T, Edelman S. Inhaled insulin: a breath of fresh air? A review of inhaled insulin. Clin. Ther., 36, 1275–1289 (2014).
- 32) Jensen KT, Larsen FH, Cornett C, Lobmann K, Grohganz H, Rades T. Formation mechanism of coamorphous drug–amino acid mixtures. Mol. Pharm., 12, 2484–2492 (2015).
- 33) Avdeef A, Strafford M, Block E, Balogh MP, Chambliss W, Khan I. Drug absorption in vitro model: filter-immobilized artificial membranes: 2. Studies of the permeability properties of lactones in Piper methysticum Forst. Eur. J. Pharm. Sci., 14, 271–280 (2001).
- 34) Derakhshandeh K, Karimi M, Azandaryani AH, Bahrami G, Ghanbari K. Pharmacokinetic study of furosemide incorporated PLGA microspheres after oral administration to rat. Iran. J. Basic Med. Sci., 19, 1049–1055 (2016).
- 35) Matsuda Y, Tatsumi E. Physicochemical characterization of furosemide modifications. Int. J. Pharm., 60, 11–26 (1990).
- 36) Fioritto AF, Bhattachar SN, Wesley JA. Solubility measurement of polymorphic compounds via the pH-metric titration technique. Int. J. Pharm., 330, 105–113 (2007).
- 37) Pade V, Stavchansky S. Link between drug absorption solubility and permeability measurements in Caco-2 cells. J. Pharm. Sci., 87, 1604–1607 (1998).
- 38) Zvonar A, Berginc K, Kristl A, Gasperlin M. Microencapsulation of self-microemulsifying system: improving solubility and permeability of furosemide. Int. J. Pharm., 388, 151–158 (2010).
- 39) Kaukonen AM, Laitinen L, Salonen J, Tuura J, Heikkila T, Limnell T, Hirvonen J, Lehto VP. Enhanced in vitro permeation of furosemide loaded into thermally carbonized mesoporous silicon (TCPSi) microparticles. Eur. J. Pharm. Biopharm., 66, 348–356 (2007).
- 40) Rege BD, Yu LX, Hussain AS, Polli JE. Effect of common excipients on Caco-2 transport of low-permeability drugs. J. Pharm. Sci., 90, 1776–1786 (2001).
- 41) Fujikawa M, Ano R, Nakao K, Shimizu R, Akamatsu M. Relationships between structure and high-throughput screening permeability of diverse drugs with artificial membranes: application to prediction of Caco-2 cell permeability. Bioorg. Med. Chem., 13, 4721–4732 (2005).
- 42) Avdeef A, Artursson P, Neuhoff S, Lazorova L, Grasjo J, Tavelin S. Caco-2 permeability of weakly basic drugs predicted with the double-sink PAMPA pKa(flux) method. Eur. J. Pharm. Sci., 24, 333–349 (2005).
- 43) Kansy M, Senner F, Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J. Med. Chem., 41, 1007–1010 (1998).
- 44) Zhu C, Jiang L, Chen TM, Hwang KK. A comparative study of artificial membrane permeability assay for high throughput profiling of drug absorption potential. Eur. J. Med. Chem., 37, 399–407 (2002).
- 45) Asai A, Okuda T, Sonoda E, Yamauchi T, Kato S, Okamoto H. Drug permeation characterization of inhaled dry powder formulations in air–liquid interfaced cell layer using an improved, simple apparatus for dispersion. Pharm. Res., 33, 487–497 (2016).
- 46) De Backer L, Cerrada A, Perez-Gil J, De Smedt SC, Raemdonck K. Bio-inspired materials in drug delivery: Exploring the role of pulmonary surfactant in siRNA inhalation therapy. J. Control. Release, 220 (Pt B), 642–650 (2015).
- 47) Hidalgo A, Cruz A, Perez-Gil J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm., 95 (Pt A), 117–127 (2015).
- 48) Weers JG, Miller DP. Formulation design of dry powders for inhalation. J. Pharm. Sci., 104, 3259–3288 (2015).
- 49) Kawashima Y, Yamamoto H, Takeuchi H, Fujioka S, Hino T. Pulmonary delivery of insulin with nebulized DL-lactide/glycolide copolymer (PLGA) nanospheres to prolong hypoglycemic effect. J. Control. Release, 62, 279–287 (1999).
- 50) Kaminskas LM, McLeod VM, Ryan GM, Kelly BD, Haynes JM, Williamson M, Thienthong N, Owen DJ, Porter CJ. Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy. J. Control. Release, 183, 18–26 (2014).