Abstract
Cocaine-associated environmental cues elicit craving and relapse to cocaine use by recalling the rewarding memory of cocaine. However, the neuronal mechanisms underlying the expression of cocaine-associated memory are not fully understood. Here, we investigated the possible contribution of γ-aminobutyrate (GABA)ergic neurons in the nucleus accumbens (NAc), a key brain region associated with the rewarding and reinforcing effects of cocaine, to the expression of cocaine-associated memory using the conditioned place preference (CPP) paradigm combined with designer receptors exclusively activated by designer drugs (DREADD) technology. The inhibitory DREADD hM4Di was selectively expressed in NAc GABAergic neurons of vesicular GABA transporter-Cre (vGAT-Cre) mice by infusing adeno-associated virus (AAV) vectors. Ex vivo electrophysiological recordings revealed that bath application of clozapine-N-oxide (CNO) significantly hyperpolarized membrane potentials and reduced the number of spikes induced by depolarizing current injections in hM4Di-positive NAc neurons. Additionally, systemic CNO injections into cocaine-conditioned mice 30 min before posttest session significantly reduced CPP scores compared to saline-injected mice. These results indicate that chemogenetic inhibition of NAc GABAergic neurons attenuated the expression of cocaine CPP, suggesting that NAc GABAergic neuronal activation is required for the environmental context-induced expression of cocaine-associated memory.
Cocaine addiction is a chronic relapsing disease characterized by compulsive drug seeking and taking. The rewarding and reinforcing properties of cocaine are readily associated with the environmental contexts where cocaine is experienced. The cocaine-associated environmental cues elicit craving and relapse to cocaine use by recalling the rewarding memory of cocaine. However, the mechanisms by which environmental cues induce the expression of cocaine-associated memory are not fully understood. To investigate these mechanisms, the conditioned place preference (CPP) test, a widely used Pavlovian conditioning paradigm is considered to be useful.1) In the conditioning session of the CPP, animals learn to associate the rewarding effects of cocaine and a neutral environmental context that has been paired with cocaine administration by investigators. Thereafter, in the posttest session, animals exhibit a preference for the cocaine-paired environment, likely due to the expression of cocaine-associated memory. Although previous studies revealed that the mesocorticolimbic reward system consisting of the ventral tegmental area (VTA), medial prefrontal cortex (mPFC), and nucleus accumbens (NAc) is involved in the expression of cocaine-associated memory,2–4) the precise neuronal and circuit mechanisms underlying this behavior remain unclear.
The NAc plays an important role in the expression of cocaine-seeking behavior and cocaine-associated memory.5–7) Recent studies have shown that activation of glutamatergic projections from the prelimbic subregion (PL) of the mPFC to the NAc core is required for cue-induced reinstatement of cocaine seeking.8,9) In addition, intra-NAc infusion of an AMPA receptor antagonist was found to block the expression of cocaine CPP.2) These findings suggest that excitation of the NAc is involved in the expression of cocaine-associated memory. Considering that the vast majority of NAc neurons are γ-aminobutyrate (GABA)ergic medium spiny neurons (MSNs),10,11) these GABAergic neurons are likely to contribute to the expression of cocaine CPP. Thus, in the present study, we addressed this issue using designer receptors exclusively activated by designer drugs (DREADD) technology.12) We selectively expressed Gi/o-coupled inhibitory DREADD hM4Di in NAc GABAergic neurons of vesicular GABA transporter-Cre (vGAT-Cre) mice, and examined the effects of inhibition of these neurons with systemic clozapine-N-oxide (CNO) injections on the expression of cocaine CPP.
MATERIALS AND METHODS
AnimalsMale vGAT-Cre mice (C57BL/6J background; 6–10 weeks) were bred in-house at Kanazawa University, and group-housed at a constant ambient temperature (22±2°C) under 12-h light/dark cycle (lights on 08 : 45) with food and water available ad libitum. All experiments were performed with the approval of the Institutional Animal Care and Use Committee at Kanazawa University.
