2019 Volume 42 Issue 9 Pages 1517-1523
2019 Volume 42 Issue 9 Pages 1517-1523
Atherosclerosis (AS) is a chronic inflammatory disease threatening human health, and vascular smooth muscle cells (VSMCs) are involved in AS processes. Baicalin is a flavonoid compound, which has anti-atherosclerotic effect. The aim of our study was to explore the molecular mechanism of baicalin on AS. The expression of miR-126-5p was measured in peripheral blood of AS patients and healthy control. We found miR-126-5p expression was decreased in AS. Then, high-mobility group box 1 (HMGB1) was verified as a target of miR-126-5p and its expression was increased in AS. Similarly, miR-126-5p and HMGB1 expression was downregulated and upregulated in oxidized low-density lipoprotein treated VSMCs (ox-LDL-VSMCs), respectively. Furthermore, baicalin upregulated miR-126-5p and downregulated HMGB1 expression. Functionally, baicalin significantly inhibited ox-LDL-VSMCs proliferation and migration, and miR-126-5p targets HMGB1 to enhance the inhibition induced by baicalin. Taken together, baicalin is able to prevent AS, which suppressed the proliferation and migration of ox-LDL-VSMCs through upregulating miR-126-5p by targeting HMGB1. These findings suggested that baicalin is an effective drug to alleviate AS, and miR-126-5p is a novel therapeutic target for AS.
Atherosclerosis (AS) is a chronic inflammatory disease, which damages integrity at the arterial surface and then induces the formation of a thrombus.1) The two main reasons of atherothrombosis are plaque rupture and endothelial erosion. It is reported that AS is the major process of cardiovascular disease, which associated with high rate mortality, leading to angina pectoris, myocardial infarction and even death.2,3) Vascular smooth muscle cells (VSMCs) are involved in remodelling of arterial wall to maintain blood flow in atherosclerosis.4) Like the processes of cancer, increased VSMCs proliferation and migration, changes in transcription factors and adhesion molecules synthesis play important roles in AS.4–6) Therefore, it is critical to understand the pathogenesis of AS and the effect of VSMCs on AS.
Baicalin, one of the flavonoid compounds, is a major active ingredient in Chinese medicinal plant Radix Scutellariae (Huangqin).7) Baicalin has various pharmacological activities, such as anti-inflammation, anti-oxidation, anti-cancer and anti-viral.8–11) Previous study has revealed that baicalin has anti-adipogenic effect, anti-oxidation and anti-inflammatory effect in AS.12) However, the underlying molecular mechanism of baicalin on AS is still largely unknown.
microRNAs (miRNAs) are a family of endogenous, small and non-coding RNAs, which binds with complementarity in the seed region, leading to mRNA degradation and translation inhibition.13) Accumulating evidences show that miRNAs are important regulators in AS pathophysiological processes, such as cell proliferation, lipoprotein homeostasis, endothelial cell inflammation and plaque progression.14) Previous study has revealed that miR-126 expression was decreased in AS.15) However, whether miR-126 associates with the role of baicalin in AS remains unclear.
In the present study, we explored the effect of baicalin on oxidized low-density lipoprotein treated VSMCs (ox-LDL-VSMCs) proliferation and migration, as well as its underlying molecular mechanism. We revealed that miR-126-5p involved in the inhibition in proliferation and migration induced by baicalin in ox-LDL-VSMCs.
Peripheral blood samples were obtained from 30 healthy control and 30 patients with AS who diagnosed at Zhongda Hospital. The blood samples were placed in eppendorf tubes without pyrogen and endotoxin. This study protocol was approved by the Ethics Committee of Zhongda Hospital. The signed consent form was provided by each participator. The clinical feature of patients with AS and healthy control was provided in Table 1.
| AS (n = 30) | Control (n = 30) | p Value | |
|---|---|---|---|
| Age (y) | |||
| <50 | 8 | 9 | 0.7745 |
| ≥50 | 22 | 21 | |
| Gender | 0.7961 | ||
| Male | 14 | 15 | |
| Female | 16 | 15 | |
| Hypertension | 0.4383 | ||
| Yes | 16 | 13 | |
| No | 14 | 17 | |
| Diabetes | 0.4884 | ||
| Yes | 6 | 4 | |
| No | 24 | 26 | |
| Smoke history | 0.7866 | ||
| Yes | 10 | 11 | |
| No | 20 | 19 |
Human-vascular smooth muscle cells (HA-VSMCs) were obtained from American Type Culture Collection (ATCC, Manassas, VA, U.S.A.). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, U.S.A.) and 1% penicillin and streptomycin (V/V, Gibco). The cultural condition of the incubator maintained at 37°C with 5% CO2.
