2019 年 42 巻 7 号 p. 1155-1163
2019 年 42 巻 7 号 p. 1155-1163
A conjugate between chondroitin sulfate (CS) and glycyl-prednisolone (GP), named CS-GP, was produced by carbodiimide coupling at a high GP/CS ratio. CS-GP was not water-soluble and gave a nanogel (NG) in aqueous solution. Two types of nanogels, NG(I) and NG(II), with prednisolone (PD) contents of 5.5 and 21.1% (w/w), respectively, were obtained. They had particle sizes of approximately 280 and 570 nm, respectively, and showed negative ζ-potentials of approximately −40 mV. The PD release rate was slower in the nanogels than in a solution of CS-GP with a PD content of 1.4% (w/w). The PD release rate was slower in NG(II) than in NG(I), and was elevated at pH 7.4 than at pH 6.8. NG(II) was applied in vivo to rats with trinitrobenzene sulfonic acid (TNBS)-induced colitis, and its therapeutic efficacy and pharmacokinetic features were investigated. The therapeutic efficacy of NG(II) was slightly better than that of PD alone. Drug delivery to the lower intestines was enhanced with NG(II). The CS-GP nanogel has potential as a potent DDS for the treatment of ulcerative colitis.
Autoimmune diseases, such as rheumatoid arthritis and ulcerative colitis, are major health issues because they are chronic, often refractory, and difficult to treat.1–5) Many intractable diseases are closely related to autoimmune diseases. Although bio-pharmaceuticals, including antibody drugs, have recently been developed as novel highly active agents against autoimmune diseases, they may cause severe toxicities in rare cases, and their cost has become an obstacle in the economics of medicine.6,7) Therefore, conventional anti-inflammatory drugs are important in pharmacotherapy for these autoimmune diseases. Glucocorticoid drugs (GCs) play an important role in the treatment of rheumatoid arthritis and ulcerative colitis because they rapidly and strongly exert suppressive effects.8–11) However, since GCs may cause severe systemic side effects, including diabetes, osteoporosis, and adrenal failure, at frequent and high dosages, their use is limited.12–14) These side effects are caused by systemic absorption, namely, a non-selective body distribution.15–17)
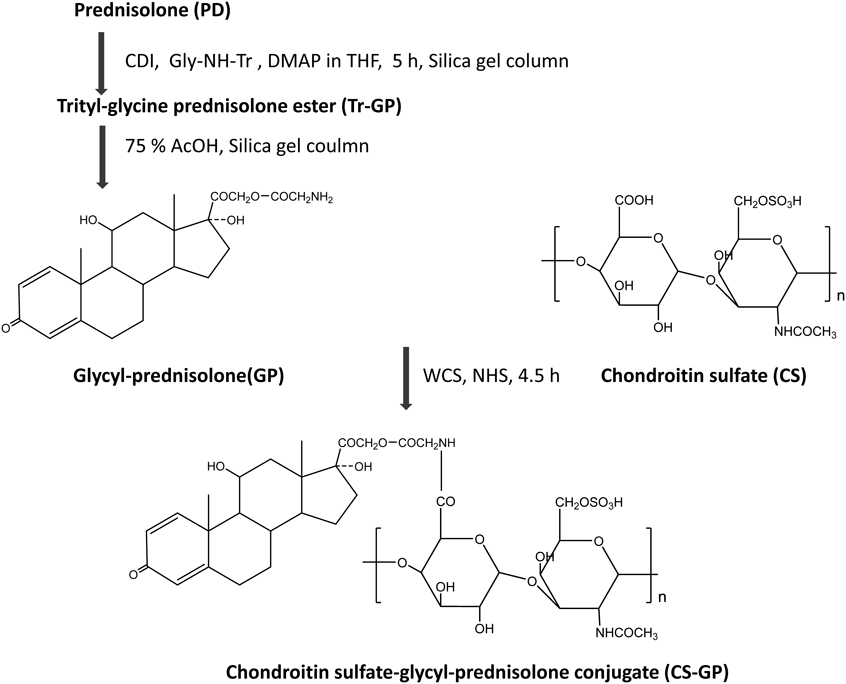
Drug delivery systems (DDSs) have recently been attracting attention because they improve the behaviors of drugs. For example, micro- or nano-size devices have been developed to modify therapeutic features.18,19) DDSs have been developed for GCs, such as prednisolone (PD) and betamethasone (BM), by several research groups. We also previously developed a chondroitin sulfate-glycyl-prednisolone conjugate (CS-GP). This conjugate enhanced the therapeutic efficacy of treatments in animal models of rheumatoid arthritis.20) Furthermore, CS-GP functioned as a macromolecular prodrug of PD and promoted its localization to inflammatory sites.21) In those studies, CS-GP with a low drug content of 4% was used and mostly dissolved in saline. However, CS-GP with a PD content of more than 4–5% (w/w) was suspended in aqueous solution. This may be due to the low water solubility of the PD moiety of CS-GP. Conjugates of water-soluble polysaccharides and hydrophobic organic molecules, such as cholesterol, appear to have the ability to form nanogels due to the hydrophobic interaction of the latter groups and the formation of a water-soluble outer layer by the former groups.22–25) As CS-GP has similar chemical structures to them, the nanoparticulate products were considered to be formed by hydrophobic assembly of PD. Therefore, in this study, the CS-GP nanoparticles were called CS-GP nanogels (NGs). CS-GP was found to form a nanogel in the presence of a moderate or high load of PD in CS-GPs. The CS-GP nanogels (NGs) can be prepared simply and reproducibly by the combination with a specified amount of PD. In addition, the NG was expected to clearly exhibit reproducible release profiles, dependent on the stability of the ester, mainly on pH values; therefore, the NG biodistribution can be synchronized with the drug action due to the release of free PD there. These points were considered to be advantage of the present NG. In the present study, NGs were prepared and their particle characteristics and in vitro release were examined. The therapeutic efficacy, toxic side effects, and pharmacokinetic characteristics of the nanogels were also investigated in vivo using rats with trinitrobenzene sulfonic acid (TNBS)-induced colitis.26–28)
