2018 年 41 巻 6 号 p. 869-876
2018 年 41 巻 6 号 p. 869-876
Pinelliae Rhizoma Praeparatum (PRP) as traditional Chinese medicine had been used for hepatic diseases in combinative forms. However, the effect of PRP was not clear when used alone. So to explore the hepatoprotective/hepatotoxin of PRP is necessary. The activities of PRP were investigated in acetaminophen-induced hepatic injury mice. Liver function markers, hepatic oxidative stress markers were evaluated. Bile acids metabolic transports and nuclear factor erythroid 2-related factor 2 (Nrf2) were detected. As a drug for the treatment of liver diseases, PRP slightly restored the parameters towards normal in model mice only in low dosage, and also had no antioxidant activity and regulate Nrf2. Cholestasis was significantly elevated in model mice when pretreatment with routine or high dosage of PRP, but had no effect on normal mice. Bile salt export pump (Bsep) and multidrug resistance-associated protein 2 (Mrp2) in model mice were markedly increased when pretreatment with low dose PRP, but significantly decreased when pretreatment in routine or high dosage. Mrp3 was significantly induced in model mice after pretreatment of PRP. But the adjustment effect to bile acids transporters by PRP was not significant in normal mice. These results reveal that PRP has the different effects on bile acids transporter in hepatic injury mice, and therefore, the dosage of PRP need to be paid attention to when it is used in clinical hepatic injury.
Pinelliae rhizoma is the dried tuber of Pinellia ternate (THUNB.) BREIT, it is the most commonly traditional herb medicine which has been used for the treatment of cough, infection and inflammation since ancient times. Nowadays, it has been widely used in clinic for antiemetic, antitussive, sedative and anti-inflammatory purposes in China, Korea, Japan and other Asian countries.1–3) Raw Pinelliae Rhizoma could cause irritant toxicity to oral, throat and gastrointestinal mucosa,4,5) so it is always used in its preparation forms in clinic. Pinelliae Rhizoma Praeparatum (PRP) is the product of raw Pinelliae Rhizoma processed with alkaline solution and Radix Glycyrrhiza. The preparation method was according to the Chinese Pharmacopoeia (2005 and 2010 Edition). Modern pharmacological studies showed that PRP possessed multiple activities, such as antitussive, expectorant, antiemetic, antitumor, antibacterial, anti-inflammation, and sedative-hypnotic.6–10) As a main component in traditional Chinese medicine, PRP has been used for treatment acute and chronic gastroenteritis, chronic colitis, hepatitis, and early hepatocirrhosis in combinative forms, such as Banxia–Xiexin-Decoction, Banxia–houbu-Decoction, Banxia–Kucao-Decoction11) and so on. At present, It has also been widely used in the treatment of clinical hepatic disease currently (Dictionary of Chinese Traditional Medicine 2006). Traditional Chinese medicine (TCM) is mostly prescribed in combination to obtain synergistic effects and reduce possible adverse reactions. Hence, the compatibility of Chinese medicinal herbs is an important theory in the combination of TCM. The PRP has been used in clinic for a long time in combinative forms, but the therapeutic effect of PRP is not clear, It has also been reported that PRP could cause hepatic toxicity, the activities of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) could significantly increase after pretreatment of 59.6 g/kg PRP.12,13) So whether PRP is effective or toxic to hepatic injury still need to be further investigated.
Liver disease has been a major health problem globally.14) Oxidative stress induced by large number of highly reactive free radicals, is the starting point for hepatic injury which later advances to serious chronic conditions. Acetaminophen induced hepatic injury is the main inducement of acute hepatic injury in clinical and also is one of the commonly used hepatotoxic model in experimental systems.15) PRP as traditional Chinese medicine has been used for hepatic diseases in combinative forms. But the effect of PRP was not clear when used as single. So to explore the hepatoprotective/hepatotoxin of PRP is necessary. Therefore, in the light of all these bases, it could be much more meaningful to study the hepatoprotective/hepatotoxin active of PRP on acetaminophen-induced hepatotoxicity in mice and explore the possible mechanisms of the action.
Acetaminophen of analytical grade was purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Superoxide dismutase (SOD), malondialdehyde (MDA) detection kits, glutathione peroxidase (GSH-Px), glutathione (GSH) detection kits and Membrane and Cytosol Protein Extraction Kit were purchased from Beyotime Biotech Co. (Shanghai, China). Multidrug resistance-associated protein 2 (Mrp2, Lot: GR141887-12), multidrug resistance-associated protein 3 (Mrp3, Lot: GR129658-10), bile salt export pump (Bsep, Lot: GR32413-13), nuclear factor erythroid 2-related factor 2 (Nrf2, GR298097-2) were purchased from Abcam, U.K.; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (0228BR) was purchased from CMCTA G, USA. All other chemicals and solutions were of the highest quality available commercially.