The cells in logarithmic growth were exposed to 100 µg/mL ox-LDL (Solarbio, Beijing, China) for 48 h, called ox-LDL-VSMCs. After that, the cells were treated with 20 µM baicalin for 24 h.
Dual-Luciferase Reporter ActivityTo predict the target gene of miR-126, the online tool TargetScan (http://www.targetscan.org/) was used. Subsequently. to assess the prediction dual-luciferase reporter assay was performed. The 3′UTR of high-mobility group box 1 (HMGB1) containing miR-126-5p binding site was cloned into pGL3 Basic vector (Promega, Madison, WI, U.S.A.), so as the HMGB1 3′UTR mutant. HEK293 cells were seeded in 24-well plates and incubated at 37°C with 5% CO2 for 24 h. Then the cells were co-transfected with miR-126-5p mimics or miR-NC mimics and pGL3-WT-HMGB1 3′UTR or pGL3-Mut-HMGB1 3′UTR using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, U.S.A.). Forty-eight hours post-transfection, firefly luciferase and renilla luciferase activity were measured by a Dual Luciferase Reporter Assay kit (Promega). Renilla luciferase activity was used for the endogenous control.
Cell TransfectionmiR-126-5p mimics and miR-NC mimics were obtained from Ribobio (Guangzhou, China). pcDNA3.1 was obtained from Thermo Fisher Scientific, Inc. The coding sequence of HMGB1 was cloned into pcDNA3.1 plasmid. Ox-LDL-VSMCs were seeded in 96-well plates and were transfected with Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Inc.). After 6 h of transfection, the cells were cultured in complete medium and continuously incubated for 48 h before further analysis.
RT-Quantitative (q)PCRTotal RNA was extracted from blood samples and transfected cells by using Trizol reagent (Invitrogen, Thermo Fisher Scientific, Inc.). Subsequently, total RNA was reverse-transcribed to cDNA using All-in-OneTM First-Strand cDNA Synthesis kit (GeneCopoeia, Rockville, MD, U.S.A.) following the manufacturer’s protocol. Then, qPCR was performed on an ABI PRISM 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, U.S.A.) by using All-in-One™ miRNA qPCR Kit (GeneCopoeia) to detect miRNA expression and All-in-One qPCR mix (GeneCopoeia) to detect mRNA expression according to the manufacturer’s protocol. The sequences of the specific primers (Invitrogen, Thermo Fisher Scientific, Inc.) were as follows: miR-126-5p: F, 5′-CCG ACA CGG GAG ACA ATG-3′; R, 5′-TCT GGA AGT GAG CCA ATG TG-3′, U6: F, 5′-CTG TGC CCA TCT ACG AGG GCT AT-3′; R, 5′-TTT GAT GTC ACG CAC GAT TTC C-3′, HMGB1: F, 5′-CTC AGA GAG GTG GAA GAC CAT GT-3′; R, 5′-GGG ATG TAG GTT TTC ATT TCT CTT TC-3′, glyceraldehyde-3-phosphate dehydrogenase (GAPDH): F, 5′-TTT GGA AGA CCC AGT TCA GA-3′; R, 5′-AGT CCT TCC ACG ATA CCA AAG T-3′. The reaction conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 10 s, 60°C for 20 s and 72°C for 15 s. The relative expression levels were calculated by using the comparative 2−ΔΔCt method,16) with miRNA normalized to U6 and mRNA normalized to GAPDH.