Chondroitin sulfate C (CS) sodium salt (CS-Na; MW 40000, derived from shark cartilage, N and S contents = 2.6 and 6.6%, respectively, and a ratio of 6-sulfate to 4-sulfate of 9 : 1 (mol/mol)), PD, N,N-carbonyldiimidazole (CDI), 4-dimethylaminopyridine (DMAP), N-hydroxysuccinimide (NHS), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (WSC), and tetrahydrofuran (THF) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). N-Trityl-glycine (Tr-G) and TNBS were purchased from Sigma Chemical Company (St. Louis, MO, U.S.A.). All other chemicals were of reagent grade.
AnimalsMale Wistar rats (7 weeks old, 200–210 g) were purchased from Tokyo Laboratory Animal Science Co., Ltd. (Tokyo, Japan), and used in in vivo experiments. Animals were kept on the breeding diet MF supplied by Oriental Yeast Co., Ltd. (Tokyo, Japan) with water available ad libitum in a room in which temperature and relative humidity were maintained at 23 ± 1°C and 60 ± 1%, respectively. The light-dark cycle was 12 h. The experimental protocol was approved by the Committee on Animal Research of Hoshi University (Tokyo, Japan), and animal experiments were performed according to the Guiding Principles for the Care and Use of Laboratory Animals of Hoshi University.
Instruments1H- and 13C-NMR spectra were measured with a JEOL Lambda-500 (500 MHz) spectrometer, in which TMS was used as a reference with a chemical shift of 0 ppm. UV-Vis absorption spectra were recorded using a Beckman DU640 spectrophotometer. Particle sizes and ζ-potentials were analyzed using an ELS-Z2 apparatus (Otsuka Electronic Co., Ltd., Osaka, Japan).
Synthesis of CS-GPCS-GP was synthesized as described previously and shown in Fig. 1.20,29) In the first step, GP was synthesized as an intermediate compound. The Tr-G ester of PD, named Tr-GP, was synthesized as follows. PD (270 mg) was reacted with Tr-G (477 mg) using CDI (243 mg) as a catalytic agent in 10 mL THF containing DMAP (15 mg) at 0°C for the initial 0.5 h, and at room temperature for the subsequent 4.5 h. After evaporation of the solvent, the residue was washed with methanol, and Tr-GP was recrystallized using methanol. The Tr-GP (300 mg) obtained was dissolved in 75% (v/v) aqueous acetic acid (10 mL) by heating at 75°C. Immediately after the white precipitate appeared, the mixture was cooled on ice and kept for 30 min. After the white precipitate was removed by filtration, the solvent of the filtrate was evaporated. The residue was dissolved in several milliliters of a mixture of chloroform and methanol (15 : 1, (v/v)) and subjected to column chromatography with a silica gel column using a mixture of chloroform and methanol (15 : 1, (v/v)) as the elution solvent. The fractions in which Rf was 0.23 [chloroform–methanol (15 : 1, (v/v))] were collected, and the solvent was evaporated to obtain the product, GP. The chemical structure of GP was confirmed from 1H- and 13C-NMR spectra, as previously reported.29)
CS and GP were then conjugated by carbodiimide coupling with WSC in the different formulations. These reaction formulations are shown in Table 1. In the first formulation, CS (100 mg), dissolved in 5 mL water, was mixed with 5 mL THF solution containing GP (100 mg). After NHS (300 mg) and WSC (400 mg) were added to the mixture, the resultant solution was stirred at room temperature for 24 h. The solution was then chromatographed using a Sephadex G50 column with 0.1 M NaCl as the elution solvent. Macromolecular fractions were gathered and dialyzed extensively against water using a cellulose tube. The residue in the tube was then lyophilized to yield the first conjugate, CS-GP(1). In the second formulation, CS (60 mg) was dissolved in 4 mL of water, and the solution was added to THF solution (6 mL) containing GP (120 mg). After NHS (360 mg) and WSC (480 mg) were added, the conjugate was purified in a similar manner. The suspension remaining in the cellulose tube was lyophilized to obtain the second conjugate, CS-GP(2). The chemical structure of each conjugate was confirmed by 1H-NMR spectra.