Maintenance of AnimalsMale ICR mice (18–22 g) were purchased from animal center of the Fourth Military University, and housed under controlled environment (22±2°C, 12 h light/dark cycle, free access to food and water). The mice were fasted for 12 h before the experimental procedures. All experiments were carried out between 8:00 and 13:00 in a quiet room with temperature 22–24°C. Procedures involving animals and their care were conducted in conformity with NIH guidelines (NIH Pub. No. 85-23, revised 1996) and approved by the fourth military university committee on animal care and use.
PRP DecoctionPRP (100 g, Batch Number: 250231), provided by XiaoZao Pharmaceutical Company (Xi’an, China) and identified by College of Pharmacy, Fourth Military Medical University, Xi’an, China. The Pinelliae Rhizoma Praeparatum decoction was prepared according to the pharmacopoeial procedures.5) PRP was crushed into crude powders. The aqueous extract was prepared by reflux of 100 g of powdered sample with 800 mL water, then reflux for 30 min and repetition twice. The extracted solutions were combined, filtered and concentrated volume to 500 mL.
Experimental ProtocolAfter acclimation for 1 week, mice were divided into six groups of ten animals each as follows: Group A (normal control) was given distilled water (10 mL/kg body weight). Group B (PRP control) received PRP decoction (10 g/kg). Group C (acetaminophen model) was given distilled water. Groups D to F was respectively administrated PRP decoction (0.4, 2, 10 g/kg, and middle dosage 2 g/kg in accordance with the human daily dose). The daily dose of PRP recommended by the Chinese Pharmacopoeia is 0.25 g/kg raw herb for human. After the oral administration for 7 d, the animals were treated as described previously with some modifications. Two hours after the final administration, mice in Groups C–F were injected intraperitoneally (i.p.) with acetaminophen (300 mg/kg, dissolved in olive oil) which is documented to induce acute hepatotoxicity in mice,16) while the mice in Groups A and B received appropriate vehicle (olive oil, i.p.). Twenty-four hours after the acetaminophen challenge followed by fasting, the animals were anesthetized for obtaining the blood and sacrificed to collect the livers.17) Blood samples were centrifuged to obtain serum at 10000×g for 10 min at 4°C. The right lobe of the liver was fixed in 10% formalin to prepare paraffin sections and the remaining parts were stored at −80°C for the other assays.
Serum AnalysisThe serum activity of AST, ALT, total bile acid (TBA), total bilirubin (TBIL), direct bilirubin (DBIL), γ-glutamyltranspeptidase (γ-GT), alkaline phosphatase (ALP) was measured to evaluate hepatotoxicity. An auto analyzer (200FR, TOSHIBA, Japan) was used in the experiments.
Histopathology AnalysisA sample of liver obtained after decapitation was washed in saline and fixed in 10% formalin for the routine haematoxylin–eosin (HE) staining technique and histopathological examinations. Fixed tissues were processed routinely, embedded in paraffin wax, sectioned into 5 µm thick sections in a rotary microtome and then stained with HE dye. At least three different sections were examined per sample of liver.
Biochemical MeasurementsEstimation of MDA level, the activities of SOD, GPx, and GSH content in liver by using commercial assay kits (Biyuntian Co., China). The experimental method was operated according to the instruction of the commercial assay kits.18–20) Protein content was determined by a Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL, U.S.A.). All values were normalized to hepatic total protein.
ImmunohistochemistryLiver tissues from mice were perfused with phosphate-buffered saline (PBS), sliced and fixed with 4% paraformaldehyde for immunohistochemical experiments. The liver samples were incubated overnight in PBS with 6.8% sucrose, dehydrated with acetone and paraffin-embedded. Before staining, semi-thin sections were incubated for 5 min at 37°C in 0.01% trypsin/0.1% CaCl2 (pH 7.8). Sections were incubated for 5 h at 37°C with Mrp2, Mrp3, Bsep and Nrf2 antibodies (Abcam, U.K.) and then treated with corresponding secondary antibodies, followed by incubations with peroxidase–antiperoxidasea at dilution of 1 : 100 for 1 h at 37°C. At last, the immunolabeling was visualized by incubation with 3,3-diaminobenzidine–H2O2 medium for 10 min at room temperature. Images were captured with a Nikon Photo Micro-scope equipped with a digital camera.
