2019 年 42 巻 2 号 p. 299-302
2019 年 42 巻 2 号 p. 299-302
While the use of in vitro-transcribed mRNA (IVT-mRNA) in therapeutics is a rapidly expanding area, the transfection of the exogenous IVT-mRNA is accompanied by a risk of immune activation. This immunological defense mechanism suppresses cellular translation process and can reduce transfection efficiency to a considerable extent. In the present study, we investigated the in vitro effects of Integrated Stress Response Inhibitor (ISRIB), and dexamethasone, a steroidal anti-inflammatory drug, on the transfection activity of a lipid nanoparticle (LNP) that was composed of ionizable lipids and IVT-mRNA. In the case of transfection to mouse embryonic fibroblast (MEF) cells, ISRIB mainly enhanced the transfection activity at an early stage of transfection (0–6 h). In contrast, dexamethasone caused an increase in transfection activity at intermediate-late stages of transfection (4–48 h). We also investigated the in vivo effects of dexamethasone using an LNP on that the IVT-mRNA and lipid-conjugated dexamethasone (Dex-Pal) were co-loaded. The intravenous administration of the LNP successfully enhanced the protein expression in a mouse liver by up to 6.6-fold. Collectively, the co-delivery of an anti-inflammatory drug is a promising approach for enhancing transfection efficiency of IVT-mRNA.
The usage of in vitro-transcribed mRNA (IVT-mRNA) as a tool for introducing proteins into cells/organs is now rapidly expanding in the field of biology. Since the IVT-mRNA is a vulnerable and membrane-impermeable molecule, the development of a delivery system that can efficiently deliver IVT-mRNA to the cytoplasm of a cell is prerequisite. We developed a series of ionizable lipids (SS-cleavable and pH-activated lipid-like material; ssPalm) that is equipped with hydrophobic scaffolds, proton-sponging tertiary amines and cleavable disulfide bonding for achieving the cytoplasmic delivery of nucleic acids.1–3) The ssPalm can form an mRNA-encapsulating lipid nanoparticle that possesses a neutral surface charge in a physiological environment (LNPssPalm). Once taken up by cells, the LNPssPalm develops a positive charge via protonation of the tertiary amines in the acidic endosomal compartments. The positive charge acts as a driving force for endosomal escape and allows the IVT-mRNA to reach the cytoplasm. In the cytoplasm, the cleavage of the disulfide bonding in the ssPalm by natural reducing agents in cells (i.e., glutathione) accelerates the collapse of the LNPssPalm and efficient translation.
The transfection of exogenous IVT-mRNA is attended by a risk of activating innate immunity. Pattern recognition receptors (PRRs) such as endosomal Toll-like receptors (TLRs) and cytoplasmic RIG-I like receptors (RLR) are responsible for this immune activation.4) This self-adjuvant effect makes the IVT-mRNA suitable for nucleic acid vaccines against cancers/infections. However, the immune activation induced by the IVT-mRNA would be detrimental for other applications since the cellular anti-viral responses causes significant down-regulation in cellular translation. One strategy for avoiding immune activation and for enhancing enhance protein production is to reduce the immune activating properties of the IVT-mRNA. This strategy includes the purification of the IVT-mRNA5) and/or the incorporation of chemically modified nucleotides.6) Another strategy is the combination use of low molecular weight immune-suppressive molecules during the transfection of the IVT-mRNA.7) In this study, we investigated the effects of Integrated Stress Response Inhibitor (ISRIB) and dexamethasone, a steroidal anti-inflammatory drug, on the transgene activity of the LNPssPalm both in vitro and in vivo.
IVT-mRNA coding luciferase (Luciferase-mRNA, 1931 bases including 5′/3′ untranslated regions) was prepared using mMESSAGE mMACHINE T7 Ultra Kit (Fig. S1). The LNPssPalm used in the present study was composed of ssPalmO-P4C2 (COATSOME® SS-18/4PE-13, Fig. 1a), a helper lipid (1,2-dioleoyl sn-glycero-3-phosphoethanolamine; DOPE), a cholesterol, and a polyethylene glycol conjugated lipid (1-(monomethoxy polyethyleneglycol2000)2,3-dimyristoylglycerol; DMG-PEG2000). The LNPssPalm (ssPalmO-P4C2/DOPE/cholesterol/DMG-PEG2000 = 30/30/40/3) was 64.6 ± 18.3 nm in size with a unimodal size distribution (Fig. S2). The encapsulation efficiency was 79.3 ± 4.8% and the surface charge was neutral (Table 1).