| Conjugate | CS (mg) | GP (mg) | WSC (mg) | NHS (mg) | Yield (mg) | PD content (%) |
|---|---|---|---|---|---|---|
| CS-GP(1) | 100 | 100 | 800 | 600 | 102 | 5.5 |
| CS-GP(2) | 60 | 120 | 480 | 360 | 83 | 21.1 |
The PD content of each conjugate was investigated as follows. CS-GP was added to 0.1 M NaOH aqueous solution. After the mixture was heated at 45°C for 10 min, it was centrifuged and the supernatant was measured spectrophotometrically at 246 nm. The content of PD was calculated from the absorbance of free PD in 0.1 M NaOH aqueous solution.
Preparation of NanogelsSince CS-GP(1) and CS-GP(2) were suspended in water, they were used to produce nanogels. Nanogels were prepared by dissolving CS-GP in the water–THF mixture and subsequently evaporating the organic solvent. After 5 mg of CS-GP(1) was dissolved in 2 mL of the water–THF (1 : 1, (v/v)) mixed solution, the organic solvent was removed by evaporation at 35°C using a rotary evaporator. The nanogel obtained was named NG(I). The nanogel was also produced in a similar manner using CS-GP(2). A total of 30 mg of CS-GP(2) was dissolved in 6 mL of the THF–water (1 : 1, (v/v)) mixture, and THF was then evaporated at 35°C using a rotary evaporator. The resultant nanogel was called NG(II).
Particle CharacteristicsThe particle sizes and ζ-potentials of nanogels were investigated using an ELS-Z2 dynamic light scattering apparatus (Otsuka Electronic Co., Ltd.).
In Vitro Release StudiesThe solution form of CS-GP with PD content of 1.4% (w/w), NG(I), and NG(II) were incubated at 37°C and physiological pH 7.4, and then, the release rates were compared among them. Each preparation was diluted so that the total PD concentration was 20 µg/mL in phosphate buffered saline (PBS). At appropriate time points, aliquots (100 µL) were withdrawn. Immediately after each sampling, 0.1 M acetate buffer of pH 4 (200 µL) was added, and the mixture was centrifuged using a Millipore ultrafilter unit (MW cut-off 30000; Millipore, U.S.A.). The filtrate was analyzed by HPLC to assess PD concentrations.
Regarding NG(II), release rates were compared between pH 6.8 and 7.4. NG(II) suspensions were prepared using the Japanese Pharmacopoeia (JP) second fluid (pH 6.8) and PBS (pH 7.4), and incubated in the same manner as described above. The released PD was examined after sampling at appropriate time points. Furthermore, after CS-GP(2) was dissolved in the mixed solution of buffer–THF (1 : 1, (v/v)), the resultant solution was incubated in a similar manner to investigate the release of PD.
NG(II) was also incubated in the Japanese Pharmacopoeia (JP) first fluid (pH 1.2) and its second fluid (pH 6.8), and release profiles were examined. Release studies were performed in a similar manner to that described above.
In Vivo Studies on Efficacy and Toxic Side EffectsIn the in vivo experiments, therapeutic efficacy and toxic side effects were investigated. As to NGs, NG(II), with high drug content, was used because it could be administered at moderate particle concentration. The animal disease model was produced using rats as follows: After rats had been fasted for 48 h, TNBS (20 mg) dissolved in 0.25 mL of 50% (v/v) ethanol was instilled into the colon of each rat 8 cm from the anus with a catheter to induce ulcerative colitis.26–28) Three days after the TNBS treatment, rats were divided into the following four groups (n = 3): Control (saline), PD suspension in saline (5 mg/kg), NG(II) aqueous suspension (5 mg PD eq./kg), and CS aqueous solution (24 mg/kg). Healthy rats were used as the fifth group. Administration was performed via gastric intubation once daily for three consecutive days. Five days after the final administration, rats were sacrificed by excessive ether anesthesia, and the colon and thymus were excised.
Therapeutic efficacy was evaluated from the ratio of (colon weight)/(body weight) and visible severity of colitis. Immediately after the collection of tissues, (proximal colon weight: Cp)/(body weight: B) and (distal colon weight: Cd)/(body weight: B) were examined. Regarding the severity of colitis, stool consistency (SC) and colonic damage were observed visually. SC was scored as 0, 2, and 4 for the pellet form, soft form (not stuck to the anus), and liquid form (stuck to the anus), respectively. Colonic damage scores (CDS) were given as follows: 0, no damage; 1, hyperemia with no ulcers; 2, one small ulcer with no inflammation; 3, one small ulcer with inflammation; 4, two or more small ulcers with inflammation; 5, Ulcers including large (> 1 cm) one with inflammation. The ratio of (thymus weight: T)/(body weight: B) was investigated as an index of the toxic side effects.