DrugsCocaine hydrochloride (Takeda Pharmaceutical, Osaka, Japan) and CNO (Cayman Chemical, Ann Arbor, MI, U.S.A.) were dissolved in saline. For electrophysiological experiments, CNO, picrotoxin (Sigma, St. Louis, MO, U.S.A.), and kynurenic acid (Sigma) were dissolved in recording solution.
Preparation of the Adeno-Associated Virus (AAV) VectorLent-X 293T cells were transfected with pAAV-hSyn-DIO-hM4Di-mCherry (#44362, Addgene, Cambridge, MA, U.S.A.), pAAV-DJ, and pHelper using polyethylenimine (polyethylenimine “Max,” Polysciences, Warrington, PA, U.S.A.), and 72 h after transfection, the cells were gently scraped with a gradient buffer (15 mM NaCl, 1 mM Tris, and 1 mM MgCl2). The buffer was freeze-thawed four times between liquid nitrogen and a 55°C water bath to break the cell membrane. The lysates were treated with benzonase nuclease (Sigma), and cell debris was removed by centrifugation at 3000×g for 15 min. The supernatants containing AAV vector were purified using discontinuous iodixanol (Opti Prep, Sigma) gradient (15, 25, 40, and 58%). After ultracentrifugation for 105 min at 48000 rpm (50.2Ti rotor; Beckman Coulter, Brea, CA, U.S.A.), the viral fraction was extracted from the interface between the 40 and 58% layers. The purified AAV vector was aliquoted, and stored at −80°C.
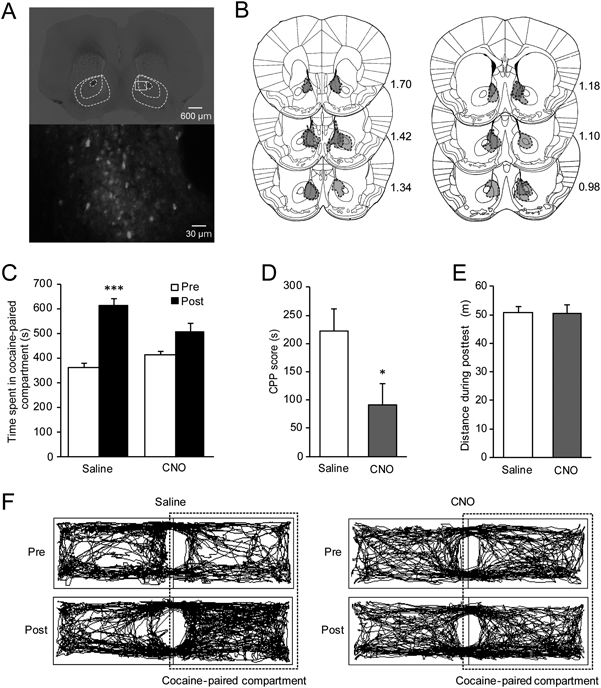
AAV Vector InfusionUnder chloral hydrate anesthesia (400 mg/kg, intraperitoneally (i.p.); Nacalai tesque, Kyoto, Japan), mice were infused with 0.5 µL of AAV-DJ-hSyn-DIO-hM4Di-mCherry bilaterally into the NAc (AP: +1.4 ML: ±0.8 DV: −4.3 from bregma).13) For behavioral analyses, the vector was infused at a rate of 0.075 µL/min, while the vector was infused at a rate of 0.1 µL/min for electrophysiological experiments. The spread of the vector was similar between the two injection speeds. After infusions, mice were housed individually, and maintained for more than 2 weeks to allow expression of viral constructs.