Immunoblot AnalysisThe protein expressions of Mrp2, Mrp3, Bsep and Nrf2 in liver tissue were analyzed by Western blot. This method was performed as described previously.21) The total protein concentrations of liver tissue extracts were determined using a bicinchoninic acid (BCA) protein concentration assay kit (Comwin Biotech, Beijing, China). A membrane-enriched fraction (50 µg protein) by Membrane and Cytosol Protein Extraction Kit (Beyotime Biotech, Shanghai, China) were separated on a 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and used Bio-Rad electrophoresis system (Bio-Rad Laboratories, Inc., Hercules, CA, U.S.A.). After being transferred to a nitrocellulose membrane, the proteins were blocked for 1 h at room temperature with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TBST). The membrane was then incubated with anti-Mrp2, anti-Mrp3, anti-Bsep, anti-Nrf2 primary antibodies at 4°C overnight. After the membrane was washed four times for 10 min each with TBST buffer, and then it was incubated in the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 1 h. After washing the membrane four times with TBST buffer, chemiluminescence was developed using an ECL reagents (Millipore immobilon™ HRP Substrate). The immunoreactive bands on the autoradiography films were scanned with calibrated densitometer ChemiDoc™ XRS+ (Bio-Rad Imaging System) and quantified using the QuantityOne imaging software (Bio-Rad Laboratories). Equal loading of proteins onto the gel was confirmed by immunodetection of GAPDH.
Statistical AnalysisData are analyzed statistically by one-way ANOVA followed by multiple comparison Dunn’s test. Two independent groups were compared by t-test. A value of p<0.05 was considered as statistically significant. Data are expressed as mean±standard error of the mean (S.E.M.).
Pinelliae Rhizoma Praeparatum. (Banxia, 100 g) extractions were detected by HPLC and GC/MS. Quantitation results indicated that the concentrations of which contained approximately oxalic acid 1.37 mg/mL, citric acid 3.12 mg/mL, malic acid 6.41 mg/mL and succinic acid 2.46 mg/mL and β-sitosterol 12.3 mg/mL. HPLC Methods: Gemini-C18 analytical column (4.6×250 mm, 5 µm) with 0.03 mol/L ammonium dihydrogen phosphate buffer solution (adjusted pH to 2.0 with phosphoric acid)–methanol (97 : 3) as the mobile phase at a flow rate of 0.8 mL/min. The detection wavelength was 210 nm and the column temperature was 30°C. GC/MS Methods: The GC-MS analysis was performed using a Shimadzu GC-2010 equipped with a Shimadzu AOC-20i auto sampler system and interfaced with a Shimadzu QP 2010 mass spectrometer (Shimadzu, Kyoto, Japan). A SH-Rxi-5Sil MS column (30 m×0.25 mm i.d., and 0.25 µm film thickness) supplied by Shimadzu Technologies was used. The carrier gas was 99.99% high purity Helium with a flow rate of 1.1 mL/min. The injection volume was 1 µL. Oven temperature program was initially set at 210°C for 10 min, then to 290°C (10 min) at 20°C/min, and finally to 300°C (1 min) at 10°C/min. The total run time was 26 min with a solvent delay of 2.5 min. The injection temperature and interface were 300 and 280°C.
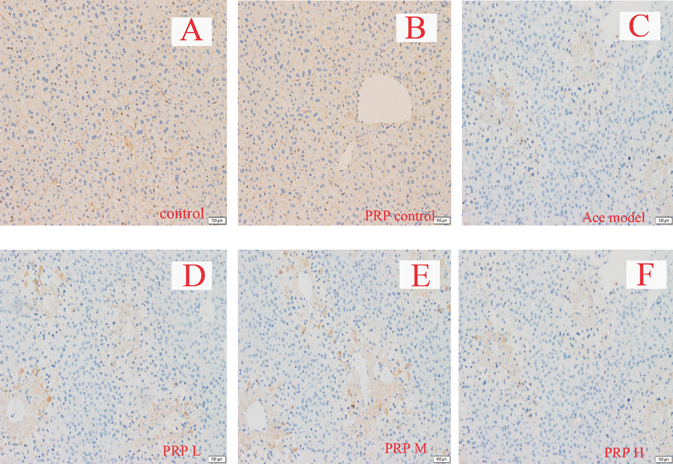
PRP Slightly Alleviated Hepatic Injury in Low Dosage, but Obviously Enhancement the Cholestasis in High DosageAs represented in Fig. 1, the liver samples from the control group (A) and the PRP group (B) showed normal cellular structures with distinct hepatic cells and sinusoidal spaces. The liver of acetaminophen-treated group (C) exhibited significantly injury (Fig. 1C), widespread necrosis with acute inflammation and hepatic tissue structure arrangement are present. Compared with the acetaminophen group samples, the low dosage of PRP pretreatment group (D) showed low density of cellular lesions, and had less neutrophil infiltration and subtle necrotic. But pretreatment with middle or high dosage of PRP had no significant improvement compared with acetaminophen model group. Even liver pathological changes significantly increased in high dosage group, the range of necrosis with acute inflammation in liver tissues increased significantly.