Studies on Gastrointestinal Distribution after Oral IngestionTransfer in the gastrointestinal (GI) tract was investigated after the intragastric administration of NG(II) at 5 mg PD eq/kg. Regarding GI tract transfer, drug biodisposition was examined for free and total (incorporated and free) PD in each GI tract region 4, 8 and 24 h after administration. Briefly, the whole content of each GI part was removed with a spatula and phosphate-buffered saline (pH 7.4) was added as follows: 8 mL to the contents of the stomach (ST), cecum (CE), and distal colon (CD), and 4 mL to the contents of the proximal small intestine (SIP), distal small intestine (SID), and proximal colon (CP). The resultant suspension was homogenized with a glass homogenizer with a Teflon pestle.
Free PD in the homogenate was assessed as follows: 100 µL of saturated NaCl aqueous solution, 100 µL of 6% (w/v) phosphoric acid, and 4 mL of the mixture of t-butylmethyl ether and pentane (3 : 2, (v/v)) were added to 100 µL of the final tissue homogenate, and the resultant mixture was shaken vigorously. Two milliliters of the organic phase were dried under nitrogen gas at room temperature. The resultant residue was dissolved in the HPLC mobile phase, and analyzed for PD by HPLC. The amount of total (free and incorporated) PD in the content suspension was measured as follows: 50 µL of 0.1 M NaOH aqueous solution was added to 50 µL of the content suspension and maintained at 45°C for 10 min to hydrolyze the ester. The extraction of PD and preparation of samples for the HPLC assay were then performed in the same manner. The recovery study was conducted by the addition of a known amount of PD to drug-free tissue homogenates in the range around the observed concentration. The recovery ratio was calculated as the ratio of the observed amount to the calculated amount, and used for data correction.
The colonic delivery ratio (%) was calculated as the ratio of (total PD amount in (CP + CD)) to (total PD amount administered).
Studies on Plasma Concentration after Oral IngestionFour days after the treatment with TNBS, rats were fasted for 24 h. NG(II) was then administered intragastrically at a dose of 5 mg PD eq./kg as a suspension in saline (0.5 mL). Immediately before and 0.5, 1, 2, and 5 h after administration, blood samples (0.3 mL) were withdrawn via the jugular vein under light anesthesia with ethyl ether inhalation. Plasma was obtained after the centrifugation of blood at 3000 rpm for 10 min. After plasma (100 µL) and water (100 µL) had been mixed with a vortex mixer, 140 µL of methanol was added and also mixed. Trichloroacetic (40 µL) was then added, and the mixture was shaken vigorously for 2 min. After the mixture was centrifuged at 3500 rpm for 10 min, the supernatant was collected. Twenty microliters of the resultant supernatant was analyzed by HPLC. PD concentrations were calculated using the absolute calibration curve method.
HPLC AssayHPLC assays were performed at room temperature. Regarding the apparatus, a Shimadzu LC-6AD pump was used with a Shimadzu SPD-10AV VP UV-Vis detector set at a wavelength of 246 nm and a Shimadzu C-R7A plus Chromatopac (Shimadzu, Kyoto, Japan).
In in vitro studies, a YMC Pack ODS-AM column (i.d. of 6 mm, length of 150 mm; YMC Co., Ltd., Kyoto, Japan) was used as the analytical column. A column (i.d. of 6 mm, length of 250 mm; YMC Co., Ltd.) was employed in drug distribution studies as the analytical column. In both experiments, a 26% (v/v) 2-propanol aqueous solution containing 0.1% (v/v) trifluoroacetic acid was used as the mobile phase, and the flow rate was set at 0.9 mL/min with an injection volume of 40 µL. PD concentrations were assessed using the absolute calibration curve.
In the plasma concentration assay, a YMC Pack ODS-AM column (6 mm i.d., 150 mm length; YMC Co., Ltd.) was used as the analytical column. A mixture of acetonitrile and 50 mM citrate buffer with pH adjusted to 4.1 with phosphoric acid (35 : 65, (v/v)) was used as the mobile phase. The flow rate was set at 1 mL/min and an injection volume of 20 µL. PD concentrations were assessed using an absolute calibration curve.
Statistical AnalysisData were compared using ANOVA followed by the Dunnett’s post hoc test. In all cases, a significant difference was set as p < 0.05.
CS-GP was synthesized according to the scheme shown in Fig. 1. The chemical structures of GP and conjugates were confirmed by 1H-NMR spectra. As reported previously,26) ester formation at the C21 position of PD was confirmed, and the 1H-NMR spectra of the conjugate showed a pattern in which the 1H-NMR spectrum of GP was superimposed on that of CS. The formulations, yields, and PD contents of the CS-GP conjugates are shown in Table 1. Conjugates with PD contents of 5.5 and 21.1% (w/w) were named CS-GP(1) and CS-GP(2), respectively. Neither conjugate was soluble in water because PD contents were not low. However, each conjugate dissolved in a mixture of water and an organic solvent such as THF. As reported previously,20) the CS-GP conjugate, produced under the formulation of CS (120 mg), GP (15 mg), NHS (300 mg), and WSC (500 mg), had a PD content of only 1.4% (w/w) and was water-soluble. On the other hand, conjugates with a PD content of more than 3–4% (w/w) appeared to give a suspension in water, in which the suspension particles showed a submicron size.