Slice Preparation and ElectrophysiologyMice were anesthetized with isoflurane and transcardially perfused with ice-cold modified Ringer’s solution containing (in mM): N-Methyl-D-glucamine (NMDG), 92; glucose, 25; N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 20; NaHCO3, 30; CaCl2, 0.5; MgSO4·7H2O, 10; KCl, 2.5; NaH2PO4·2H2O, 1.25; Thiourea, 2.0; ascorbate, 5.0; pyruvate, 3.0; N-Acetyl-L-cysteine, 12; and bubbled with 95% O2/5% CO2 (pH 7.4 with HCl). Brains were removed and coronal slices (250 µm thick) containing the NAc were made using a microslicer (VT1200S, Leica, Wetzlar, Germany), and incubated at 32–34°C for 10 min and then kept at room temperature for 5 min in a chamber containing modified Ringer’s solution. Afterward, slices were stored with standard Ringer’s solution containing (in mM): NaCl, 125; KCl, 2.5; NaH2PO4, 1.25; MgCl2, 1.0; CaCl2, 2.0; NaHCO3, 26; and glucose, 25; and bubbled with 95% O2/5% CO2 (pH 7.4) at room temperature. The slices were mounted in a recording chamber on an upright microscope (BX-51WI, Olympus, Tokyo, Japan). The chamber was continuously superfused with standard Ringer’s solution at a flow rate of 2–2.5 mL/min. Whole-cell current-clamp recordings were obtained from the NAc neurons by visual control of patch pipettes, which were prepared from borosilicate glass capillaries and filled with an internal solution containing (in mM): KOH, 150; KCl, 10; MgCl2, 2; Na2ATP, 2; Na3GTP, 0.3; ethylene glycol bis (2-aminoethyl ether)-N,N,N′,N′-tetra acetic acid (EGTA), 0.2; HEPES, 10 and spermine, 0.1 (pH 7.3 with gluconic acid). To stain the recorded neurons, biocytin (1–3 mg/mL; Sigma) was also contained in the internal solution. The resistance of the electrodes was 3–9 MΩ in the Ringer’s solution. All recordings were performed at 32–34°C.
At the start of recordings, membrane potentials were adjusted at approximately −70 mV by current injections in the presence of kynurenic acid (2 mM) and picrotoxin (50 µM), which block ionotropic glutamate and GABAA receptors, respectively. Membrane potentials were measured in the periods of 0–30 s before and 90–120 s after CNO (10 µM) application. To evaluate changes in firings, a series of 400-ms depolarizing current pulses (40 pA steps, 0–280 pA) were injected and the number of evoked spikes was counted before and after CNO application.
Conditioned Place PreferenceThe CPP apparatus consisted of two equal-sized compartments (15×24×30 cm) with distinct tactile and visual cues. One compartment was white with a textured floor and the other one was black with a smooth floor. Each mouse was handled 1 min/d for 2 consecutive days before the CPP test. In morning (habituation) and afternoon (pretest) sessions on day 1, mice were allowed to explore two compartments freely for 900 s, and time spent in each compartment during the exploration period was measured using Smart 3.0 software (Panlab Harvard Apparatus, Holliston, MA, U.S.A.). Mice that spent more than 80% (>720 s) of the total time in one compartment in the pretest or that showed a difference of >200 s in time spent in one compartment between the habituation and pretest were eliminated from the following procedures. We used a bias-like protocol14) and designated the compartment in which each mouse spent less time (<450 s) in the pretest as the cocaine-paired compartment for that animal. On day 2–5 (conditioning), mice were given an i.p. injection of saline (10 mL/kg) and immediately confined to non-cocaine-paired compartment for 30 min. After at least 4 h, each mouse received i.p. cocaine (20 mg/kg) injection and then confined to the cocaine-paired compartment for 30 min. On day 6 (posttest), mice were given an i.p. injection of either saline or CNO (1 mg/kg). Thirty minutes later, mice were allowed to freely explore the two compartments for 900 s, and the time spent in each compartment during the exploration period were measured. The CPP scores were calculated by subtracting the time spent in the cocaine-paired compartment during the pretest from that during the posttest.