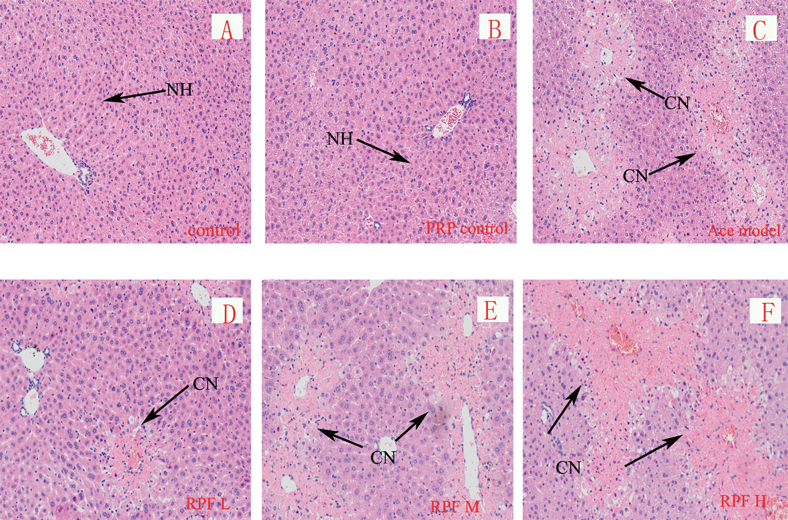
Control (A), PRP control (B), acetaminophen model (C) and PRP pretreatment from low to high dosage (D–F) groups. Normal hepatocytes (NH), Centrilobular necrosis (CN), Representative liver sections of each group are shown (original magnification: A–F, 100).
As the results in Table 1, the serum biochemical parameters of PRP group (B) compared to control has no significant different (p>0.05). In acetaminophen intoxicated mice (C), both AST and ALT activities were significantly elevate (p<0.05 vs. control). Pre-treatment of low dosage PRP to the model mice (D), AST and ALT activities were slightly decreased compared to model group (p<0.05) (C). But there was no significant difference in group (E) compared to model mice (C), the activities of AST and ALT even increased after pre-treatment of high dosage of PRP (F).
| Animal groups | Control group (A) | PRP alone (B) | Acetaminophen group (C) | PRP pre-treated group (D) | PRP pre-treated group (E) | PRP pre-treated group (F) |
|---|---|---|---|---|---|---|
| AST (U/L) | 199.3±10.2 | 210±17.3 | 15474±175.2* | 11278±121.4*,** | 14564±177.1* | 18020±201.8*,** |
| ALT (U/L) | 75.6±10.3 | 77.4±8.9 | 11934±144.2* | 7692±107.2*,** | 10598±155.8* | 15826±211.4*,** |
| T-BIL (µmol/L) | 1.2±0.04 | 0.9±0.03 | 3.7±0.80* | 3.2±0.32* | 6.3±1.28*,** | 9.6±2.17*,** |
| D-BIL (µmol/L) | 0.5±0.03 | 0.5±0.01 | 1.5±0.27* | 1.1±0.30*,** | 4.3±1.12*,** | 7.6±1.81*,** |
| TBA (µmol/L) | 7.6±0.8 | 8.1±1.1 | 49.4±4.2* | 36.8±3.4*,** | 59.5±7.2*,** | 92.9±17.4*,** |
| γ-GT (U/L) | 2.26±0.51 | 2.18±0.46 | 3.78±0.67* | 2.93±0.42*,** | 4.55±0.57*,** | 5.67±0.71*,** |
| ALP (U/L) | 275±17.8 | 301±22.8 | 487±42.7* | 366±37.2*,** | 498±57.9* | 567±67.1*,** |
Data are reported as mean±S.D. (n=10 in each group). * p<0.05 versus control group. ** p<0.05 versus acetaminophen group.
Alkaline phosphates (ALP) and γ-glutamyl trans-peptidase (γ-GT) levels, which are essential enzymes in cholestasis progress, significantly increased in acetaminophen-treated mice than in control (p<0.05). The serum levels of ALP and γ-GT slightly decreased when given low dose PRP to acetaminophen-intoxicated mice (p<0.05), but significantly increased in high dose group compared with model mice (p<0.05) (Table 1). It is important to note that PRP did not affect the ALP or γ-GT lever in normal mice.