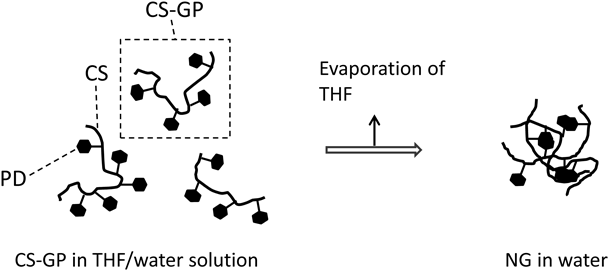
The nanoparticles were formed by hydrophobic assembly of PD. The nanoparticle formation was similar to that of nanogels such as cholesterol-bearing pullulan23) and hexadecenoic acid-bearing glycol chitosan.24) In this study, the CS-GP nanoparticles were called CS-GP nanogels (NGs). The nanogel preparation was performed as follows. The obtained conjugates were dissolved in an organic solvent-water mixture. Then, by removing the organic solvent from the conjugate solution, a nanogel was formed due to the association of hydrophobic molecules, as shown in Fig. 2. The nanogels prepared using CS-GP(1) and CS-GP(2) were called NG(I) and NG(II), respectively. Their particle characteristics are shown in Table 2. NG(I) with a PD content of 5.5% had a particle mean size of 276 nm and mean ζ-potential of −40.5 mV. NG(II) had a mean size of 570 nm and its ζ-potential was −41.1 mV. The size distributions of NG(I) and NG(II) are shown in Fig. 3. Elevations in the content of PD increased the particle size to some extent, while the ζ-potential was not influenced. These particle features were reproducible.
| Nanogel | CS-GP(mg) | Solvent volume (mL/mL) | Particle sizea) (nm) | ζ-Potentiala) (mV) |
|---|---|---|---|---|
| NG(I) | 5 | 1/1 | 276 ± 51 | −40.5 ± 14.7 |
| NG(II) | 30 | 3/3 | 570 ± 6 | −41.1 ± 3.5 |
a) The results are expressed as the mean ± S.D. (n = 3).
A: NG(I), B: NG(II). Size distributions were shown as light scattering intensities.
Release profiles in PBS at 37°C were examined for NG(I) and NG(II). Results were compared with previous findings obtained from CS-GP solution,20) prepared using CS-GP with a PD content of 1.4% (w/w) (Fig. 4). Release from CS-GP solution reached approximately 80% after 24 h. The release rate was suppressed for NG(I) and NG(II). The release rate of NG(I) was slower, with approximately 30% being liberated at 24 h, while NG(II) showed an initial burst at approximately 10%, followed by a markedly slower release. Thus, PD release was found to be suppressed in the nanogel state. This result suggested the poorer cleavage of the ester bond of the GP moiety in the nanogel and/or the trapping of the released PD inside the nanogel. Since NG(II) had a markedly higher content of PD, PD residues were considered to aggregate more tightly, resulting in a greater reduction in the release of PD.
The CS-GP solution was made using the conjugate with a PD content of 1.4% (w/w). Results are expressed as the mean ± standard deviation (S.D.) (n = 3).
The effects of pH and solubilization on the release of PD were investigated using NG(II). NG(II) also showed pH-dependent release. The release rate from NG(II) was slightly slower at pH 6.8 than at pH 7.4 (Fig. 5A). When THF was added to the aqueous suspension of NG(II) up to a ratio of 1 : 1 (v/v), NG(II) was visually recognized to be mostly solubilized. From the HPLC studies in the solution state of NGs, free GP and PD, generated by hydrolysis of GP, were approximately 10% at most, respectively. Therefore, their burst release was not observed markedly. The release of PD was considered to be promoted to some extent in the solubilized state than in the nanogel state, from the results in Fig. 5B.
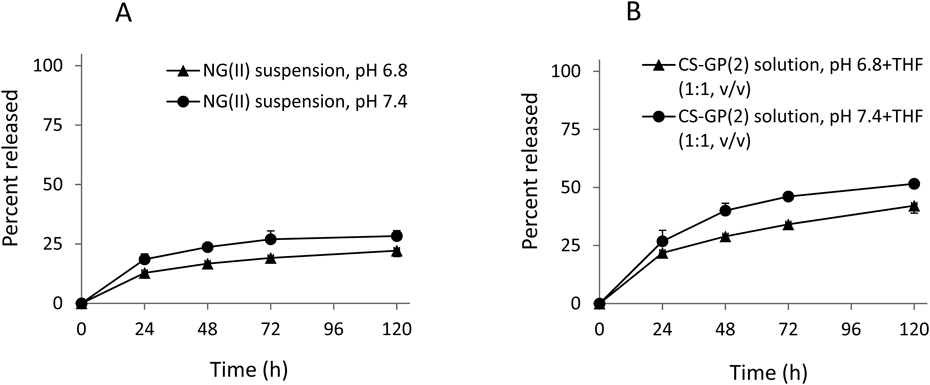
Results are expressed as the mean ± S.D. (n = 3).