Western BlotTotal protein was extracted by using RIPA Lysis Buffer (Beyotime, Shanghai, China), and the concentration was measured by using Enhanced BCA Protein Assay Kit (Beyotime). Then, 30 µg protein was separated by 10% sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Beyotime). After blocking with Quickblock Blocking Buffer (Beyotime), the membranes were incubated with primary antibodies (anti-HMGB1, ab77302, 1 : 2000; anti-GAPDH, ab8245, 1 : 10000, Abcam, Cambridge, MA, U.S.A.) overnight at 4°C and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Goat Anti-Mouse immunoglobulin G (IgG), ab97040, 1 : 10000, Abcam) for 1 h at room temperature. The protein bands were visualized using BeyoECL Plus reagent (Beyotime) and the intensity of each protein was quantified using ImageQuant LAS 4010 Imaging System (GE Healthcare Life Sciences, Piscataway, NJ, U.S.A.). The relative expression was normalized to the internal control GAPDH.
Cell Proliferation AssayThe capability of cell proliferation was analyzed by Cell Counting Kit-8 (CCK-8; Beyotime). Briefly, transfected ox-LDL-VSMCs were seeded in 96-well plates at the density of 2 × 103 cells/well. At 0, 12, 24 and 48 h of incubation, 10 µL CCK-8 solution was added into each well. The absorbance was detected using a microplate reader (Thermo Fisher Scientific, Inc.) at 450 nm after incubation for 2 h.
Cell Migration AssayFor migration assay, 2 × 105 transfected ox-LDL-VSMCs cells were seeded in 6-well plates and incubation overnight. Then a wound was made by 10 µL sterile pipette tip and the cell debris were washed by phosphate buffered saline (PBS) twice. The cells were continuously cultured for 24 h. The photographs were taken at 0 and 24 h under an inverted microscope (Olympus Corp., Tokyo, Japan). The percent of the wound was quantified.
Statistical AnalysisOur data was analyzed by GraphPad Prism7 software (GraphPad Software, Inc., La Jolla, CA, U.S.A.). The data was expressed as mean ± standard error of the mean (S.E.M.) from at least three independent experiment. Student’s t-test was utilized to analyze data between two groups. One-way ANOVA followed by Newman–Keuls post hoc analysis was used to asses the data among multiple groups. p < 0.05 was considered as a statistical significant.
For this study, the background of 30 pairs of patients with AS and healthy control was provided (Table 1). Patients with AS and healthy control have no significant difference among age, gender, hypertension, diabetes and smoke history. To explore miR-126-5p expression in peripheral blood of patients with AS, RT-qPCR was performed. As compared to healthy control, miR-126-5p expression was greatly reduced in AS (p < 0.01, Fig. 1A). Similarly, after VSMCs treating with ox-LDL, the expression of miR-126-5p was significantly decreased (p < 0.01, Fig. 1B). These results suggested that miR-126-5p may have suppressive effect on AS.

(A) The expression of miR-126-5p was detected in peripheral blood of patients with AS and healthy control by using RT-qPCR. (B) miR-126-5p expression in control and ox-LDL-VSMCs groups was measured by RT-qPCR. VSMCs were played as the control group. ** p < 0.01.
According to the results of TargetScan, the 3′UTR of HMGB1 was directly binds to miR-126-5p (Fig. 2A). Furthermore, dual-luciferase reporter assay results suggested that overexpression of miR-126-5p markedly decreased luciferase activity when cells co-transfected with pGL3-WT-HMGB1 3′UTR, compared with miR-NC mimics (p < 0.01). However, in HMGB1 3′UTR mutant type, miR-126-5p had no effect on luciferase activity (Fig. 2B). These findings demonstrated that HMGB1 was a target gene of miR-126-5p.

(A) miR-126-5p could bind to the 3′UTR of HMGB1, which was predicted using TargetScan. (B) The prediction was confirmed by dual-luciferase reporter assay. miR-126-5p mimics or miR-NC mimics and pGL3-WT-HMGB1 3′UTR or pGL3-Mut-HMGB1 3′UTR were co-transfected into HEK293 cells, and only miR-126-5p mimics reduced luciferase activity in WT HMGB1 3′UTR. ** p < 0.01.