Serum total bilirubin (TBIL), direct bilirubin (DBIL) and total bile acid (TBA) are the crucial indices of bile markers. The activity of total bilirubin (T-BIL) value was increased in acetaminophen model group compared to control (p<0.05). Pretreatment of low dose PRP could slightly decrease the levels of TBA and DBIL (p<0.05), but we note that T-BIL and TBA were significantly elevated after pretreatment of middle or high dosage PRP (Groups E and F), compared to acetaminophen intoxicated mice (C) (p<0.05). However, neither the T-BIL nor TBA was changed in single PRP group (B).
The Effect of PRP Alleviates Hepatic Injury Independent of Nrf2 Anti-oxidation PathwayExposure to acetaminophen significantly increased MDA concentrations, and the concentration of GSH decreased markedly in the mice liver, when compared to control mice (p<0.05). This result suggesting that lipid peroxidation was induced by acetaminophen poisoning. At various doses, the PRP treatment could not decrease the MDA concentration or restore the GSH level in acetaminophen-intoxicated mice. SOD and GSH-Px activities in model group were significantly lower. The same as MDA, pretreatment of PRP to acetaminophen-intoxicated mice could not enhance the activities of antioxidant enzymes in liver under any given dose of PRP (Table 2).
| Animal groups | Control group (A) | PRP alone (B) | Acetaminophen group (C) | PRP pre-treated group (D) | PRP pre-treated group (E) | PRP pre-treated group (F) |
|---|---|---|---|---|---|---|
| MDA (mmol/mg prot) | 1.53±0.06 | 1.51±0.08 | 2.87±0.52* | 2.67±0.37* | 2.48±0.67* | 3.11±0.49* |
| SOD (U/mg prot) | 41.42±2.21 | 42.21±3.72 | 22.43±3.32* | 25.13±2.65* | 26.37±1.84* | 27.32±2.99* |
| GSH (mg/g prot) | 52.51±2.45 | 48.25±3.71 | 31.21±4.24* | 35.48±2.75* | 29.22±6.47* | 33.17±4.11* |
| GSH-Px (U/mg prot) | 682.4±23.4 | 667.1±31.2 | 512.4±29.7* | 565.7±11.4* | 533.4±33.8* | 545.7±38.9* |
Data are reported as mean±S.D. (n=10 in each group). * p<0.05 versus control group.
Immunohistochemistry and protein quantitative assay results also showed that, Nrf2 was significantly raised in acetaminophen-intoxicated mice, but pretreatment of PRP in model mice resulted in no significant influence on Nrf2, and also, PRP had no significant effect on normal mice (Figs. 2, 3).
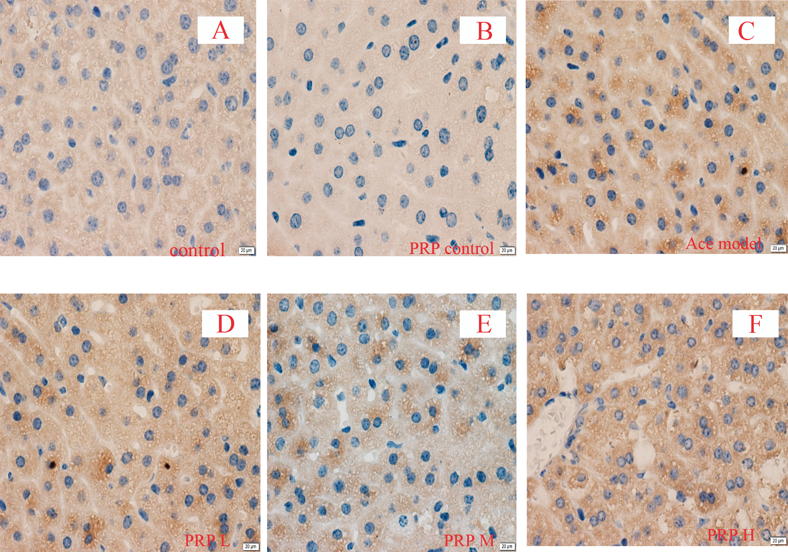
Original magnification ×400.