The release profiles of PD from NG(II) were compared using JP 1st and 2nd fluids as artificial gastric (pH 1.2) and intestinal (pH 6.8) liquids, respectively. Release was gradual, and was enhanced at pH 6.8 (Fig. 6). These release patterns suggested that the drug absorption from the gastrointestinal tract was lowered and PD was efficiently transported to the lower intestines. Glucocorticoids (GCs) exhibit various functions including anti-inflammation in the following manner; GC is initially transported into cells and binds to the receptor (RC) in the cytosol and the GC-RC complex further moves and binds to DNA.30) Therefore, the PD derivatives with large molecules such as CS and its fragments could not seem to exhibit the pharmacological action due to their steric hindrance and large molecular size. The released PD was considered to be dominantly related to the anti-inflammatory function.
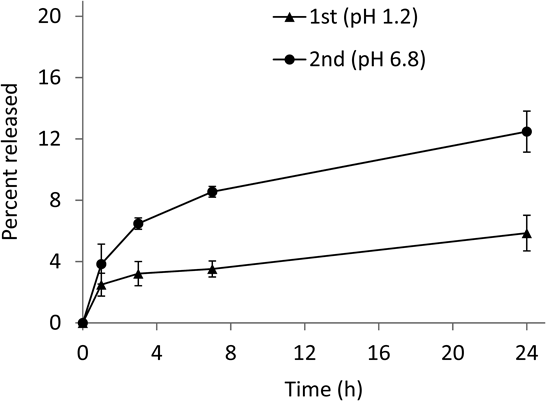
Results are expressed as the mean ± S.D. (n = 3).
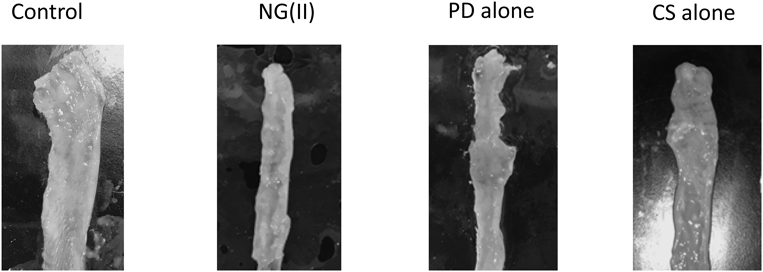
Experiments on therapeutic efficacy and toxic side effects were performed as shown in Fig. 7. Therapeutic efficacy was evaluated from colonic weight and colitis severity indices, the methods for which were reported previously.27,28) The results obtained are shown in Fig. 8. No significant differences were observed in the indices shown as Cp/B and Cd/B. However, NG(II) slightly tended to reduce Cd/B values, which suggests the greater therapeutic efficacy of the nanogel. Although the colonic length was checked in the preliminary study, the tendency of elongation or shortening was not observed clearly due to large variation (data not shown). Regarding T/B values, used often as the index of toxic side effect, thymic atrophy was not significantly induced, and this may have been due to the moderate dose of 5 mg/kg. Colitis severity was evaluated based on SC and CDS. The CDS scores of the control (Cont) were obtained as 3–4; As the values were only slightly smaller than those in the previous study,17) the experiments were performed. NG(II) clearly showed lower scores than the other substances, except for the healthy group. NG(II) exhibited significantly lower CDS than the control (Cont). These results suggested that NG(II) tended to be more effective than PD alone. The marked differences in Cd/B and T/B were not observed among NG(II), PD alone and control, which might be because the present dose would be not sufficient to obtain the large response, as shown in the other reports.15,17) As to CDS, CS tended to worse the tissue conditions. According to the study using dextran-sulfate (DSS)-induced colitis models by Hori et al.,31) CS itself seemed to give recovery effect in inflammatory bowel diseases, which was different from our results. CS was administered daily from the first day in the above DSS ingestion study, while CS was administered 3 times at the advanced stage of ulcerative colitis in the present study. These differences were considered to influence the action of CS. As CS contains many acidic sulfate moieties, it might stimulate the diseased sites of the advanced state of disease.
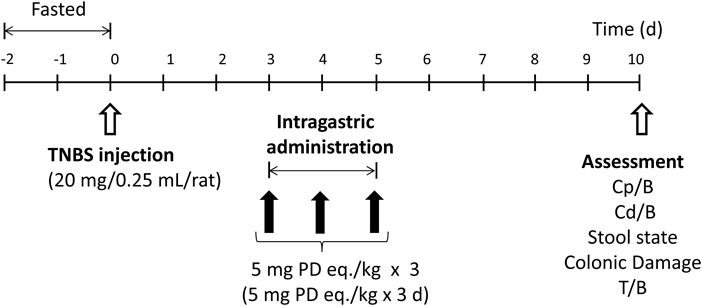
Five groups (control, NG(II), PD alone, CS, and healthy rats) were tested.