Then the expression of HMGB1 was detected in peripheral blood of patients with AS and healthy control by using RT-qPCR and Western blot. As illustrated in Fig. 3A, HMGB1 mRNA expression was significantly increased in AS, compared with that in control group (p < 0.01). Similarly, the protein expression of HMGB1 was also elevated in AS (p < 0.01, Fig. 3B). Additionally, the mRNA and protein levels of HMGB1 were upregulated in VSMCs treated with ox-LDL, compared with VSMCs (p < 0.01, Figs. 3C, D). These findings further confirmed HMGB1 is a target of miR-126-5p, and suggested it had promoting effect on AS.

The (A) mRNA and (B) protein expression of HMGB1 was examined in peripheral blood of AS and healthy control. The (C) mRNA and (D) protein levels of HMGB1 was measured in VSMCs and ox-LDL-VSMCs. ** p < 0.01.
After ox-LDL-VSMCs were treated with baicalin, the expression of miR-126-5p and HMGB1 was measured. Baicalin induced the upregulation of miR-126-5p (p < 0.01) and the downregulation of HMGB1 in both mRNA and protein levels (p < 0.01, Figs. 4A–C).
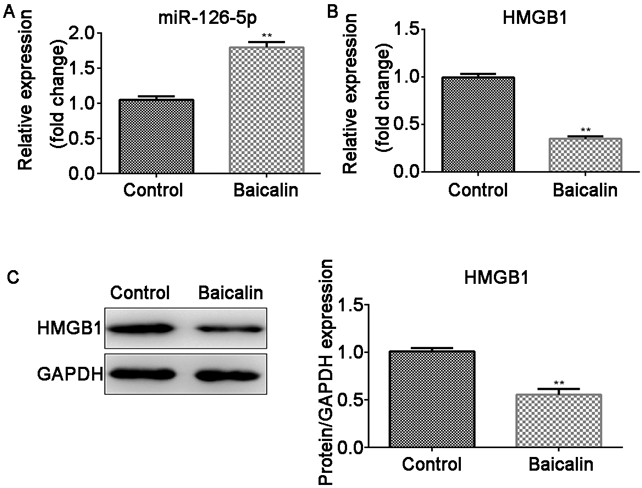
(A) miR-126-5p expression, (B) mRNA and (C) protein expression of HMGB1 were measured in ox-LDL-VSMCs treated with or without baicalin. ** p < 0.01.
Ox-LDL-VSMCs were transfected with miR-NC mimics, miR-126-5p mimics, pcDNA3.1 and pcDNA3.1-HMGB1 after treated with baicalin, then transfection efficiency was examined. The expression of miR-126-5p was increased after ox-LDL-VSMCs transfected with miR-126-5p mimics, compared with miR-NC mimics (p < 0.01, Fig. 5A). The expression of HMGB1 was elevated in pcDNA3.1-HMGB1 transfected ox-LDL-VSMCs, compared with pcDNA3.1 (p < 0.01, Fig. 5B). The results from CCK-8 demonstrated that baicalin markedly inhibited cell proliferation (p < 0.01). miR-126-5p enhanced the inhibition induced by baicalin (p < 0.01), while HMGB1 reversed the effects of miR-126-5p (p < 0.01, Fig. 5C). Similarly, cell migration capability was suppressed by baicalin (p < 0.01), and it was further reduced by miR-126-5p (p < 0.01), which was reversed by HMGB1 (p < 0.01, Fig. 5D). Collectively, baicalin inhibited the proliferation and migration by upregulation of miR-126-5p via targeting HMGB1.

(A) The transfection efficiency was examined after ox-LDL-VSMCs transfected with miR-NC mimics and miR-126-5p mimics by RT-qPCR. (B) The transfection efficiency was examined after ox-LDL-VSMCs transfected with pcDNA3.1 and pcDNA3.1-HMGB1. (C) Cell proliferation in transfected ox-LDL-VSMCs was detected by CCK-8. (D) Cell migration was measured by wound healing assay in transfected ox-LDL-VSMCs. ** p < 0.01.