** p<0.01, * p<0.05.
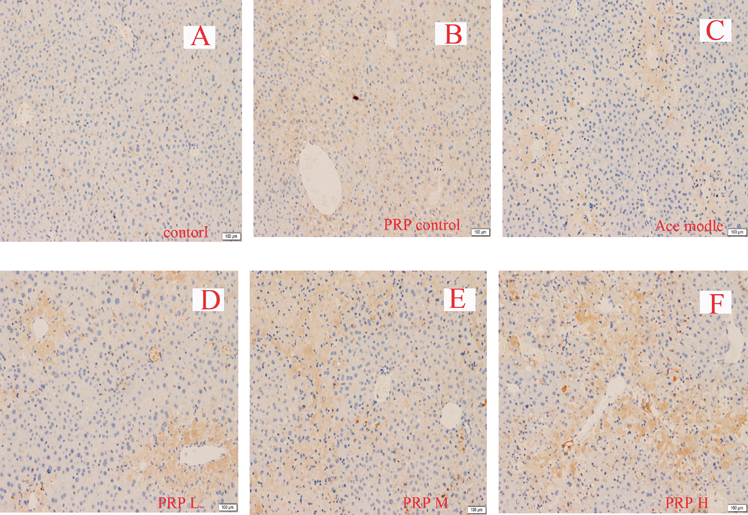
Immunohistochemical data showed that Mrp2 and Bsep in control mice livers were found on canalicular membranes in hepatocytes. Mrp2 staining had no significant difference in the acetaminophen-intoxicated mice compared to, but Bsep staining was obviously less than the control mice. Co-administration of low dosage of PRP markedly increased Bsep and Mrp2 staining compared with acetaminophen-intoxicated mice alone, but significantly decreased in middle or high dosage of PRP group. Mrp3 staining was less in normal liver tissue, while significantly increased in liver damage. PRP could significantly increase the content of Mrp3 when hepatic injury, and with dose dependently (Figs. 4–6).
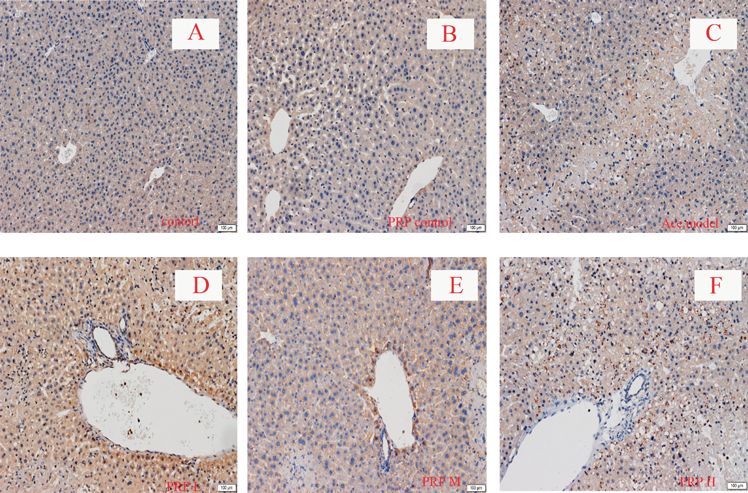
Original magnification ×100.
Original magnification ×100.
Original magnification ×100.
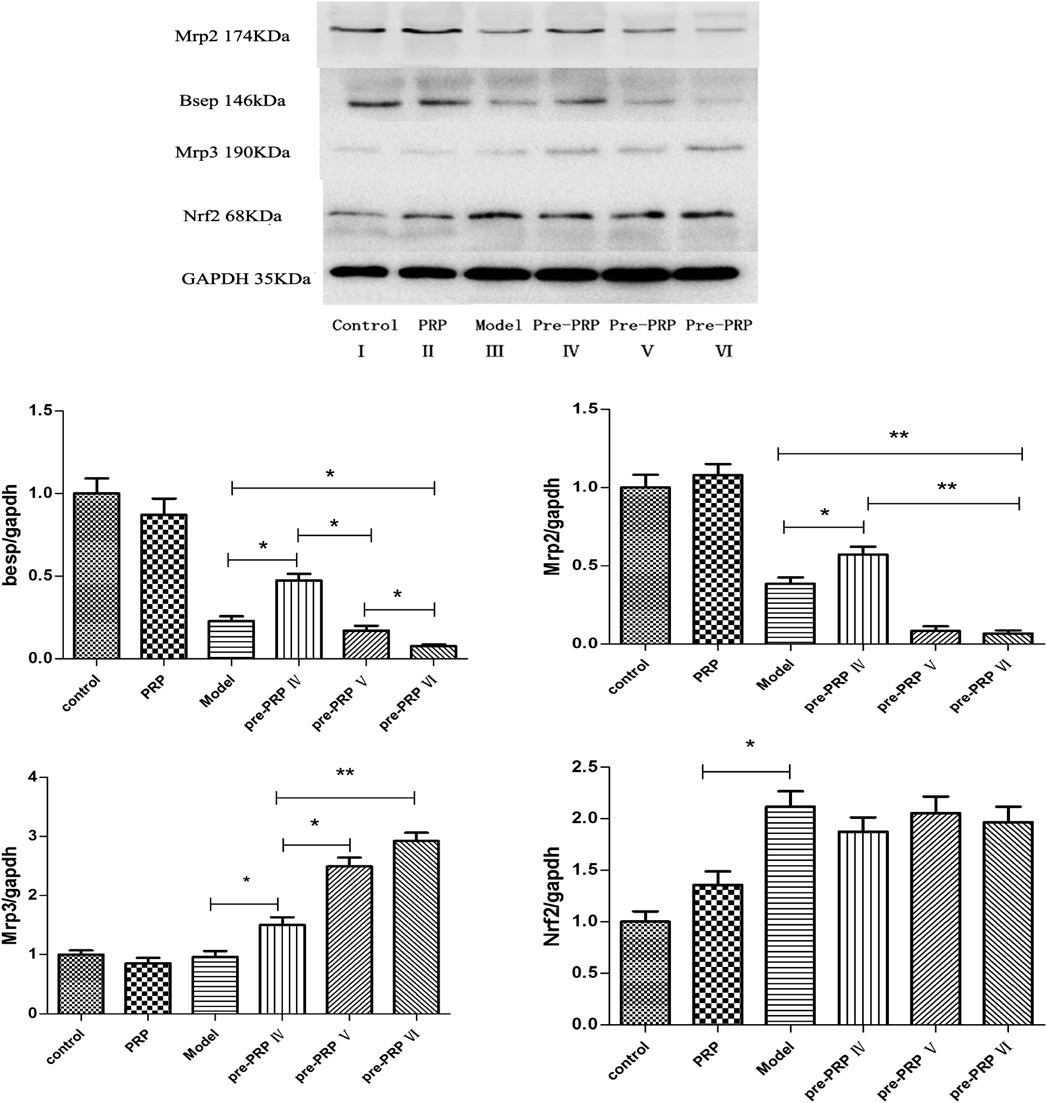
We also determined the potential influence of the protein expression of Mrp2, Mrp3 and Bsep by Western blot analysis. As demonstrated in Fig. 3, PRP inhibited Mrp2 and Bsep expression in liver with middle and high dosage but up-regulation of Mrp2 and Bsep only in low dosage. Meanwhile, the expression of Mrp3 in liver was significantly induced in PRP pretreatment groups and increased with the dosage. It’s also important to need note that PRP had no significant impact on normal liver, but its influence significantly enlarged under liver injury (Fig. 3).
PRP has been used for the treatment of hepatic disease and always in combinative form with other herbs such as Banxia–Xiexin-Decoction, Banxia–houbu-Decoction and Banxia–Kucao-Decoction since ancient times. These traditional Chinese medicines were used for the treatment of acute and chronic gastroenteritis, chronic colitis, hepatitis, and early hepatocirrhosis.11) Therefore it should be more meaningful to study the hepatoprotective/hepatotoxin active of PRP.