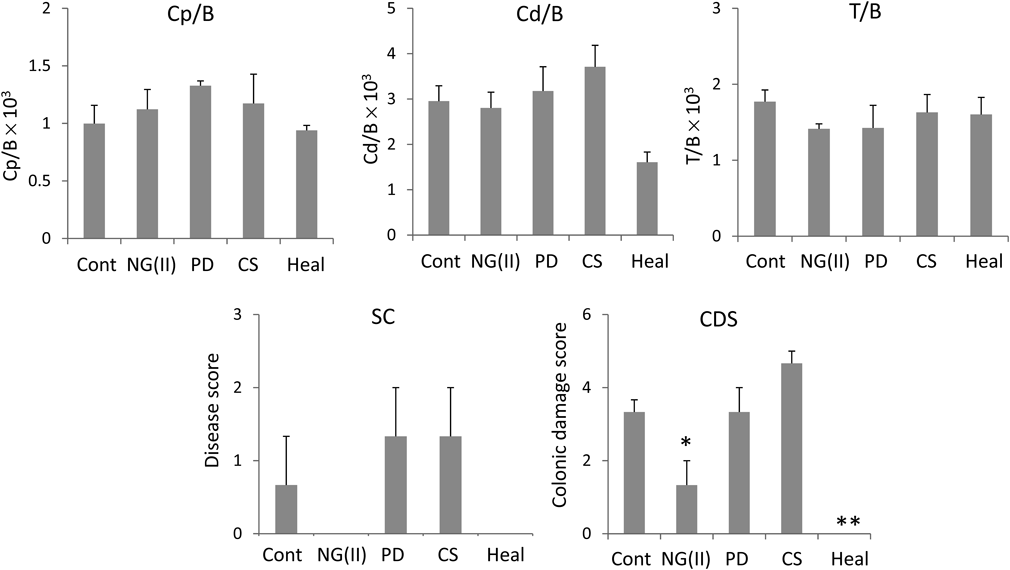
Cont: Control, Heal: Healthy rats. A: (Proximal colon weight)/(body weight) × 103, B: (Distal colon weight)/(body weight) × 103, C: (Thymus weight)/(body weight) × 103, D: Stool consistency, E: Colonic damage score. Results are expressed as the mean ± standard error (S.E.) (n = 3). * p < 0.05, ** p < 0.01 vs. Cont (control) by Dunnett’s test.
Figure 9 shows a representative image of the distal colon of each group. Colitis damage was visually observed to be suppressed the most by NG(II).
Four days after the TNBS instillation, the NG(II) aqueous suspension was administered intragastrically at 5 mg PD eq./kg. Free and total (conjugated + free) PD were investigated 4, 8 and 24 h after administration. Drug distribution profiles were obtained as shown in Fig. 10. At 4 h, free PD was detected in each GI part, and the distributed amount of free PD was not large in any part; however, for ST and SID, free PD was distributed in the range of 10–20 µg. Total PD was mainly observed in ST, SID, and CE. Its distribution was the greatest in SID. Drug distribution profiles showed that NG(II) was mainly distributed in SID at 4 h. Regarding the intragastric administration of PD alone, it was previously reported that PD was mainly observed in ST and upper intestine at 4 h,16,28) in which more than 100 µg was obtained. The present results indicated that NG(II) was rapidly transferred to the lower GI tract, and did not remain in the stomach. These characteristics differed from those of chitosan-succinyl-prednisolone conjugate microparticles, with PD linked to chitosan remaining in the stomach longer due to its positive charge caused by the many amino groups of chitosan.32) Thus, NG(II) appeared to deliver PD efficiently to the lower intestines. At 8 h, the drug moved to further lower intestinal parts. The relative ratio of drug distribution in the large intestine appeared to increase at 8 h as compared to that at 4 h. According to PD alone, no PD was reported to observed in the large intestine.16) Furthermore, the drug was only observed in the CP region at 24 h. Regarding PD alone, no drug was reportedly detected in any region.16,28) The present results supported NG(II) prolonging the retention of the drug in the lower intestines, including the colon. From the literature, including the gastrointestinal distribution studies in the administration of PD alone,16,28) the PD distribution in the colonic part (CP and CD) was less than detection level (0.01%) at 4, 8 and 24 h. From these results, the colonic delivery ratios were obtained in Fig. 10. NG(II) exhibited the delivery of PD to the diseased area, while the drug hardly reached the colitis region after the administration of PD alone. The distributed amount was much less than the administered amount, and it was eliminated considerably rapidly. The NG(II) and its related products, distributed to the tissue parts other than gastrointestinal contents and mucus, were not included in the present analysis. Furthermore, the physicochemical or biological degradation of PD and its related compounds would be caused, and the gastrointestinal content should be excreted as feces with time. These were considered to affect the considerably rapid elimination of the gastrointestinal distributions.