b) Cumulative luciferase activity from 0 to 6 h. c) Cumulative luciferase activity from 0 to 48 h. MEF cells were transfected with the LNPssPalm (0.4 µg of mRNA). Luciferase activity was monitored hourly starting immediately after transfection. The cumulative luciferase activity was normalized by a control group (PBS treated). The data are represented as the mean ± standard deviation (S.D.) (n = 3). Statistical analyses were performed by One-way ANOVA followed by Dunnett’s test (*; p < 0.05, **; p < 0.01). d) Relative luciferase was activity normalized by a control group. The data are represented as the average values of three independent experiments.
| Sample | Size (nm)a) | PdIa) | Zeta-potential (mV)a) | Encapsulation efficiencyb) |
|---|---|---|---|---|
| LNPssPalm | 64.6 ± 18.3 | 0.199 ± 0.098 | −2.1 ± 1.6 | 79.3 ± 4.8 |
| LNPssPalm(Dex-Pal) | 64.8 ± 18.2 | 0.147 ± 0.032 | −2.3 ± 4.7 | 78.8 ± 1.6 |
a) Particle properties were determined using dynamic light scattering. b) Encapsulation efficiency was determined by Ribogreen® assay.
The LNPssPalm corresponding to 0.4 µg of the IVT-mRNA was transfected to mouse embryonic fibroblast (MEF) cells that intrinsically express a series of cytoplasmic nucleic acid sensors.8) The transfection experiments were carried out with or without 200 nM ISRIB, 100 nM dexamethasone, or a combination of these compounds. Luciferase activity was monitored at hourly intervals starting immediately after transfection. The cumulative luciferase activity was calculated and normalized by the luciferase activity of control cells (phosphate buffered saline (PBS) treated). The cumulative luciferase activity during an early stage of transfection (0–6 h, Fig. 1b) indicated that ISRIB enhanced the transfection activity of the LNPssPalm by 1.4-fold. On the other hand, the total luciferase activity (0–48 h, Fig. 1c) indicated that dexamethasone eventually increased the transfection activity by 1.6-fold. The relative luciferase activities at each time point, normalized values compared to those without drug treatment (PBS-control group), are shown in Fig. 1d. The time-dependency for the variation in luciferase activity indicated that ISRIB mainly enhances translation at an early stage of transfection (1–6 h) while dexamethasone exerts a long-lasting enhancing effect on transgene activity (4–48 h). The use of the combination of ISRIB and dexamethasone clearly indicated a biphasic up-regulation of translation. In the following in vivo application, dexamethasone was selected as an enhancer of transfection since the enhancing effect of dexamethasone was superior to that of ISRIB.
An in vivo demonstration of the enhancing effects on transfection efficiency by the small molecule is highly important for extending this strategy to practical applications. In the case of dexamethasone, the molecule readily dissociates from LNPssPalm in blood circulation because of its low hydrophobicity. Thus, for achieving the in vivo co-delivery of the IVT-mRNA and dexamethasone to the same cells/organs, the hydrophobicity of the drug would need to be increased. We previously reported that dexamethasone-palmitate (Dex-Pal), an active ingredient in a rheumatoid arthritis medicine, can be successfully mounted in the lipid structure of an LNPssPalm (LNPssPalm(Dex-Pal)).9) Thus, we evaluated the effects of Dex-Pal co-loading on the in vivo hepatic transfection efficiency after intravenous administration. The physicochemical properties of the particle were not altered regardless of whether or not the system contained co-loaded Dex-Pal (Table 1). Mice were injected with the LNPssPalm or LNPssPalm(Dex-Pal) at a dose of 0.25 mg/kg of mRNA. The dose of dexamethasone palmitate was 0.65 mg/kg in dexamethasone amount. At 3 h after administration, the luciferase activity in the liver was determined. As a result, in both cases of Balb/c mice and C57BL6/J mice, protein production was augmented (Fig. 2, 2.7-fold and 6.6-fold, respectively).
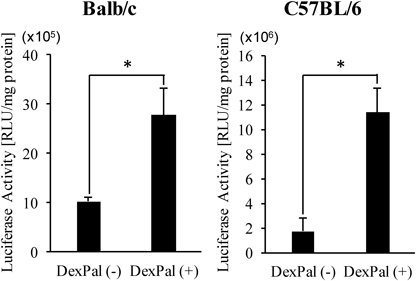
An LNPssPalm modified with or without 15% of Dex-Pal (LNPssPalm(Dex-Pal)) was intravenously administrated via the tail vein. Mice were injected with the LNPssPalm or LNPssPalm(Dex-Pal) at a dose of 0.25 mg/kg of mRNA. The dose of the dexamethasone palmitate was 0.65 mg/kg in dexamethasone amount. At 3 h after administration, the mouse liver was exercised, and the luciferase activity was determined. The data are represented as the mean ± S.D. (n = 3). Statistical analyses were performed by Student’s t-test (*; p < 0.05). The data are represented as the average values of three independent experiments.