Serum ALT and AST are indication of stabilization of plasma membrane with hepatic tissue damages caused by acetaminophen.22) However, PRP as a therapeutic drug for clinical liver diseases, contrary to routine recognition, the ALT and AST activities were no significantly declined when pretreatment middle dosage of PRP to acetaminophen-intoxicated mice, the activities even significantly increased in high dose PRP group. ALT and AST were slightly decreased compared with model mice only in low dosage. It should be noted that high dosage of PRP did not induce liver damage in normal mice, but could promote liver damage in mice which had undergone liver injury.
The hepatic toxicity of acetaminophen probably depends on the formation of N-acetyl-p-benzoquinone imine (NAPQI), a highly electrophilic metabolite. Subsequently, NAPQI binds to cellular proteins and causes GSH depletion and oxidative stress, which may trigger signaling pathways that cause mitochondrial toxicity and ultimately result in cell injury.23,24) In order to reveal the antioxidant activity of PRP, SOD, MDA, GSH-Px and GSH were detected. Furthermore, Nrf2, acts through the antioxidant response element (ARE) to regulate the expression of many intracellular detoxifying and antioxidant genes was also detected.25) Studies have shown that PRP has certain antioxidant activity in vitro.26) Our results showed that the levels of SOD, GSH, GSH-Px were significantly reduced and the level of MDA was increased in acetaminophen treated mice compared with control group. Pretreatment with PRP had no significant change in the SOD, GSH, GSH-Px and MDA in acetaminophen-induced hepatic injury. The result indicated that PRP showed no significantly antioxidant activity against acetaminophen-induced oxidative damage. Nrf2 is the key protein of the body to resist oxidative stress. Nrf2 regulates the expression of antioxidant proteins via interaction with the ARE. Under normal conditions, the cytoplasmic Nrf2 mostly combines with Keap1 and is rapidly degraded by the ubiquitin-proteasome. Oxidative stress or stimulation of nucleophilic substances may trigger dissociation of Nrf2 from Keap1 and release free Nrf2. Nrf2 is the key protein to resist the oxidative stress. Immunohistochemical and Immunoblot analysis results also showed that PRP had no significant influence on the nuclear transfer of Nrf2 in liver tissue.