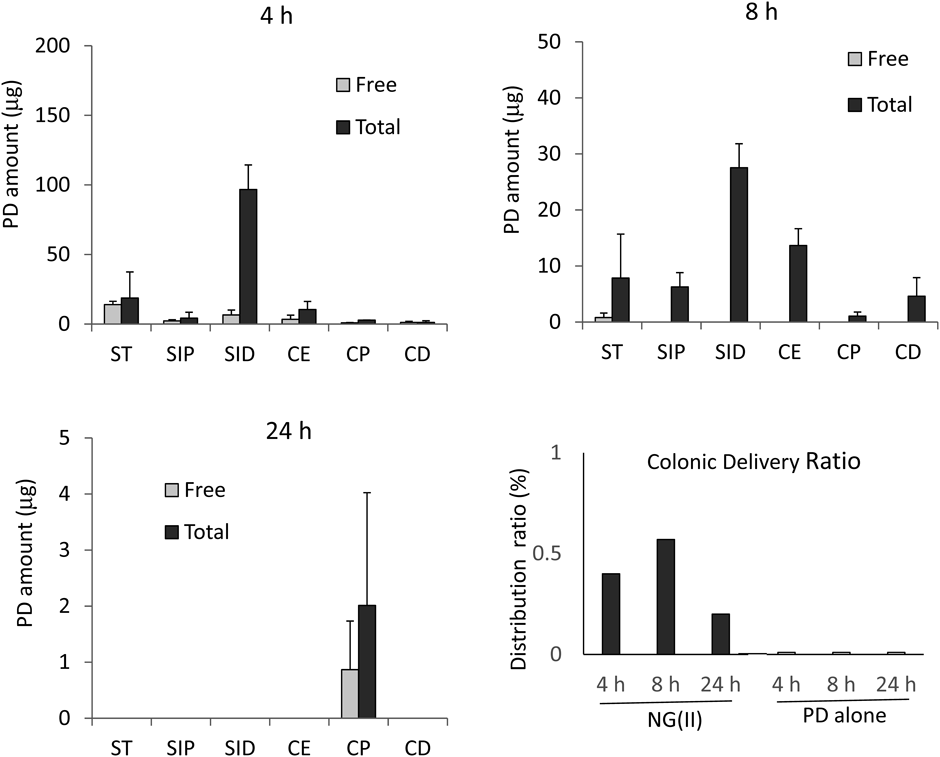
ST: Stomach, SIP: Proximal small intestine, SID: Distal small intestine, CE: Cecum, CP: Proximal colon, CD: Distal colon. The data of gastrointestinal distributions at 4, 8 and 24 h are expressed as the mean ± standard error (S.E.) (n = 3), and the results of the colonic delivery ratio are shown as the mean values (n = 3).
Plasma levels were monitored for 5 h after the intragastric administration of NG(II). The mean concentration was lower than 0.15 µg/mL at every time point (Fig. 11). The plasma concentration-time profile for the intragastric administration of PD alone was previously reported,16) and was shown with a broken line in Fig. 11; plasma levels peaked at approximately 1.5 µg/mL after 1–2 h and then declined. Based on the results for GI tract distribution (Fig. 10), the release of PD was suppressed with NG(II) administration, and the drug was rapidly transferred to the lower intestines. These drug behaviors in the GI tract were considered to be related to the slight absorption associated with the NG(II) administration.
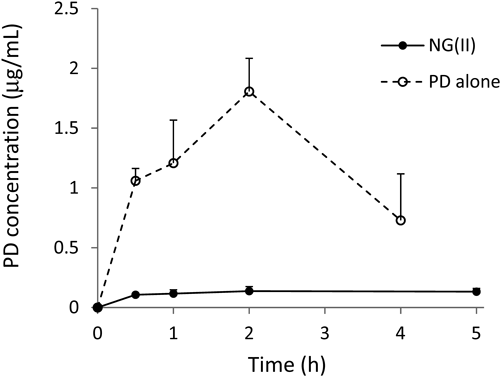
The broken line shows mean plasma concentration–time profiles after the intragastric administration of the PD saline suspension at 5 mg/kg to rats with TNBS-induced colitis, which was reported in the ref. 16. Results are expressed as the mean ± S.E. (n = 3).
The results obtained for therapeutic efficacy, toxic side effects, drug distribution, and pharmacokinetics suggested that NG(II) was superior to PD alone for the transfer and localization of the drug to the lower intestines, including the large intestine. The present nanogel system, produced without treatments such as enteric coating, was confirmed to have a targeting potential to areas affected by colitis. Administration schedules, including doses and frequencies, need to be examined in more detail in order to achieve optimal use for the nanogel in the future.
Nanogels were formed in aqueous media using CS-GP with a moderate or high drug content. The nanogel showed a mean size of 300–600 nm and mean ζ-potential of approximately −40 mV. The PD release rate was dependent on medium pH, being increased at higher pH; approximately 5 and 10% were released at gastric pH (1.2) and intestinal pH (6.8), respectively, per day and this was attributed mainly to the chemical stability of the ester bond of the GP moiety. The therapeutic efficacy of the CS-GP nanogel was better than that of PD alone. GI tract distribution and pharmacokinetic features of the CS-GP nanogel were superior for delivery to the large intestine. The present results suggested that CS-GP nanogel should have the good potential to treat ulcerative colitis.
The authors declare no conflict of interest.