The combination use of immune suppressive reagents for improving IVT-mRNA transfection process has been originally investigated in the area of regenerative medicine. For the successful development of induced pluripotent stem (iPS) cells, repetitive transfection of IVT-mRNA is generally needed. However, the repetitive transfection of the IVT-mRNA resulted in a development of severe cytotoxicity due to the cellular anti-viral responses. It was reported that the knockdown of Ifnb1, Eif2ak2 (PKR), and Stat2 mRNA resulted in the complete rescue of the cells after the repetitive transfection.10) Furthermore, Warren et al. reported that the combination use of chemically modified nucleotides (5-methylcytidine and pseudouridine) and a vaccinia virus-derived decoy receptor for interferon β (IFNβ) (B18R) allowed the repetitive transfection of the IVT-mRNA.11) These reports clearly indicated that the suppression of the type-I interferon signaling is necessary for the repetitive transfection. However, it was also reported that small molecule inhibitors of type-I interferon signaling showed no enhancing effects on the overall production of proteins in single transfection of IVT-mRNA.12) On the other hand, Awe et al. reported that inhibitors of nuclear factor-kappaB (NF-κB) signaling, BAY11-708 and BX795, significantly elevated the transfection efficiency of the IVT-mRNA.13) Other possible targets are the proteins that are involved in integrated stress responses (ISRs), since cellular stresses including the recognition of exogenous RNA induce the suppression of canonical cap-dependent translation via the phosphorylation eIF2α .
In this study, we investigated the effects of ISRIB, a unique small molecule that counteracts the phosphorylation of eIF2α14), and dexamethasone, a widely used anti-inflammatory drug. Neither ISRIB nor dexamethasone affected cellular viability (Fig. S3a), the cellular uptake of the LNPssPalm (Fig. S3b) or the homogeneity of protein expression (Fig. S3c). It is thus assumed that the changes in transfection activity reflected the changes in the translation activity of the cells. The results for ISRIB indicated that the transgene activity of the IVT-mRNA was enhanced at the early stages of transfection, up to 6 h. In contrast to ISRIB, dexamethasone caused a long-lasting up-regulation of the transgene activity starting at 4 h. Since the expression of the luciferase gradually increased and culminated at 20 h after transfection (Fig. S4), dexamethasone would be expected to contribute to total protein production more effectively.
The up-regulation of translation by ISRIB can be attributed to its counteracting the effects on the phosphorylation of eIF2α and the subsequent formation of stress granules. On the other hand, detailed molecular mechanisms regarding the up-regulation of translation by dexamethasone remained to be clarified. However, a previous report indicated that BAY-11, an inhibitor of NF-κB signaling, also increased the expression of IVT-mRNA13) Thus, it is probable that the up-regulation of translation by dexamethasone was mainly due to its inhibition of the NFκB pathway. Although the combination of ISRIB and dexamethasone indicated a clear biphasic up-regulation of translation in both early and intermediate-late stages of transfection (Fig. 1d), no additive or synergistic increase in luciferase activity was observed. Detailed molecular mechanisms responsible for the up-regulation of translation, especially in the case of dexamethasone, will be necessary to develop a clear understanding of this observation.
Taken our findings and previous literatures into account, we speculated that the cellular responses against transfected IVT-mRNA involved three stages on different time scales; [i] The PKRs are immediately activated after transfection and subsequently down-regulate cap-dependent translation via the phosphorylation of eIF2α (on the order of minutes to hours), [ii] The inflammatory responses induced by the activation of NF-κB inhibit an mRNA translation process (on the order of tens of hours), and [iii] The type-I interferon signaling mediates an establishment of the anti-viral immunity that is accompanied with the lethal cellular signaling in case of repetitive transfections (on the order of days).
For the in vivo application of dexamethasone as an enhancer for the transfection of IVT-mRNA, we incorporated Dex-Pal into the LNPs for the stable co-loading. We recently reported that the combination of Dex-Pal improved the transfection activity of DNA-encapsulating LNPs by 25-fold. In this situation, the co-loading of Dex-Pal significantly reduced the cytokine production triggered by the i.v. injection of DNA-encapsulating LNPs. Therefore, the use of Dex-Pal can be a common strategy for improving the in vivo transfection activity of DNA and RNA.
In conclusion, we demonstrate herein that the combination of dexamethasone resulted in the enhancement in protein production after the transfection of IVT-mRNA. The co-delivery of dexamethasone can be successfully applied to the in vivo mRNA delivery by using the hydrophobic derivative of dexamethasone. Collectively, this process is a potent strategy for the use in gene therapy based on IVT-mRNA.
Detailed materials and methods including sequence information of mRNAs are described in Supplementary Materials.
Animal ExperimentsThe experimental protocols were reviewed and approved by the Chiba University Animal Care Committee in accordance with the “Guide for Care and Use of Laboratory Animals.”
T. Ohto, M. Konishi, and H. Tanaka contributed equally to this work. The authors wish to thank Dr. M. S. Feather for his helpful advice in writing the English manuscript.
T. Ohto, M. Konishi, K. Onomoto, M. Yoneyama, Y. Nakai, and H. Yoshioka declare no conflict of interest. H. Tanaka, K. Tange, and H. Akita have a patent (PCT/JP2012/079160) for ssPalm chemicals. H. Tanaka received research Grant from JSPS KAKENHI [Grant numbers 17H06558, 18K18377]. H. Akita received research Grant from JST CREST [Grant number JPMJCR17H1], and the Asahi Glass Foundation.
The online version of this article contains supplementary materials.