HistologyHistological analyses were performed to confirm viral expression in the NAc. Briefly, under chloral hydrate anesthesia, mice were transcardially perfused with 0.1 M phosphate-buffered saline (pH 7.4) followed by 4% paraformaldehyde. Brains were removed, post-fixed in the same fixative, cryoprotected with 15% sucrose, and then frozen in powdered dry ice. Coronal sections (30 µm) were prepared on a cryostat, mounted on slides and coverslipped with Fluoromount-G (SouthernBiotech, Birmingham, AL, U.S.A.). Images were obtained with a fluorescent microscope (BZ-9000; Keyence, Osaka, Japan). Data from mice with failed expression were excluded from the statistical analyses.
Statistical AnalysesData are expressed as means±standard error of the mean (S.E.M.) and were compared using paired t-test, Student’s t-test, or two-way repeated measures ANOVA followed by the Holm–Sidak post hoc test using GraphPad Prism 6 (GraphPad software, La Jolla, CA, U.S.A.). Differences with p<0.05 were considered statistically significant.
RESULTS
Activation of hM4Di Suppresses the Activity of GABAergic Neurons in the NAcWe first examined the effects of hM4Di activation by CNO on membrane potential and spiking activity of NAc neurons using whole-cell recordings from hM4Di-mCherry-positive neurons that were obtained from vGAT-Cre mice bilaterally infused with AAV-DJ-hSyn-DIO-hM4Di-mCherry into the NAc (Fig. 1A). Bath application of CNO (10 µM) hyperpolarized hM4Di-mCherry-positive neurons in a small but significant manner (Control, −70.0±0.4 mV; CNO, −71.3±0.5 mV, t5=2.855, p=0.0356, paired t-test; Figs. 1B, C). In addition, CNO application significantly reduced the number of spikes induced by depolarizing current injections (160 to 280 pA; interaction, F6,18=6.394, p=0.0010; CNO, F1,3=12.57, p=0.0382; injected current, F6,18=9.791, p<0.0001; Figs. 1D, E). These results indicate that CNO application inhibits the activity of hM4Di-mCherry-positive NAc neurons.
Chemogenetic Inhibition of GABAergic Neurons in the NAc Attenuates Cocaine CPP ExpressionTo determine the role of NAc GABAergic neurons in cocaine CPP expression, vGAT-Cre mice were infused with AAV-DJ-hSyn-DIO-hM4Di-mCherry into the NAc more than 2 weeks before the pretest (Figs. 2A, B). To inhibit NAc neuronal activity during the posttest, we injected CNO (1 mg/kg) or saline 30 min before the posttest. In saline-treated mice, the time spent in the cocaine-paired compartment was significantly longer during the posttest session (615±25.8 s) than during the pretest session (364±15.1 s; t5=7.86, p=0.0005, paired t-test; Figs. 2C, F). On the other hand, in CNO-treated mice, no significant difference was observed in the time spent in the cocaine-paired compartment between the posttest (506±35.7 s, n=7) and pretest sessions (416±14.1 s; t6=2.361, p=0.0562, paired t-test; Figs. 2C, F). Consistent with these findings, CNO injections significantly reduced CPP scores compared to saline-treated mice (saline, 222±39.3 s; CNO, 90.9±38.5 s; t11=2.37, p=0.0372, Student’s t-test; Fig. 2D). We confirmed that there was no significant difference in locomotor activity during the posttest between CNO- and saline-treated mice (saline, 50.68±2.36 m; CNO, 50.55±2.91 m; t11=0.03251, p=0.9746, Student’s t-test; Figs. 2E, F). These results indicate that NAc GABAergic neuronal activity is critical for the expression of cocaine CPP.
DISCUSSION
Our electrophysiological analyses revealed that CNO-induced activation of hM4Di in the NAc GABAergic neurons dramatically reduced the number of spikes evoked by depolarizing current injections, although CNO produced only a small, but significant, hyperpolarization of membrane potential. Similar reductions in firing were reported in neurons of the retrosplenial cortex15) and the mPFC.16) These results suggest that CNO-induced activation of hM4Di reduces NAc GABAergic neuronal activity through the suppression of neuronal firing.