Hyperbilirubinaemias can result from any defects in the steps of bilirubin metabolism. Thus overproduction, impaired uptake and conjugation lead to unconjugated/indirect hyperbilirubinaemia, and impaired excretion of bilirubin from damaged hepatocytes or bile ducts leads to direct/conjugated hyperbilirubinaemia.27) The present study showed that hepatic injury by acetaminophen resulted in the elevation of T-BIL/D-BIL and total bile acid (TBA) levels, which could reflect a pathological alteration in biliary flow. ALP and γ-GT as essential enzymes in cholestasis progress were also significantly increased in acetaminophen-treated mice compared with control mice (p<0.05). Compared with acetaminophen group, the serum levels of ALP and γ-GT activities slightly decreased in low dose PRP group (p<0.05), but significantly increased in high dose group (p>0.05) (Table 1). D-BIL and TBA were also elevated significantly when pretreatment middle or high dose of PRP. Meanwhile, ALP and γ-GT, bilirubin and bile acids were not significantly changed when given PRP to normal mice.
Mrp2, a member of the ABC transporter family, transports organic anions residing on hepatocellular bile canalicular (BC) membranes, it plays an indispensable role in the biliary excretion of a wide variety of organic anions, including glutathione, glutathione conjugates, and sulfated and glucuronidated bile acids. Together with the function of the bile salt export pump (in humans, BSEP; in rodents, Bsep), promoting the combined bile salts with renal excretion when cholestasis.28–30) Mrp3 exists in the liver cell side of the base film, weak expression in normal liver, but significant expression when cholestasis. It is the major transporter to absorb the bile salts from bile, and then excretes to portal vein circulation when cholestasis.28,31) In this experiment, Mrp2, Mrp3 and Bsep were detected by immunohistochemistry and protein quantitative assay. The results showed that pretreatment low dosage of PRP significantly increased Mrp2, Bsep protein expressions in model group, which was helpful to ameliorate cholestasis in mice with liver injury. But in middle or high dosage group, the Mrp2 and Bsep protein expressions were significantly inhibited. The result directly influenced the elimination process of bilirubin from the liver. Meanwhile, it was worth noting that PRP also induced the expression of Mrp3 in the liver when damage occurred, and in a dose dependent manner. Therefore, the patients with clinical liver injury should pay enough attention to the treatment of PRP. Studies have shown that Mrp3 could be up-regulated by Nrf2 in acetaminophen liver damage. In addition, Nrf2 also participates in the adjustment of Mrp2 and Bsep in liver.32,33) but our study showed that the regulation of Mrp2, Mrp3 and Bsep by PRP had no correlation with Nrf2.
Nuclear bile acid receptor (FXR) plays a key role in mediating the effects of bile acids synthesis and transport. Bsep expression strictly depends on the presence of FXR. Mrp2 and Mrp3 also could regulate by FXR.34,35) Studies have shown that inflammation is involved in the regulation of FXR inflammation may provide a microenvironment to reduce the expression of FXR. Hepatic nuclear factor 1α (HNF1α) was major regulator on FXR. Proinflammatory cytokines, such as tumor necrosis factor alpha (TNFα), interleukin-1 beta (IL-1β), and IL-6 could reduce the FXR expression via inhibiting the transactivity of HNF1α.36–38) TNFα and IL-1β also could reduce the expression of BESP by unregulated the FXRα1/ FXRα2 ratio.39) At the same time, studies have shown that nuclear factor-kappa B (NF-κB) is also involved in the regulatory role of FXR.40) Our research showed that when single administration of PRP could not change the expression levels of these bile acid transporters. So whether the regulation of bile acid metabolism by PRP is involved in FXR or changes the inflammatory state of the liver still needs to be further explored. At the same time, as a clinical drug, the role of PRP in the herbal formulae in TCM needs to be further studied.
PRP as traditional herb medicine always combined with other herbs used for the treatment of hepatic disease. Our present studies found that PRP slightly alleviated acetaminophen-induced hepatic damage only in low dosage, high dosage of PRP led to aggravate liver damage instead, but nontoxic to normal mice. Meanwhile, PRP significantly regulated bile acids metabolic in hepatic injury mice, but not in normal mice. So as a traditional hepatic disease treatment medicine, the dosage needs to be paid attention to in clinical use. Both the role of PRP in the treatment of hepatic disease combined with other medicines and the potential side effects need to be further researched.
This article was supported by the National Natural Science Foundation of China (Nos. 81503327, 81403170 and 81274171).
The authors declare no conflict of interest.