In this study, we focused on the molecular mechanism underlying baicalin effects on ox-LDL-VSMCs. We found miR-126-5p expression was decreased and HMGB1 was increased in both peripheral blood of AS and ox-LDL-VSMCs. Baicalin upregulated miR-126-5p and downregulated HMGB1 expression in ox-LDL-VSMCs. Furthermore, the proliferation and migration of ox-LDL-VSMCs was inhibited by baicalin, and the inhibition was enhanced by miR-126-5p, which was reversed by HMGB1.
There are several evidences that baicalin is able to prevent AS.12,17) Baicalin attenuates atherosclerotic lesions progression through lipids regulation and immunoregulation.18) Additionally, baicalin could combined with geniposide to mediated the phenotype of dendritic cells in bone marrow and anherosclerotic plaque, which reduced atherosclerotic lesions.19) Moreover, Baicalin inhibits ox-LDL treated VSMCs proliferation and promotes apoptosis through MEG3/p53 pathway.20) Our study also revealed that baicalin inhibited the proliferation and migration in ox-LDL-VSMCs, which confirmed the anti-AS effect of baicalin. However, the underlying molecular mechanism needs to be further studied.
miRNAs expression in peripheral blood were significantly different in patients with or without AS, and this study indicated that the level of miR-126 is markedly decreased in AS patients.15) On the other hand, previous studies have shown that HMGB1 level in serum of patients with AS was significantly increased.21,22) Our study also showed that miR-126-5p was downregulated and HMGB1 was upregulated in peripheral blood of patients with AS, which were consistent with previous findings.
Then, we further found the expression of miR-126-5p was downregulated in ox-LDL-VSMCs. Previous studies on miR-126-5p has focused on tumors, such as non-small cell lung cancer,23,24) giant cell tumor,25,26) followed by Parkinson’s disease.27) In AS, miR-126 could decrease the AS progression through reducing the cytokine release.28) Furthermore, Administration of miR-126-5p reduces endothelial cell proliferation and limits AS.29) Additionally, miR-126 expression was altered by baicalin, which could alleviate interleukin 1β (IL-1β)-induced inflammatory injury by regulating miR-126.30) Otherwise, miRNAs expression could be regulated by baicalin, such as Wang et al. reported that baicalin obviously downregulates miR-294 expression in mouse embryonic stem cells D3.31) Baicalin treated hepatic stellate cells increased the expression of miR-3595.32) In this study, baicalin induced the increased expression of miR-126-5p in ox-LDL-VSMCs. Moreover, baicalin inhibited ox-LDL-VSMCs proliferation, and overexpression of miR-126-5p enhanced the inhibition.
HMGB1 is a nuclear protein, which plays an important role in AS,33) especially in ox-LDL induced AS.34) HMGB1 is involved in the maintaining and amplifying inflammation in AS,35) as well as in some cellular processes. For example, HMGB1 is a target of miR-22-3p, which regulates arterial smooth muscle cell proliferation and migration in AS obliterans.36) In addition, miR-24 targets HMGB1 to inhibit high glucose-induced VSMCs proliferation and migration.37) In the present study, HMGB1 was identified as a target of miR-126-5p. The level of HMGB1 was increased in ox-LDL-VSMCs, and was downregulated by baicalin. Moreover, it abolished the inhibition in proliferation and migration induced by miR-126-5p. These results suggested that baicalin suppresses ox-LDL-VSMCs proliferation and migration through regulating miR-126-5p via targeting HMGB1.
In conclusion, miR-126-5p expression was decreased in peripheral blood of patients with AS and ox-LDL-VSMCs, and the expression of HMGB1, a target of miR-126-5p, was increased. Moreover, Baicalin induced upregulation of miR-126-5p and downregulation of HMGB1. Functionally, this study is the first to find that baicalin inhibited ox-LDL-VSMCs proliferation and migration by upregulating miR-126-5p via targeting HMGB1. Therefore, baicalin could be a promising drug and miR-126-5p may be a novel target for AS treatment.
This study is funded by the National Nature Science Foundation of the People’s Republic of China (No. 81500204 and No. 81600227).
The authors declare no conflict of interest.