Previous studies suggest that enhanced glutamatergic transmission from the mPFC to NAc may mediate the activation of NAc GABAergic neurons and consequently promote cocaine CPP expression. Pharmacological silencing of the VTA, the origin of dopaminergic afferents to the mPFC and NAc, and intra-mPFC infusion of a dopamine D1 receptor (D1R) antagonist blocked the expression of cocaine CPP.4) Additionally, the induction of c-Fos, a marker of neuronal activation, in the PL mPFC was associated with cocaine CPP expression.4) Moreover, optogenetic stimulation of VTA-mPFC projections was found to increase c-Fos expression in the PL mPFC in a D1R-dependent manner.4) These findings imply that D1R-mediated mPFC neuronal activation is critical for the expression of cocaine CPP. Consistent with this, dopamine-dependent activation of PL mPFC glutamatergic neurons that project to the NAc core is involved in cue-induced reinstatement of cocaine self-administration.8) Moreover, activation of glutamatergic projections from the PL mPFC to NAc core is necessary for cue-induced reinstatement of cocaine seeking and induction of synaptic potentiation of MSNs in the NAc.9) Considering that intra-NAc infusion of an AMPA receptor antagonist blocks cocaine CPP expression,2) these findings suggest that enhanced glutamatergic transmission and subsequent activation of NAc GABAergic neurons mediate the expression of cocaine-associated memory, although Brown et al.17) demonstrated that exposure of rats to an environment that had previously been paired with cocaine did not induce the expression of c-Fos in the NAc.
A previous study demonstrated that in control mice which express GFP in the lateral nucleus of amygdala, CNO injection did not affect the expression of cocaine CPP,18) suggesting that CNO itself does not produce the deficit in spatial cognition-associated behavior. Thus, although we did not examine the effect of CNO on cocaine CPP expression in control mice, the suppressive effects of CNO on the expression of cocaine CPP in hM4Di-expressing mice may not be due to nonspecific effects of CNO.
The MSNs in the NAc express either D1R (D1R-MSNs) or dopamine D2 receptor (D2R-MSNs). D1R-MSNs and D2R-MSNs play opposing roles in cocaine-associated behaviors. Optogenetic activation of D1R-MSNs during cocaine conditioning enhances cocaine CPP, while activation of D2R-MSNs attenuates cocaine CPP.19) Moreover, chemogenetic inhibition of D1R-MSNs, but not D2R-MSNs, before the posttest blocks the expression of cocaine CPP.20) Thus, although we expressed hM4Di in all vGAT-positive GABAergic neurons in the NAc, these findings suggest the involvement of subpopulations of MSNs in the expression of cocaine CPP. However, it should be noted that recent studies have shown that silencing NAc GABAergic parvalbumin (PV) interneurons blocks CPP induced by amphetamine,21) while optogenetic activation of NAc PV interneurons produces aversion.22) Thus, further studies are needed to examine the contribution of NAc PV interneurons to the expression of cocaine-associated memory.
Contrary to the present results, Wang et al.23) reported that optogenetic stimulation of NAc GABAergic neurons blocked cocaine CPP expression in vGAT-channelrhodopsin-2 (ChR2)-enhanced yellow fluorescent protein (EYFP) mice in which ChR2-EYFP fusion protein is expressed in GABAergic neurons under the control of the vGAT promoter. Differences in experimental design could explain the contradicting results. In the present study, the expression of hM4Di was limited to NAc GABAergic neurons, but not GABAergic neurons projecting to the NAc, because the retrograde transport efficacy of the AAV-DJ serotype used in the present study is low.24) Thus, CNO injections may selectively inhibit NAc GABAergic neurons. On the other hand, in vGAT-ChR2-EYFP mice, all GABAergic neurons in the brain express ChR2. Therefore, optogenetic stimulation activates not only NAc GABAergic neurons, but also afferent GABAergic fibers to the NAc, which makes it difficult to interpret the net effect of photo-stimulation on NAc GABAergic neuronal activity.
In conclusion, the present study demonstrated that chemogenetic inhibition of NAc GABAergic neurons attenuated the expression of cocaine CPP, indicating that NAc GABAergic neuronal activation is required for the environmental context-induced expression of cocaine-associated memory.
Acknowledgments
This study was supported by Grant-in-Aid for Scientific Research (C) (K.K., 15K06765) from the Japan Society for the Promotion of Science (JSPS), Hoansha Foundation (K.K.), Astellas Foundation for Research on Metabolic Disorders (K.K.) and The Natio Foundation (K.K.).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol., 12, 227–462 (2007).
- 2) Kaddis FG, Uretsky NJ, Wallace LJ. DNQX in the nucleus accumbens inhibits cocaine-induced conditioned place preference. Brain Res., 697, 76–82 (1995).
- 3) Ranaldi R. Dopamine and reward seeking: the role of ventral tegmental area. Rev. Neurosci., 25, 621–630 (2014).
- 4) Shinohara F, Kamii H, Minami M, Kaneda K. The Role of dopaminergic signaling in the medial prefrontal cortex for the expression of cocaine-induced conditioned place preference in rats. Biol. Pharm. Bull., 40, 1983–1989 (2017).
- 5) Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci., 2, 119–128 (2001).
- 6) Nestler EJ. Cellular basis of memory for addiction. Dialogues Clin. Neurosci., 15, 431–443 (2013).
- 7) Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur. J. Pharmacol., 526, 65–76 (2005).
- 8) McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G. Prelimbic to accumbens core pathway is recruited in a dopamine-dependent manner to drive cued reinstatement of cocaine seeking. J. Neurosci., 36, 8700–8711 (2016).
- 9) Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct. Funct., 221, 1681–1689 (2016).
- 10) Gerfen CR. Synaptic organization of the striatum. J. Electron Microsc. Tech., 10, 265–281 (1988).
- 11) Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann. N. Y. Acad. Sci., 877 (1 ADVANCING FRO), 140–156 (1999).
- 12) Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U.S.A., 104, 5163–5168 (2007).
- 13) Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Third ed., Elsevier, Burlington, MA (2007).
- 14) Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol., 56, 613–672 (1998).
- 15) Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, Bucci DJ. Chemogenetic silencing of neurons in retrosplenial cortex disrupts sensory preconditioning. J. Neurosci., 34, 10982–10988 (2014).
- 16) Perova Z, Delevich K, Li B. Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. J. Neurosci., 35, 3201–3206 (2015).
- 17) Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J. Neurosci., 12, 4112–4121 (1992).
- 18) Hsiang HL, Epp JR, van den Oever MC, Yan C, Rashid AJ, Insel N, Ye L, Niibori Y, Deisseroth K, Frankland PW, Josselyn SA. Manipulating a “cocaine engram” in mice. J. Neurosci., 34, 14115–14127 (2014).
- 19) Lobo MK, Covington HE III, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science, 330, 385–390 (2010).
- 20) Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc. Natl. Acad. Sci. U.S.A., 113, 2726–2731 (2016).
- 21) Wang X, Gallegos DA, Pogorelov VM, O’Hare JK, Calakos N, Wetsel WC, West AE. Parvalbumin interneurons of the mouse nucleus accumbens are required for amphetamine-induced locomotor sensitization and conditioned place preference. Neuropsychopharmacology, 43, 953–963 (2018).
- 22) Qi J, Zhang S, Wang HL, Barker DJ, Miranda-Barrientos J, Morales M. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat. Neurosci., 19, 725–733 (2016).
- 23) Wang L, Shen M, Yu Y, Tao Y, Zheng P, Wang F, Ma L. Optogenetic activation of GABAergic neurons in the nucleus accumbens decreases the activity of the ventral pallidum and the expression of cocaine-context-associated memory. Int. J. Neuropsychopharmacol., 17, 753–763 (2014).
- 24) Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, Huang CC, Gerfen CR, Schiller J, Dudman JT, Hantman AW, Looger LL, Schaffer DV, Karpova AY. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron, 92, 372–382 (2016).